Abstract
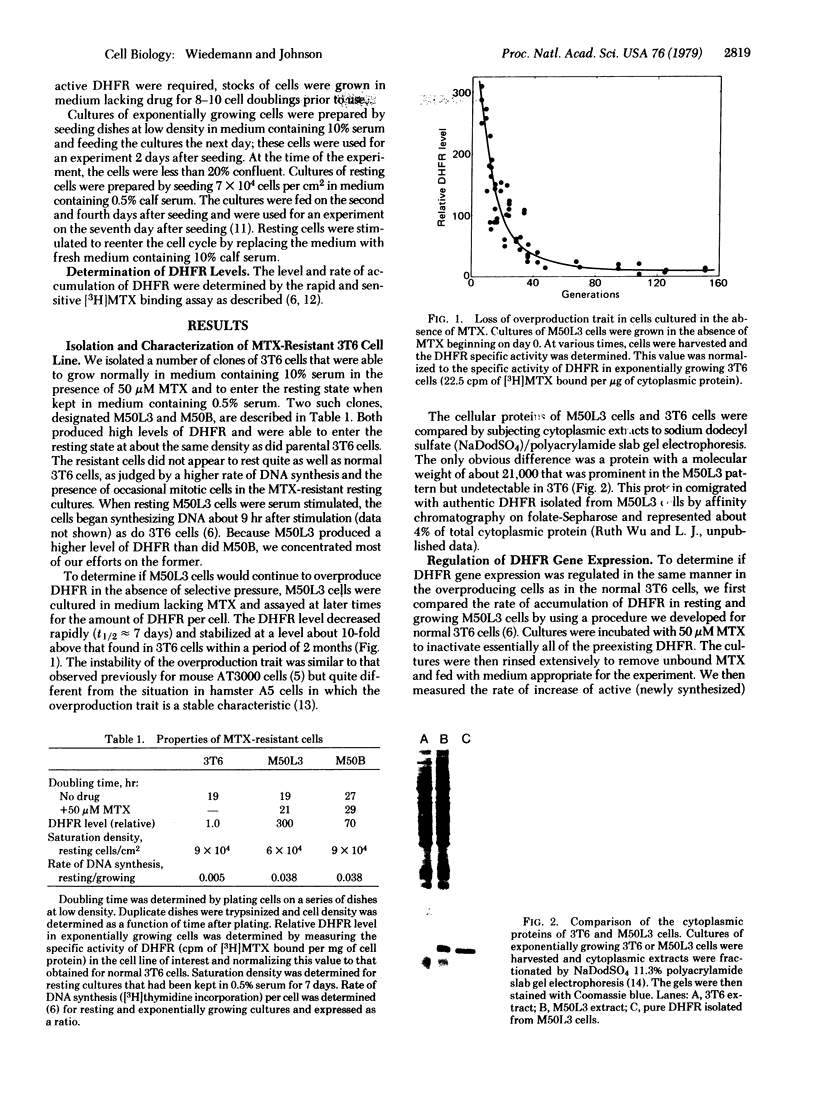
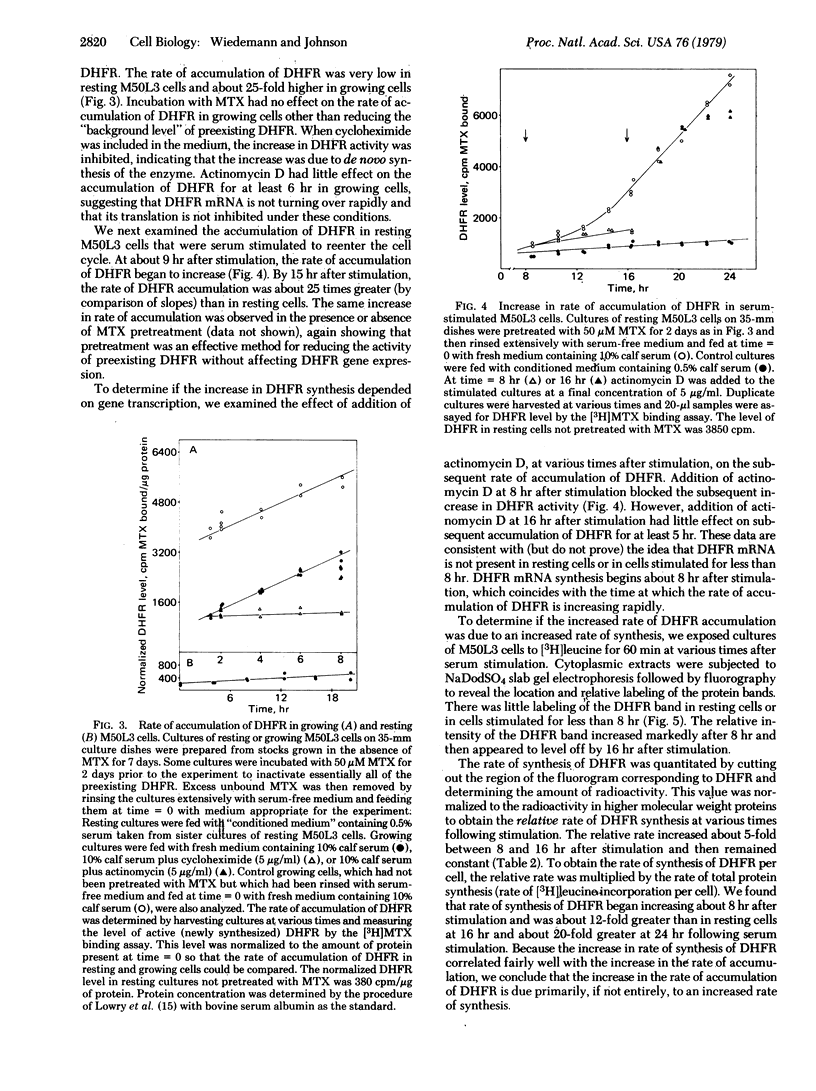
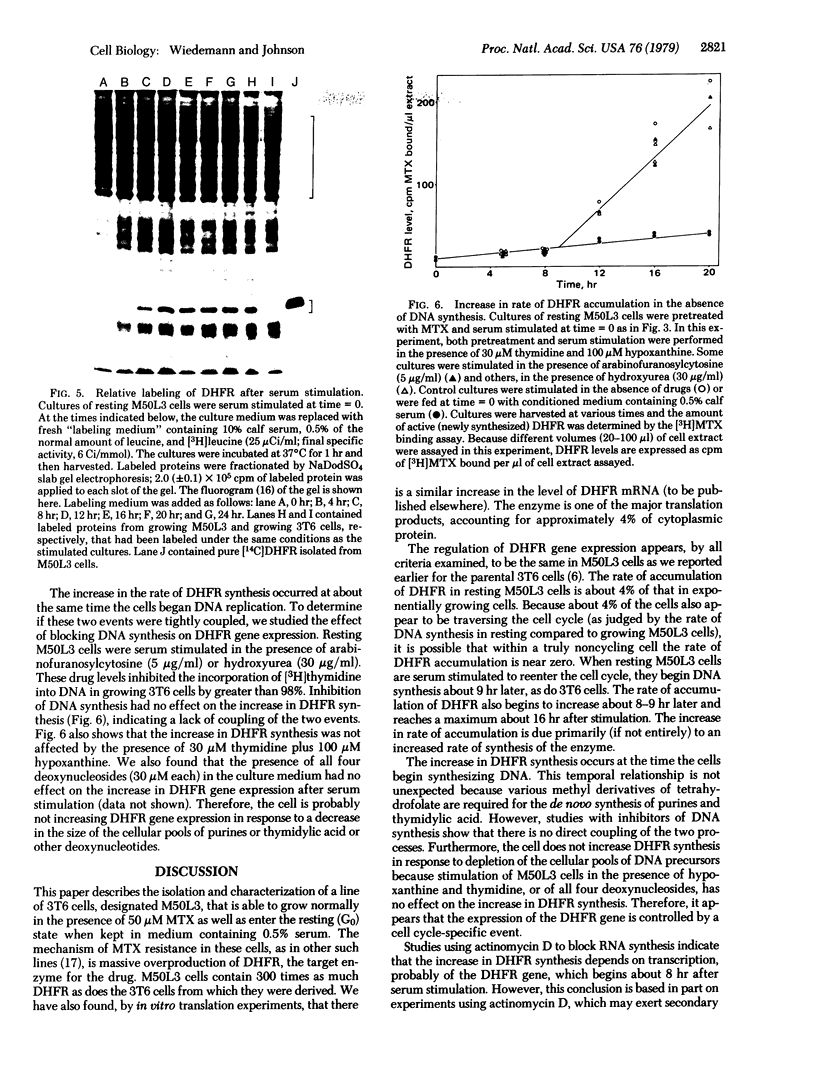
We have isolated a methotrexate (MTX)-resistant clone of mouse 3T6 cells, designated M50L3, which grows normally in the presence or absence of 50 μM MTX and produces a level of dihydrofolate reductase (DHFR; 5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase, EC 1.5.1.3) that is increased about 300-fold compared to the parental 3T6 cells. The cells retain the ability to rest in the G0 state when maintained in medium containing 0.5% calf serum and can be stimulated to reenter the cell cycle by increasing the serum concentration to 10%. The rate of accumulation of DHFR in resting M50L3 cells is about 1/25th of that in exponentially growing cells. When resting cells are stimulated to reenter the cell cycle, the rate of accumulation of DHFR starts to increase at about 8 hr and reaches a maximum (25-fold increase) at about 16 hr after stimulation. Pulse-labeling experiments show that the increase in DHFR accumulation is due to an increased rate of synthesis. This increase occurs at about the same time the cells enter S phase. However, inhibitors of DNA synthesis have no effect on the increase in DHFR accumulation after serum stimulation, indicating that there is no tight coupling of the two events. Actinomycin D inhibits the subsequent increase in DHFR accumulation if added 8 hr after stimulation but has no effect if added 16 hr after stimulation. This