In most organs, blood vessels provide a continuous supply of oxygen, nutrients, and growth factors. In the case of pancreas, such environmental signals are crucial for both the development and the function of the tissue (1). Other evidence supports the fact that paracrine signals participate in this process. Based on the existence of such signals, blood vessels are intuitively supportive for organ growth. One illustration of such positive signal is that, at early stages of development, the vascular endothelium is an inductive signal for insulin expression in the endoderm (2). However, recent findings revisited this concept and demonstrated that the previously described positive role of blood vessels is not general. Magenheim et al. (3) found, using both genetic and pharmacological approaches, that blood vessels can have a restrictive role on pancreas development at later stages. Because of this controversial effect of blood vessels at different time points of development, more investigations have been necessary to clarify the precise effect of vascularization. The pathological impact of such discoveries is important as blood vessels have been shown to be key elements in diabetes (4) and pancreatic cancer (5).
During the last decade, a number of works have shed light on the role of vascular paracrine factors on pancreas development. This includes sphingolipid sphinsosine-1 phosphate (S1P) (6) and retinoic acid signaling (7). We and others have also shown that oxygen is a crucial determinant of pancreas development (8,9). Indeed, under hypoxia rare β-cells differentiate, whereas their development is stimulated by an elevated partial pressure of oxygen. In vitro, the genetic ablation of the von Hippel-Lindau gene (Vhl) indicated that this effect of hypoxia was mediated by the hypoxia-inducible factor 1 α (HIF1α) (10). It has been shown that HIF1α is also involved in the regulation of insulin secretion in response to glucose in adult islets (11–14).
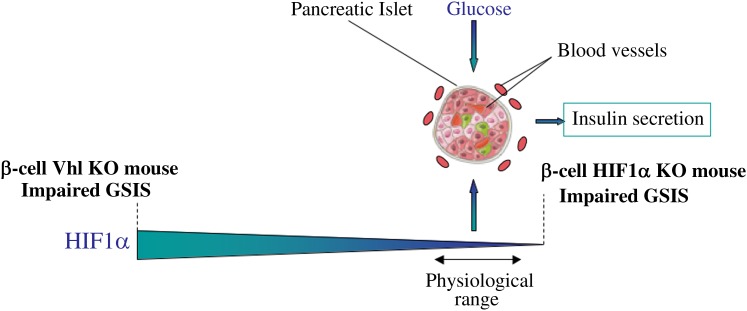
In two articles in this issue, D’Hoker et al. (15) and Reinert et al. (16) tried to clarify the exact effects of vascularization on the biology of pancreas at different periods of life by manipulating the vascular endothelial growth factor (VEGF)-A pathway. In the work of Reinert et al., a genetic approach was used to temporally inactivate VEGF-A in progenitor cells or in adult islets. The strategy of D’Hoker et al. consisted in using a new model of transgenic mice expressing sFlt1 to trap VEGF-A and inhibit vascularization in adult islets. In accord with previous studies (17,18), Reinert et al. (16) found that deletion of vascularization during early development leads to impaired β-cell proliferation and mass. Surprisingly, both Reinert et al. and D’Hoker et al. found that inactivating the VEGF-A pathway in the adult islets do not affect β-cell mass nor β-cell proliferation. In the study of D’Hoker et al. (15), insulin secretion was blunted in vivo, but the function of the isolated β-cell was not altered. In both cases, blood glucose levels also varied only slightly in the animals with hypovascularized islets compared with controls. Finally, insulin release was only mildly delayed in the animals with a mutation of VEGF-A at the adult stage (16). The conclusion was that intraislet blood vessels have a lesser role in adulthood than in embryogenesis. Such results may have an impact on islet transplantation. Indeed, it was strongly argued that low blood supply and hypoxia could affect the islets survival and function during grafts. It is thus considered that hypovascularization and hypoxia limit the survival and function of the β-cells during islets transplantation (19,20). The data from D’Hoker et al. and Reinert et al. indicate that it is not a general rule as they found only a minimal role of vascularization in the adult islets. Nevertheless, it is important to moderate such conclusion. In the work of D’Hoker et al., the authors found only a mild hypoxia and a partial stabilization of HIF1α in the islets depleted in blood vessels. This also was the case in Reinert et al. As the blood vessels were eliminated only inside the islets, a high number of blood vessels were still present at their periphery. It is not surprising to observe a gradient of hypoxia. It has been known for a long time that only cells at a distance greater than 100–150 µm from blood vessels are hypoxic (21). The recent data on the role of the oxygen-sensitive factor HIF1α on β-cell function suggest that it depends on the concentration of HIF1α (Fig. 1). When HIF1α is constitutively stabilized in β-cells, insulin secretion in response to glucose is impaired (11–14). Similarly, when HIF1α is absent, β-cell dysfunction is also observed (12). Together, these data suggest that low physiological concentrations of HIF1α are necessary for a good efficiency of β-cell function. In the work from Reinert et al. and D’Hoker et al., the intraislet hypoxia is not severe and we can hypothesize that the partial pressure of oxygen is still in a physiological range, bringing further support to the notion that the level of hypoxia is not sufficient to alter the function of β-cells.
