Abstract
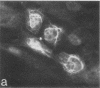
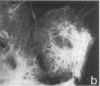
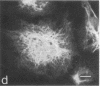
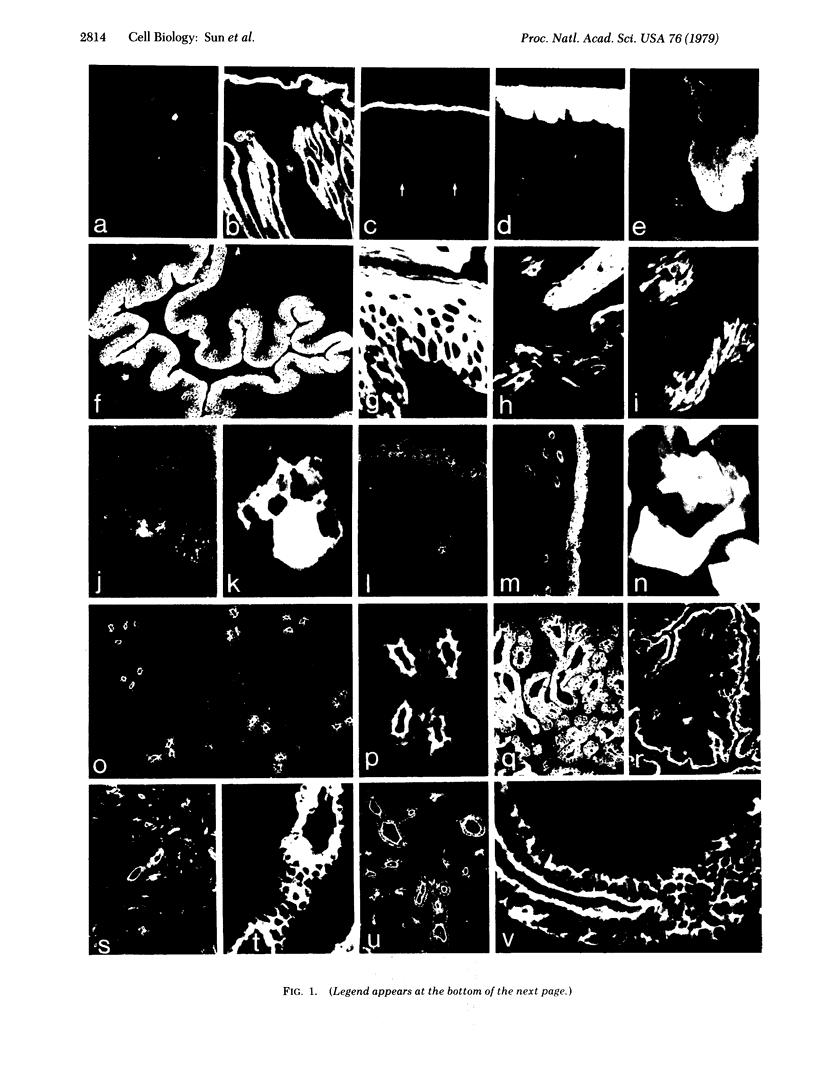
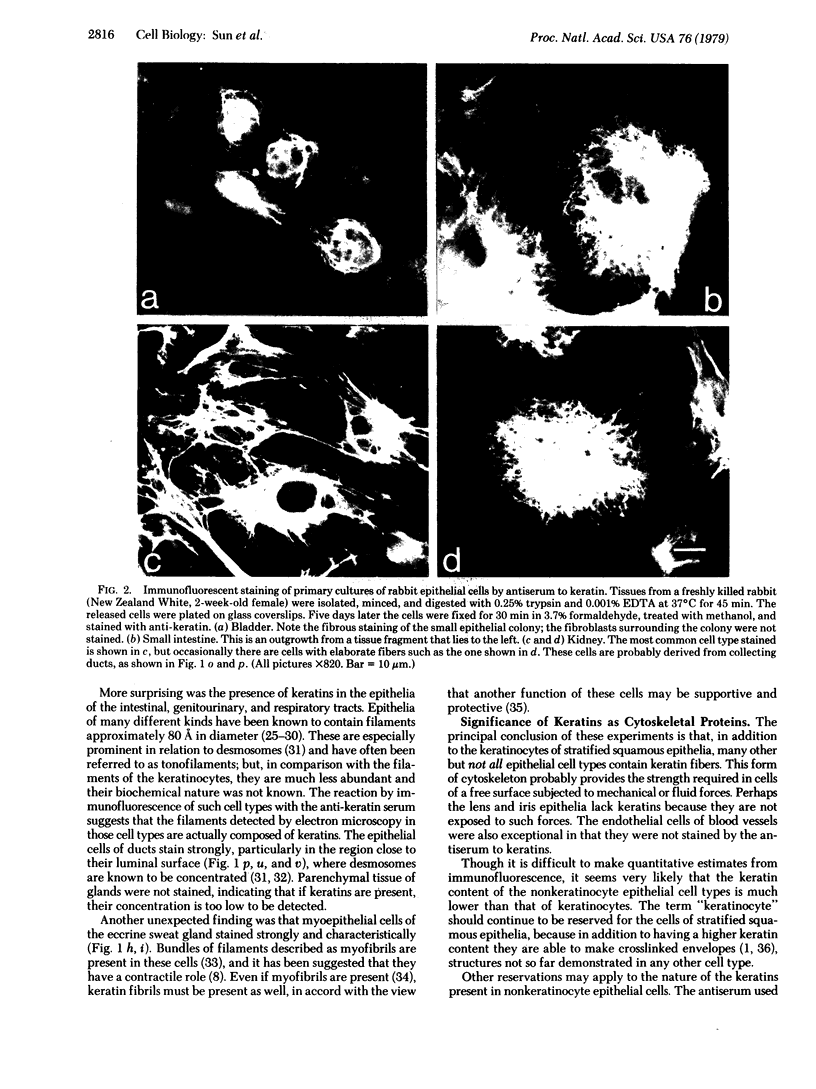
An antiserum against human epidermal keratins was used to detect keratins in frozen sections of various rabbit and human tissues by indirect immunofluorescence. Strong staining was observed in all stratified squamous epithelia (epidermis, cornea, conjunctiva, tongue, esophagus, vagina, and anus), in epidermal appendages (hair follicle, sebaceous gland, ductal and myoepithelial cells of sweat glands), as well as in Hassall's corpuscles of the thymus, indicating that all contain abundant keratins. No staining by the antiserum was observed in fibroblasts, muscle of any type, cartilage, blood vessel, nerve tissue, iris or lens epithelium, or the glomerular or tubular cells of the kidney. In contrast, the antiserum stained the cells of most epithelia of the intestinal tract, urinary tract (urethra, bladder, ureter, collecting ducts of kidney), female genital tract (cervix, cervical glands, uterus, and oviduct), and respiratory tract (trachea and bronchi). Epithelial cells of the fine ductal system in the pancreas and submaxillary gland also stained well. When primary cultures of epithelial cells derived from bladder, intestine, kidney, and trachea were grown on glass coverslips and stained with anti-keratin, fiber networks similar to those of cultured keratinocytes were observed. These results show that keratins constitute a cytoskeleton in epithelial cells of diverse morphology and embryological origin. The stability of keratin filaments probably confers the structural strength necessary for cells covering a free surface. Keratin staining can be used to obtain information about the origin of cell lines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer F. L., Beck J. S., Melvin J. M. Localization of smooth muscle protein in myoepithelium by immunofluorescence. Am J Pathol. 1971 Apr;63(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Ellis R. A. Fine structure of the myoepithelium of the eccrine sweat glands of man. J Cell Biol. 1965 Dec;27(3):551–563. doi: 10.1083/jcb.27.