Abstract
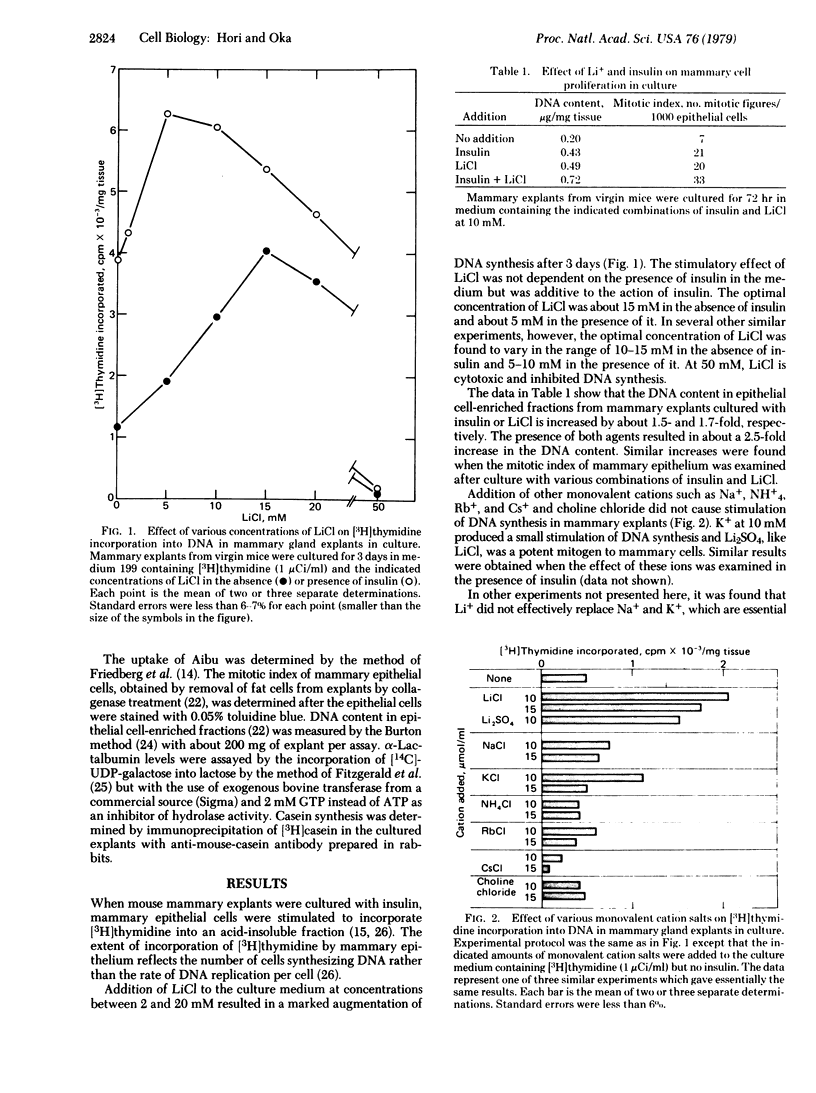
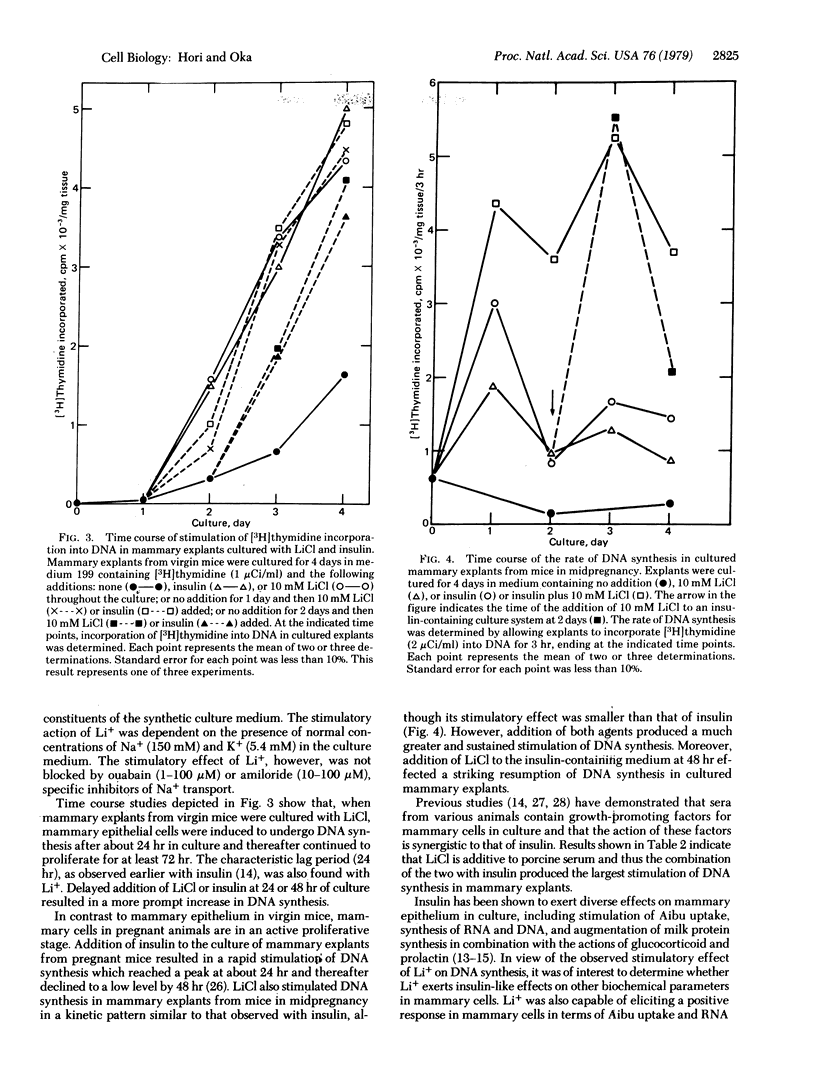
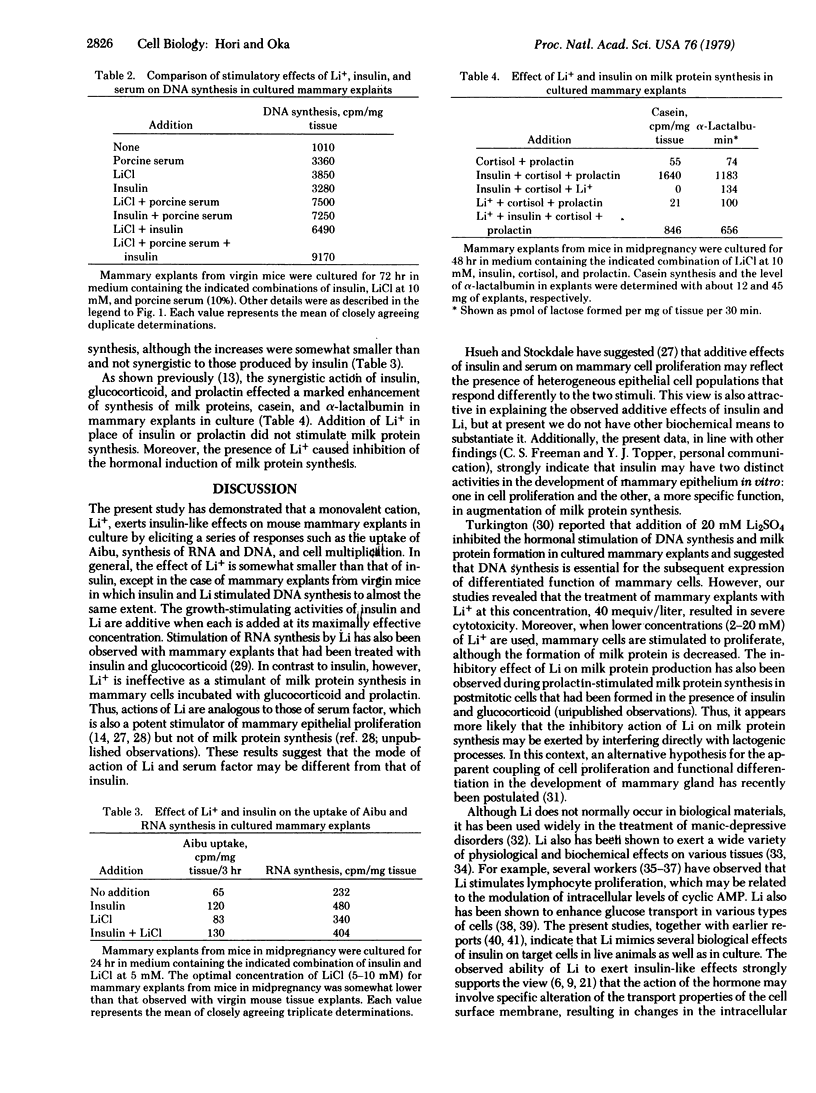
Lithium ion at concentrations between 2 and 20 mM simulated the stimulatory effects of insulin on the uptake of alpha-aminoisobutyric acid, synthesis of RNA and DNA, and cell multiplication in mouse mammary gland explants cultured in a chemically defined synthetic medium. Other monovalent cations were virtually ineffective. In most instances the stimulatory effect of lithium ion was somewhat smaller than and additive to that of insulin. However, lithium ion was incapable of substituting for the action of insulin in augmenting milk protein synthesis in mammary explants cultured with other lactogenic hormones, prolactin, and glucocorticoid. The observed similarities of the responses of mammary cells to lithium and insulin suggest that possible importance of cation(s) in the regulation of mammary cell proliferation, which may be a common basis for the action of the two agents. On the other hand, the observed inability of lithium to mimic the lactogenic effect of insulin indicates a specific function of the hormone in the functional differentiation of mammary cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BHATTACHARYA G. INFLUENCE OF LI+ ON GLUCOSE METABOLISM IN RATS AND RABBITS. Biochim Biophys Acta. 1964 Dec 9;93:644–646. doi: 10.1016/0304-4165(64)90347-2. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T., Iversen J. G. K+ transport in isolated rat liver cells stimulated by glucagon and insulin in vitro. Acta Physiol Scand. 1976 Jun;97(2):202–208. doi: 10.1111/j.1748-1716.1976.tb10253.x. [DOI] [PubMed] [Google Scholar]
- Clausen T., Elbrink J., Martin B. R. Insulin controlling calcium distribution in muscle and fat cells. Acta Endocrinol Suppl (Copenh) 1974;191:137–143. doi: 10.1530/acta.0.077s0137. [DOI] [PubMed] [Google Scholar]
