Abstract
Awake and fed thermogenesis (AFT) is the energy expenditure (EE) of the nonactive fed condition above the minimum metabolic requirement during sleep and is composed of the thermic effect of food and the cost of being awake. AFT was estimated from whole-room 24-h EE measures in 509 healthy subjects (368 Native Americans and 141 whites) while subjects consumed a eucaloric diet. Follow-up data were available for 290 Native Americans (median follow-up time: 6.6 years). AFT accounted for ∼10% of 24-h EE and explained a significant portion of deviations from expected energy requirements. Energy intake was the major determinant of AFT. AFT, normalized as a percentage of intake, was inversely related to age and fasting glucose concentration and showed a nonlinear relationship with waist circumference and BMI. Spline analysis demonstrated that AFT becomes inversely related to BMI at an inflection point of 29 kg/m2. The residual variance of AFT, after accounting for covariates, predicted future weight change only in subjects with a BMI >29 kg/m2. AFT may influence daily energy balance, is reduced in obese individuals, and predicts future weight gain in these subjects. Once central adiposity develops, a blunting of AFT may occur that then contributes to further weight gain.
Daily energy expenditure (24-h EE) can be considered to consist of the minimum energy required to sustain life, usually represented by the sleeping metabolic rate (SLEEP), the energy cost of being awake, the thermic effect of food (TEF), and the contribution from physical activity (1). Several studies have assessed the role of 24-h EE in the etiology of obesity. In a Native American population with a high prevalence of obesity, a relatively low rate of EE is a risk factor for future weight and fat mass (FM) gains (2). TEF (also known as diet-induced thermogenesis or specific dynamic action) is defined as the increase in EE in response to food intake (3).
The relationship of TEF with weight change is not clear. In cross-sectional analyses, authors report that TEF is lower in obese subjects (4–7), whereas others report no difference (8–11). The directionality of the cause-and-effect relationship that might exist between TEF and obesity has not been fully established. Brundin et al. (12) demonstrated that the postprandial rise in EE was diminished to the level of obese subjects in lean subjects with artificial thermal insulation of the abdomen. These results indicate that thermic insulation provided by abdominal adiposity may limit the body’s capacity to generate EE in response to food intake. Differing relationships between TEF and body fat distribution in obese individuals might explain the divergent results obtained in previous studies. Some studies have found that TEF increases in obese subjects after weight loss, but results are not consistent (4,13–15). In one study, TEF as calculated in a respiratory chamber over 24 h, but which also included the energy cost of being awake, was not a predictor of future weight gain (16).
In studies using 24 h of indirect calorimetry to estimate TEF, TEF is often calculated as the increase in 24-h EE over SLEEP (16–18) rather than using the awake basal EE during fasting. Therefore, this estimate of TEF also includes the energy cost of being awake (CoA). The energy CoA is defined as the difference between the basal and sleeping metabolic rate, representing the energy cost of waking conscious and unconscious activities (19). When a solitary 24-h EE measure is used, separating CoA from TEF is difficult, partly because they are overlapping biologic phenomena, with both including such bodily functions as gut motility and increased utilization of macronutrients (3). Accordingly, in this study we have defined awake and fed thermogenesis (AFT) as the difference in a subject’s EE during the fasting, sleeping state and the fed, sedentary, awake state (i.e., the energy CoA and fed exclusive of physical activity).
The aims of this study were to assess the determinants of AFT, to test whether the unexplained variability of AFT after accounting for its determinants is related to long-term weight change, and to assess whether any such relationship is affected by adiposity measures.