FIG. 1.
β-Cell function is controlled by the level of HIF1α. The presence of HIF1α in the islet cells depends on vascularization and oxygen supply. Genetic mouse models indicate that deletion of HIF1α or the constitutive stabilization of HIF1α in β-cells both lead to impaired glucose-stimulated insulin secretion (GSIS). One hypothesis is that low but physiological concentrations of HIF1α are necessary for the harmonious function of β-cells. KO, knockout.
In conclusion, D’Hoker et al. (15) and Reinert et al. (16) bring arguments on the role of blood vessels during embryogenesis and adulthood. Islet vascularization seems to be necessary for early pancreas development and may be dispensable in the adult islets. These findings improve our knowledge of the physiology of the β-cells and may diminish the importance given to blood vessels in islet transplantation. The possible role of the surrounding vascularization in the adult islets should also be taken into consideration when analyzing these results.
ACKNOWLEDGMENTS
This work was supported by a grant from the French “Association des Jeunes Diabétiques.”
No potential conflicts of interest relevant to this article were reported.
Footnotes
REFERENCES
- 1.Cleaver O, Dor Y. Vascular instruction of pancreas development. Development 2012;139:2833–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science 2001;294:564–567 [DOI] [PubMed] [Google Scholar]
- 3.Magenheim J, Ilovich O, Lazarus A, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development 2011;138:4743–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villalta SA, Lang J, Kubeck S, et al. Inhibition of VEGFR-2 reverses type 1 diabetes in NOD mice by abrogating insulitis and restoring islet function. Diabetes 2013;62:2870–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2005;23:8033–8040 [DOI] [PubMed]
- 6.Edsbagge J, Johansson JK, Esni F, Luo Y, Radice GL, Semb H. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development 2005;132:1085–1092 [DOI] [PubMed] [Google Scholar]
- 7.Kumar M, Melton D. Pancreas specification: a budding question. Curr Opin Genet Dev 2003;13:401–407 [DOI] [PubMed] [Google Scholar]
- 8.Fraker CA, Ricordi C, Inverardi L, Dominguez-Bendala J. Oxygen: a master regulator of pancreatic development? Biol Cell 2009;101:431–440 [published correction appears in Biol Cell 2009;101:555] [DOI] [PubMed]
- 9.Heinis M, Simon MT, Ilc K, et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes 2010;59:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinis M, Soggia A, Bechetoille C, et al. HIF1α and pancreatic β-cell development. FASEB J 2012;26:2734–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantley J, Selman C, Shukla D, et al. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest 2009;119:125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng K, Ho K, Stokes R, et al. Hypoxia-inducible factor-1α regulates beta cell function in mouse and human islets. J Clin Invest 2010;120:2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes 2009;58:433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zehetner J, Danzer C, Collins S, et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta cells. Genes Dev 2008;22:3135–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Hoker J, De Leu N, Heremans Y, et al. Conditional hypovascularization and hypoxia in islets do not overtly influence adult β-cell mass or function. Diabetes 2013;62:4165–4173 [DOI] [PMC free article] [PubMed]
- 16.Reinert RB, Brissova M, Shostak A, et al. Vascular endothelial growth factor-A and islet vascularization are necessary in developing, but not adult, pancreatic islets. Diabetes 2013;62:4154–4164 [DOI] [PMC free article] [PubMed]
- 17.Lammert E, Gu G, McLaughlin M, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 2003;13:1070–1074 [DOI] [PubMed]
- 18.Nikolova G, Jabs N, Konstantinova I, et al. The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell 2006;10:397–405 [DOI] [PubMed] [Google Scholar]
- 19.Carlsson PO, Mattsson G. Oxygen tension and blood flow in relation to revascularization in transplanted adult and fetal rat pancreatic islets. Cell Transplant 2002;11:813–820 [PubMed] [Google Scholar]
- 20.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 2002;51:1362–1366 [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–1186 [DOI] [PubMed] [Google Scholar]