- Clausen T. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. Biochim Biophys Acta. 1968 Jan 3;150(1):66–72. doi: 10.1016/0005-2736(68)90009-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Czech M. P. Molecular basis of insulin action. Annu Rev Biochem. 1977;46:359–384. doi: 10.1146/annurev.bi.46.070177.002043. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Elkington J. Induction of growth in resting fibroblastic cell cultures by Ca++. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1584–1588. doi: 10.1073/pnas.72.4.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. K., Colvin B., Mawal R., Ebner K. E. Enzymic assay for galactosyl transferase activity of lactose synthetase and alpha-lactalbumin in purified and crude systems. Anal Biochem. 1970 Jul;36(1):43–61. doi: 10.1016/0003-2697(70)90330-1. [DOI] [PubMed] [Google Scholar]
- Friedberg S. H., Oka T., Topper Y. J. Development of insulin-sensitivity by mouse mammary gland in vitro. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1493–1500. doi: 10.1073/pnas.67.3.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., Dosch H. M., Hastings B., Shore A. Lithium: a modulator of cyclic AMP-dependent events in lymphocytes? Science. 1979 Jan 26;203(4378):365–367. doi: 10.1126/science.216075. [DOI] [PubMed] [Google Scholar]
- Haugaard N. Metabolic and electrolyte changes produced by lithium ions in the isolated rat diaphragm. Biochem Pharmacol. 1975 Jun 15;24(11-12):1187–1191. doi: 10.1016/0006-2952(75)90060-x. [DOI] [PubMed] [Google Scholar]
- Hershko A., Mamont P., Shields R., Tomkins G. M. "Pleiotypic response". Nat New Biol. 1971 Aug;232(33):206–211. [PubMed] [Google Scholar]
- Holley R. W., Kiernan J. A. "Contact inhibition" of cell division in 3T3 cells. Proc Natl Acad Sci U S A. 1968 May;60(1):300–304. doi: 10.1073/pnas.60.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh H. W., Stockdale F. E. Serum and insulin initiation of DNA synthesis in mammary gland epithelium in vitro. J Cell Physiol. 1974 Apr;83(2):297–308. doi: 10.1002/jcp.1040830217. [DOI] [PubMed] [Google Scholar]