RESEARCH DESIGN AND METHODS
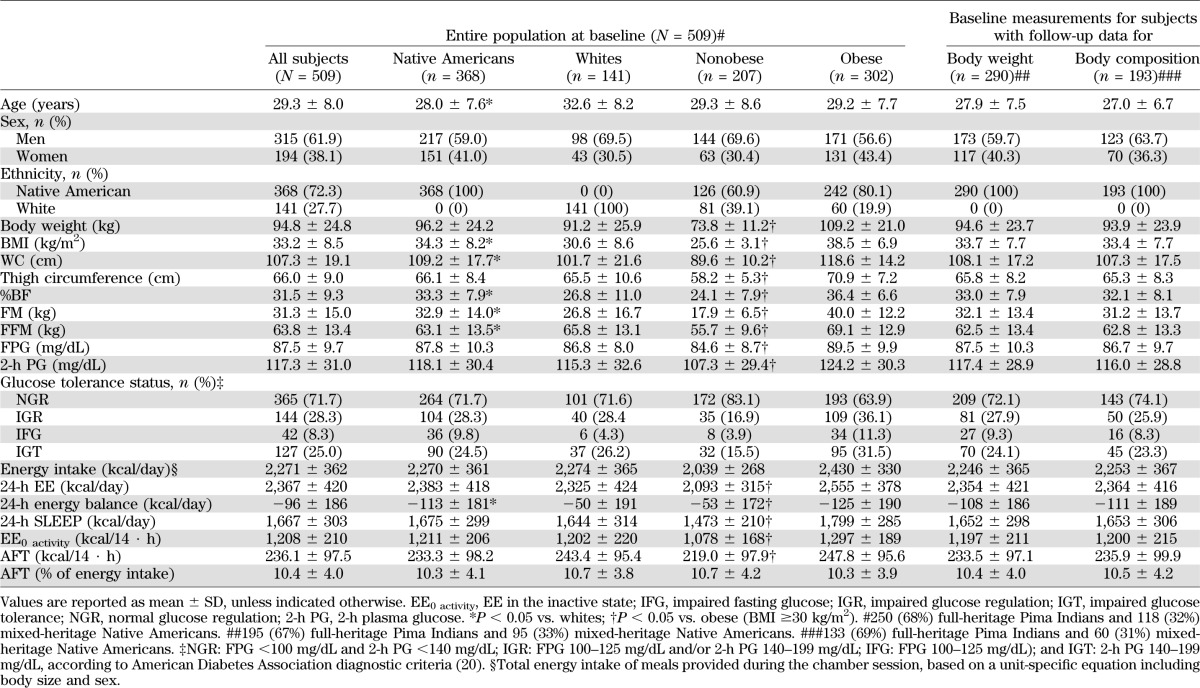
A total of 509 subjects (368 Native Americans and 141 whites; 62% men), between the ages of 18 and 55 years, were admitted to our clinical research unit in Phoenix, Arizona, between 1985 and 2005 for a longitudinal study of the pathogenesis of obesity. All subjects were determined to be healthy by physical examination, medical history, and laboratory test results. Exclusion criteria included a diagnosis of type 2 diabetes mellitus by a 75-g oral glucose tolerance test (20), other medical conditions, or use of medications known to affect energy metabolism.
Because all subjects had data available for body composition measures, obesity was defined according to the National Institutes of Health guidelines (21) as well as according to the World Health Organization criteria (22). Only results using BMI are reported because the method of classifying adiposity did not alter the results and BMI is sex-independent and used more frequently in clinical settings.
Before participation, volunteers were fully informed of the nature and purpose of the study, and written informed consent was obtained. The experimental protocol was approved by the National Institute of Diabetes and Digestive and Kidney Diseases Institutional Review Board.
Of the 509 subjects, 290 Native Americans had a follow-up visit at least 1 year after the 24-h EE measurement, with complete data for body weight and glucose tolerance (23). In a subgroup of 193 subjects, body composition data were also available. Only subjects younger than 55 years of age who remained free from diabetes and women who were not pregnant at follow-up were included in the longitudinal analysis.
Study protocol and respiratory chamber.
Upon admission, subjects were given a weight-maintaining diet (50% carbohydrate, 30% fat, and 20% protein) for 3 days before any tests were performed. The weight-maintaining energy needs were calculated as previously described based on sex, weight, and BMI (24).
