Abstract
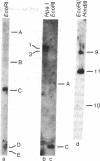
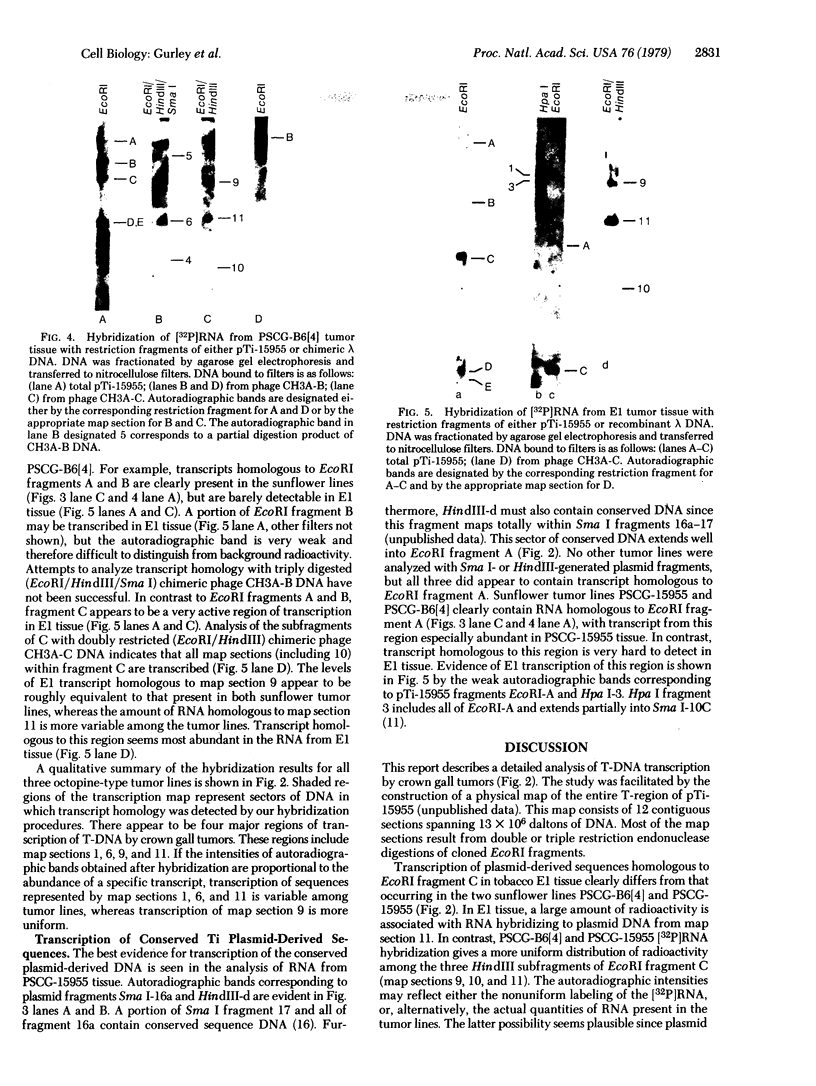
Total RNA isolated from three octopine-type crown gall lines contains sequences homologous to specific regions of the tumor-inducing (Ti) plasmid of Agrobacterium tumefaciens strain 15955. A comparison of transcripts in these three tumor lines suggests that tumor cells transcribe various sequences within a sector of plasmid DNA of 13 x 10(6) daltons and that transcription may not be uniform across the plasmid derived sequences (T-DNA). Transcription of T-DNA by octopine-type tumors occurs at four major sites. The levels of transcription occurring at three of these sites appear to vary considerably among the three tumor lines investigated. Part of this variability may reflect differences in the organization and copy number of T-DNA. One of the transcription sites maps within a region of DNA with common sequence homology with all Ti plasmids. Varying amounts of transcript homologous to this region of T-DNA are present in all three tumor lines. It is suggested that transcription of these conserved sequences in the plant may have significance regarding the mechanism of tumorigenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bomhoff G., Klapwijk P. M., Kester H. C., Schilperoort R. A., Hernalsteens J. P., Schell J. Octopine and nopaline synthesis and breakdown genetically controlled by a plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1976 May 7;145(2):177–181. doi: 10.1007/BF00269591. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Drummond M. H., Merio D. J., Sciaky D., Montoya A. L., Gordon M. P., Nester E. W. Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. Cell. 1977 Jun;11(2):263–271. doi: 10.1016/0092-8674(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Chilton M. D., Montoya A. L., Merlo D. J., Drummond M. H., Nutter R., Gordon M. P., Nester E. W. Restriction endonuclease mapping of a plasmid that confers oncogenicity upon Agrobacterium tumefaciens strain B6-806. Plasmid. 1978 Feb;1(2):254–269. doi: 10.1016/0147-619x(78)90043-4. [DOI] [PubMed] [Google Scholar]
- Drummond M. H., Chilton M. D. Tumor-inducing (Ti) plasmids of Agrobacterium share extensive regions of DNA homology. J Bacteriol. 1978 Dec;136(3):1178–1183. doi: 10.1128/jb.136.3.1178-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Kleber I., Benzinger R. Transfection of Escherichia coli spheroplasts. 3. Facilitation of transfection and stabilization of spheroplasts by different basic polymers. J Virol. 1973 Oct;12(4):741–747. doi: 10.1128/jvi.12.4.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D. In Vivo Synthesis of Crown Gall-specific Agrobacterium tumefaciens-directed Derivatives of Basic Amino Acids. Plant Physiol. 1978 Jul;62(1):26–30. doi: 10.1104/pp.62.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. D. Octopine as a marker for the induction of tumorous growth by agrobacterium tumefaciens strain B6. Biochem Biophys Res Commun. 1976 Apr 5;69(3):816–822. doi: 10.1016/0006-291x(76)90948-7. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A. M., Krol A. J., Dons J. J., Spier F., Schilperoort R. A., Zaenen I., van Larebeke N., Schell J. On the isolation of TI-plasmid from Agrobacterium tumefaciens. Nucleic Acids Res. 1976 Feb;3(2):449–463. doi: 10.1093/nar/3.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENAGE A., MOREL G. SUR LA PR'ESENCE D'OCTOPINE DANS LES TISSUS DE CROWN-GALL. C R Hebd Seances Acad Sci. 1964 Dec 21;259:4795–4796. [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Merlo D. J., Kemp J. D. Attempts to Detect Agrobacterium tumefaciens DNA in Crown-Gall Tumor Tissue. Plant Physiol. 1976 Jul;58(1):100–106. doi: 10.1104/pp.58.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya A. L., Chilton M. D., Gordon M. P., Sciaky D., Nester E. W. Octopine and nopaline metabolism in Agrobacterium tumefaciens and crown gall tumor cells: role of plasmid genes. J Bacteriol. 1977 Jan;129(1):101–107. doi: 10.1128/jb.129.1.101-107.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciaky D., Montoya A. L., Chilton M. D. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. doi: 10.1016/0147-619x(78)90042-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaenen I., Van Larebeke N., Van Montagu M., Schell J. Supercoiled circular DNA in crown-gall inducing Agrobacterium strains. J Mol Biol. 1974 Jun 15;86(1):109–127. doi: 10.1016/s0022-2836(74)80011-2. [DOI] [PubMed] [Google Scholar]