is consistent with the idea that the increase in DHFR gene expression depends on transcription of the gene and that DHFR mRNA synthesis begins at about the time the cell initiates DNA replication. DHFR gene expression appears to be regulated in the same manner in the overproducing cells as we found in the parental 3T6 cells [Johnson, L. F., Fuhrman, C. L. & Wiedemann, L. M. (1978) J. Cell. Phys. 97, 397-406]. Therefore, the alterations that are responsible for DHFR overproduction (presumably DHFR gene amplification) do not interfere with the ability of the cell to regulate the rate of synthesis of the enzyme after serum stimulation.
Keywords: gene expression, cell cycle, methotrexate
Full text
PDF

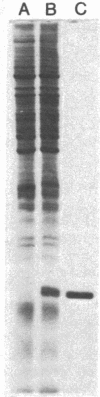
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Schimke R. T. Synthesis and degradation of folate reductase in sensitive and methotrexate-resistant lines of S-180 cells. J Biol Chem. 1976 May 25;251(10):3063–3074. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Littlefield J. W. Elevated dihydrofolate reductase messenger RNA levels in methotrexate-resistant BHK cells. Cell. 1976 Mar;7(3):391–396. doi: 10.1016/0092-8674(76)90168-9. [DOI] [PubMed] [Google Scholar]
- Chello P. L., McQueen C. A., DeAngelis L. M., Bertino J. R. Comparative effects of folate antagonists versus enzymatic folate depletion on folate and thymidine enzymes in cultured mammalian cells. Cancer Treat Rep. 1977 Jul;61(4):539–548. [PubMed] [Google Scholar]