- Majumder G. C., Turkington R. W. Stimulation of mammary epithelial cell proliferation in vitro by protein factor(s) present in serum. Endocrinology. 1971 Jun;88(6):1506–1510. doi: 10.1210/endo-88-6-1506. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehan W. L., Ham R. G. Calcium and magnesium ions and the regulation of multiplication in normal and transformed cells. Nature. 1978 Oct 26;275(5682):756–758. doi: 10.1038/275756a0. [DOI] [PubMed] [Google Scholar]
- Mickel R. A., Hallidy L., Haugaard N., Haugaard E. S. Stimulation by lithium ions of the incorporation of [U-14C]glucose into glycogen in rat brain slices. Biochem Pharmacol. 1978 Mar 1;27(5):799–800. doi: 10.1016/0006-2952(78)90524-5. [DOI] [PubMed] [Google Scholar]
- Monaco M. E., Lippmann M. E., Knazek R., Kidwell W. R. Vasopressin stimulation of acetate incorporation into lipids in a dimethylbenz(a)anthracene-induced rat mammary tumor cell line. Cancer Res. 1978 Nov;38(11 Pt 2):4101–4104. [PubMed] [Google Scholar]
- Oka T., Perry J. W., Topper Y. J. Changes in insulin responsiveness during development of mammary epithelium. J Cell Biol. 1974 Aug;62(2):550–556. doi: 10.1083/jcb.62.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillema J. A., Smith R. D. Effect of lithium ions on RNA synthesis in mammary gland explants of mice. Proc Soc Exp Biol Med. 1975 Jun;149(2):573–575. doi: 10.3181/00379727-149-38854. [DOI] [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. Do viruses use calcium ions to shut off host cell functions? Nature. 1978 Jan 12;271(5641):186–187. doi: 10.1038/271186c0. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Seifert W., Gospodarowicz D. Growth control in cultured mouse fibroblasts: induction of the pleiotypic and mitogenic responses by a purified growth factor. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2600–2604. doi: 10.1073/pnas.71.7.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Lundgren D. W., Oka T. Polyamine biosynthesis and DNA synthesis in cultured mammary gland explants from virgin mice. J Cell Physiol. 1978 Jun;95(3):259–267. doi: 10.1002/jcp.1040950303. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Membrane bound and cellular cationic changes associated with insulin stimulation of cultured cells. J Cell Physiol. 1978 Sep;96(3):265–278. doi: 10.1002/jcp.1040960302. [DOI] [PubMed] [Google Scholar]