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Junctional complexes in various epithelia. J Cell Biol. 1963 May;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. The intermediate-sized filaments in rat kangaroo PtK2 cells. II. Structure and composition of isolated filaments. Cytobiologie. 1978 Aug;17(2):392–411. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Freudenstein C., Franke W. W., Osborn M., Weber K. Reaction of tonofilament-like intermediate-sized filaments with antibodies raised against isolated defined polypeptides of bovine hoof prekeratin. Cell Biol Int Rep. 1978 Nov;2(6):591–600. doi: 10.1016/0309-1651(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- HURLEY H. J., Jr, SHELLEY W. B. The role of the myoepithelium of the human apocrine sweat gland. J Invest Dermatol. 1954 Feb;22(2):143–156. doi: 10.1038/jid.1954.19. [DOI] [PubMed] [Google Scholar]
- Hicks R. M. The fine structure of the transitional epithelium of rat ureter. J Cell Biol. 1965 Jul;26(1):25–48. doi: 10.1083/jcb.26.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Dionne G. P. Tonofilaments in normal human bronchial epithelium and in squamous cell carcinoma. Am J Pathol. 1977 Aug;88(2):345–354. [PMC free article] [PubMed] [Google Scholar]
- Jones H. W., Jr, McKusick V. A., Harper P. S., Wuu K. D. George Otto Gey. (1899-1970). The HeLa cell and a reappraisal of its origin. Obstet Gynecol. 1971 Dec;38(6):945–949. [PubMed] [Google Scholar]
- KOHNEN P., WEISS L. AN ELECTRON MICROSCOPIC STUDY OF THYMIC CORPUSCLES IN THE GUINEA PIG AND THE MOUSE. Anat Rec. 1964 Jan;148:29–57. doi: 10.1002/ar.1091480104. [DOI] [PubMed] [Google Scholar]
- Leibovitz A., McCombs W. M., 3rd, Johnston D., McCoy C. E., Stinson J. C. New human cancer cell culture lines. I. SW-13, small-cell carcinoma of the adrenal cortex. J Natl Cancer Inst. 1973 Aug;51(2):691–697. [PubMed] [Google Scholar]
- Lyampert I. M., Beletskaya L. V., Borodiyuk N. A., Gnezditskaya E. V., Rassokhina I. I., Danilova T. A. A cross-reactive antigen of thymus and skin epithelial cells common with the polysaccharide of group A streptococci. Immunology. 1976 Jul;31(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Born T., Koitsch H. J., Weber K. Stereo immunofluorescence microscopy: I. Three-dimensional arrangement of microfilaments, microtubules and tonofilaments. Cell. 1978 Jul;14(3):477–488. doi: 10.1016/0092-8674(78)90234-9. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson W. D., Jr, Stulberg C. S., Simpson W. F. A permanent heteroploid human cell line with type B glucose-6-phosphate dehydrogenase. Proc Soc Exp Biol Med. 1971 Apr;136(4):1187–1191. doi: 10.3181/00379727-136-35455. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975 Nov;6(3):317–330. doi: 10.1016/0092-8674(75)90183-x. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975 Nov;6(3):331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rhodin J. A. The ciliated cell. Ultrastructure and function of the human tracheal mucosa. Am Rev Respir Dis. 1966 Mar;93(3 Suppl):1–15. doi: 10.1164/arrd.1966.93.3P2.1. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Green H. Relation of protein synthesis and transglutaminase activity to formation of the cross-linked envelope during terminal differentiation of the cultured human epidermal keratinocyte. J Cell Biol. 1978 Mar;76(3):705–711. doi: 10.1083/jcb.76.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer W. W., Lynch R. G. Immunofluorescence studies of neurofilaments in the rat and human peripheral and central nervous system. J Cell Biol. 1977 Jul;74(1):241–250. doi: 10.1083/jcb.74.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Cultured epithelial cells of cornea, conjunctiva and skin: absence of marked intrinsic divergence of their differentiated states. Nature. 1977 Oct 6;269(5628):489–493. doi: 10.1038/269489a0. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Takigawa M., Imamura S. Experimental production of rabbit anti-guinea-pig epidermal cell sera. Comparison to pemphigus antibodies. J Invest Dermatol. 1977 May;68(5):259–264. doi: 10.1111/1523-1747.ep12506651. [DOI] [PubMed] [Google Scholar]
- Yasamura Y., Tashjian A. H., Jr, Sato G. H. Establishment of four functional, clonal strains of animal cells in culture. Science. 1966 Dec 2;154(3753):1186–1189. doi: 10.1126/science.154.3753.1186. [DOI] [PubMed] [Google Scholar]
- von Gaudecker B., Schmale E. M. Similarities between Hassall's corpuscles of the human thymus and the epidermis. An investigation by electron microscopy and histochemistry. Cell Tissue Res. 1974;151(3):347–368. doi: 10.1007/BF00224546. [DOI] [PubMed] [Google Scholar]