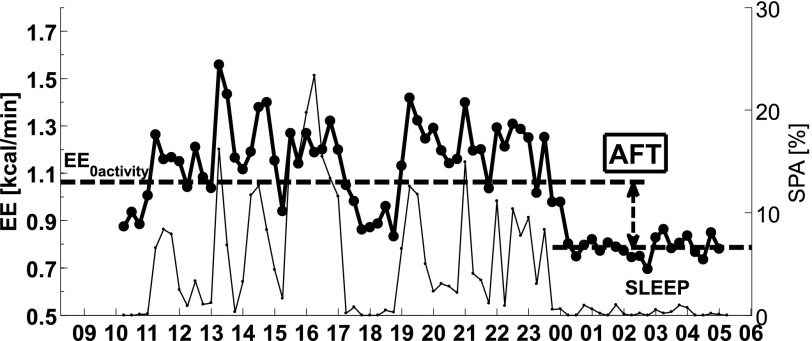
Subjects’ EE was measured by indirect calorimetry while they resided for 24 h within a respiratory chamber, and its components were derived as previously reported (1). The total energy content of the four meals given (intake) was calculated using previously described equations (25). The rate of EE was measured continuously for 23.25 h, averaged for each 15-min interval, and extrapolated to 24 h. All unconsumed food was returned to the metabolic kitchen for weighing for accurate calculation of intake. Energy balance (enbal) was calculated as the difference between intake and 24-h EE. Only subjects with enbal within 20% of 24-h EE during the stay in the respiratory chamber were included in the analysis. The EE in the inactive state was calculated as the intercept of the regression line between EE and spontaneous physical activity (SPA) between 11:00 a.m. and 1:00 a.m. (6). Subjects’ SPA was measured by radar sensors and expressed as the percentage of time when activity was detected (26). Only chambers with an average radar activity <15.0% were included in the analysis. SLEEP was defined as the average EE of all 15-min nightly periods between 1:00 a.m. and 5:00 a.m. during which SPA was less than 1.5% (<0.9 s/min). The starting point to calculate SLEEP was chosen to minimize the influence of TEF on SLEEP. AFT was defined as the increase in a subject’s EE from the sleeping condition to the awake, nonactive, and fed condition. AFT was calculated as the difference between EE in the inactive state and SLEEP (Fig. 1). The reproducibility of the AFT estimate was evaluated in 16 subjects who had two chamber sessions within 3 months of one another.
FIG. 1.
Time course of EE (thick black line, left y-axis) and SPA (thin black line, right y-axis) is shown during 24 h in a respiratory chamber. SPA is measured by a radar system based on the Doppler effect and expressed as percent over the time interval. EE in the inactive state (EE0 activity) is derived as the intercept of the regression line between EE and SPA during the daily hours of 11:00 a.m.–1:00 a.m. SLEEP is calculated as the mean EE during the nightly hours of 1:00–5:00 a.m. when SPA is lower than 1.5%. AFT is calculated as the difference between EE0 activity and SLEEP.
Body composition, body fat distribution, and analytic measurements.
Body composition was estimated by underwater weighing until August 1993 and thereafter by total-body dual-energy X-ray absorptiometry (DPX-1; Lunar Radiation Corp., Madison, WI). Percent body fat (%BF) measurements from the dual-energy X-ray absorptiometry scan were made comparable to underwater values using a conversion equation (27).
Waist circumference (WC) was measured at the umbilicus with the subject supine and thigh circumference at the gluteal fold with the subject standing. Plasma glucose concentrations were measured with the glucose oxidase method (Beckman Instruments, Fullerton, CA).
Statistical analysis.
Statistical tests used to compare groups of subjects included the Student t test for difference in mean values, the Mann-Whitney U test for skewed variables, and the χ2 test for difference in counts and frequency. The Kolmogorov-Smirnov test was used to assess normality of data; the safe logarithmic transformation, i.e., sign(rate of weight change) · LOG10[1 + abs(rate of weight change)], was applied to the rate of weight change due to its skewed distribution and in order to handle negative values. Pearson (R) and Spearman (ρ) correlation coefficients were used for Gaussian and skewed variables, respectively.