- Schou M. Pharmacology and toxicology of lithium. Annu Rev Pharmacol Toxicol. 1976;16:231–243. doi: 10.1146/annurev.pa.16.040176.001311. [DOI] [PubMed] [Google Scholar]
- Shenkman L., Borkowsky W., Holzman R. S., Shopsin B. Enhancement of lymphocyte and macrophage function in vitro by lithium chloride. Clin Immunol Immunopathol. 1978 Jun;10(2):187–192. doi: 10.1016/0090-1229(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Topper Y. J. The role of DNA synthesis and mitosis in hormone-dependent differentiation. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1283–1289. doi: 10.1073/pnas.56.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisman G., Herbert V., Rosenblatt S. Evidence that lithium induces human granulocyte proliferation: elevated serum vitamin B 12 binding capacity in vivo and granulocyte colony proliferation in vitro. Br J Haematol. 1973 Jun;24(6):767–771. doi: 10.1111/j.1365-2141.1973.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Topper R. J., Oka T., Vonderhaar B. K. Techniques for studying development of normal mammary epithelial cells in organ culture. Methods Enzymol. 1975;39:443–454. doi: 10.1016/s0076-6879(75)39039-3. [DOI] [PubMed] [Google Scholar]
- Topper Y. J. Multiple hormone interactions in the development of mammary gland in vitro. Recent Prog Horm Res. 1970;26:287–308. doi: 10.1016/b978-0-12-571126-5.50011-x. [DOI] [PubMed] [Google Scholar]
- Turkington R. W. Cation inhibition of DNA synthesis in mammary epithelial cells in vitro. Experientia. 1968 Mar 15;24(3):226–228. doi: 10.1007/BF02152783. [DOI] [PubMed] [Google Scholar]
- Vonderhaar B. K., Topper Y. J. A role of the cell cycle in hormone-dependent differentiation. J Cell Biol. 1974 Nov;63(2 Pt 1):707–712. doi: 10.1083/jcb.63.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]