- HAKALA M. T., ZAKRZEWSKI S. F., NICHOL C. A. Relation of folic acid reductase to amethopterin resistance in cultured mammalian cells. J Biol Chem. 1961 Mar;236:952–958. [PubMed] [Google Scholar]
- Hillcoat B. L., Swett V., Bertino J. R. Increase of dihydrofolate reductase activity in cultured mammalian cells after exposure to methotrexate. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1632–1637. doi: 10.1073/pnas.58.4.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- Kamen B. A., Takach P. L., Vatev R., Caston J. D. A rapid, radiochemical-ligand binding assay for methotrexate. Anal Biochem. 1976 Jan;70(1):54–63. doi: 10.1016/s0003-2697(76)80047-4. [DOI] [PubMed] [Google Scholar]
- Kellems R. E., Alt F. W., Schimke R. T. Regulation of folate reductase synthesis in sensitive and methotrexate-resistant sarcoma 180 cells. In vitro translation and characterization of folate reductase mRNA. J Biol Chem. 1976 Nov 25;251(22):6987–6993. [PubMed] [Google Scholar]
- Kellems R. E., Morhenn V. B., Pfendt E. A., Alt F. W., Schimke R. T. Polyoma virus and cyclic AMP-mediated control of dihydrofolate reductase mRNA abundance in methotrexate-resistant mouse fibroblasts. J Biol Chem. 1979 Jan 25;254(2):309–318. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Littlefield J. W. Purification, properties, and synthesis of dihydrofolate reductase from wild type and methotrexate-resistant hamster cells. J Biol Chem. 1972 Jan 10;247(1):179–187. [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]