Locally weighted regression (28) (nonparametric approach) and nonlinear regression (parametric approach) were used to detect nonlinear relationships between AFT and the anthropometric measures. The former method was used to visually detect potential nonlinear relationships on the scatterplot, with no underlying assumptions about the data distribution and without specifying a regression function. Subsequently, nonlinear regression was used to determine if a quadratic function was a statistically better fit for the data compared with the linear equation. If a nonlinear relationship was found, a spline regression analysis was then performed to objectively identify the inflection point (knot) that achieved the best fit of the data using a piecewise linear model. The equation for the one-knot spline model was:
where AFT and X are the dependent and independent variables, respectively; intercept, A, B, and knot are the model parameters; X ≥ knot is a dummy variable with value 0 for X < knot and 1 for X ≥ knot; and ε is the error term.
The reproducibility of the AFT measurement in duplicate chambers was quantified by the coefficient of variation (CV) and the intraclass correlation coefficient of the paired measurements. Multivariate regression analysis was used to identify the independent predictors of AFT among demographic, glycemic, and body composition measures. The residuals were then calculated and considered as the unexplained variability of AFT. This residual variance of AFT was tested as a potential predictor of body weight change in longitudinal analyses. A P value < 0.05 was considered significant. No correction was made for multiple tests because all analyses were preplanned, exploratory, and of independent interest (29). Data are presented as mean ± SD or median with interquartile range. Analyses were performed using SPSS 21 software (IBM Corp., Armonk, NY).
RESULTS
The baseline characteristics of the study cohort are reported in Table 1.
TABLE 1.
Demographic, anthropometric, and metabolic characteristics of the study population
Validity of AFT.
AFT varied widely among subjects, ranging from 9 to 656 kcal/14 · h and accounting, on average, for 10.0 ± 3.7% of 24-h EE (range 0.4–25.4%). In 16 subjects with repeated measures, the AFT measure had a CV of 24% and an intraclass correlation coefficient of 0.69, despite differences in body weight (CV = 2.1%) and energy intake (CV = 8.3%).
Determinants of AFT.
AFT was positively related to intake (ρ = 0.32) and fat-free mass (FFM) (ρ = 0.31) and inversely associated with age (ρ = −0.15, P = 0.001) and fasting plasma glucose (FPG) concentration (ρ = −0.09, P = 0.04). A multivariate model that included age, sex, ethnicity, %BF, FFM, and FPG explained 15% of the variance in AFT (Table 2). Similar results were obtained if FM or WC were substituted for %BF in the multivariate model or by including energy intake among the predictors, but the best-fit model was with %BF as assessed by R2 and multicollinearity.
TABLE 2.
Multivariate models for the determinants of AFT
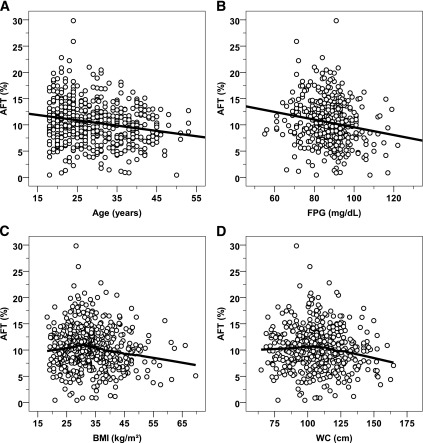
When normalized as a percentage of intake, AFT averaged 10.4 ± 4.0% and was inversely related to age (ρ = −0.19) (Fig. 2A) and FPG (ρ = −0.17, P < 0.001) (Fig. 2B). The relationship between AFT and BMI was nonlinear (Fig. 2C). The quadratic nonlinear model showed a better fit of data than the linear model (mean ± SEM, linear term: 0.171 ± 0.122, P = 0.16; quadratic term: −0.003 ± 0.001, P = 0.04), and a knot value equal to 29 kg/m2 achieved the highest R2 by spline regression analysis (Fig. 3A). AFT was inversely related to BMI for values >29 kg/m2 (ρ = −0.19, P < 0.001, n = 334), but not for BMI ≤29 (ρ = 0.10, P = 0.20, n = 175; interaction term P = 0.01). The relationship between AFT and BMI was similar if subjects were categorized as obese (BMI ≥30 kg/m2) and nonobese (interaction term P = 0.007). Sensitivity analyses using the residuals of AFT after accounting for intake in a regression model or expressing AFT as percentage of 24-h EE led to similar relationships.
FIG. 2.
Relationships between AFT (percentage of energy intake) and age, FPG concentration, BMI, and WC. Inverse associations were found between AFT (expressed as percentage of energy intake) and age (A) and FPG concentration (B). The best-fit line is displayed in both panels. Nonlinear relationships between AFT vs. BMI (C) and AFT vs. WC (D). Locally weighted regression curves are displayed (percentage of fitted points = 50%, weight function: tricube).
FIG. 3.
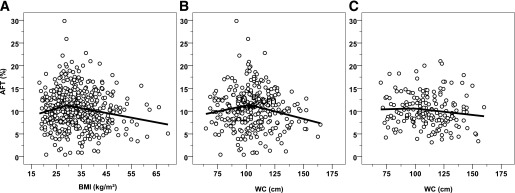
Results of spline regression analysis on the relationships between AFT (percentage of energy intake) and BMI and WC. The piecewise linear curve is shown for BMI in the global cohort of 509 subjects (knot value = 29 kg/m2) (A), and for WC in 315 men (knot value = 103 cm) (B) and 194 women (knot value = 95 cm) (C).
There was also a nonlinear relationship between AFT and WC (Fig. 2D), with a significant quadratic term by nonlinear regression (linear term: 0.182 ± 0.085, P = 0.03; quadratic term: −0.002 ± 0.001, P = 0.02). The inflection point found by spline analysis was equal to 103 cm in men (Fig. 3B) and 95 cm in women (Fig. 3C).
AFT and 24-h enbal.
The 24-h enbal ranged between −572 and 480 kcal/day and, on average, was lower than zero (−96 ± 186 kcal/day, P < 0.001). AFT was inversely related to enbal as an absolute value (ρ = −0.29) and as a percentage of intake (ρ = −0.28, P < 0.001). After adjustment for age, sex, ethnicity, FM, and FFM in a multivariate model, 50% of the variance in enbal was explained by daily mean radar activity (partial R2 = 18%, P < 0.001), SLEEP (partial R2 = 32%), and AFT (partial R2 = 23%), such that a 50-kcal increase in AFT independently corresponded to an average 42-kcal decrease (95% CI 35–49) in enbal. There was no difference in the relationship between AFT and enbal between subjects with a BMI <29 kg/m2 or >29 kg/m2 (P = 0.49).
AFT and long-term weight change.
In a longitudinal analysis of 290 Native Americans (median follow-up time: 6.6 years [interquartile range 3.9–10.7]), body weight change averaged 8.2 ± 13.0 kg (range −27.9 to 71.4, P < 0.001), or 9.4 ± 14.5% (range −33.7 to 68.9%) of initial body weight, with a mean rate of weight change of 1.3 ± 2.3 kg/year (1.4 ± 2.5% of initial weight). The rate of weight change was similar between sexes (P = 0.86). The baseline characteristics of this longitudinal cohort were not different from the Native American subjects included in the cross-sectional analyses (Table 1).
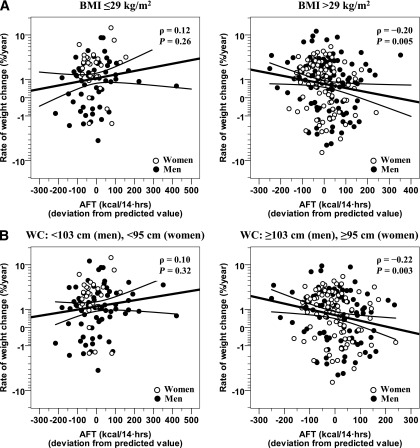
Because we found that the BMI inflection point where the relationship between BMI and AFT changed was 29 kg/m2, we assessed whether any association between the unexplained variance in AFT at baseline and rate of weight change differed for subjects with a baseline BMI above or below 29 kg/m2 (Fig. 4A). AFT was inversely related to rate of percentage weight change in subjects with a BMI >29 kg/m2 (ρ = −0.20, P = 0.005, n = 204), but not in subjects with a baseline BMI ≤29 kg/m2 (ρ = 0.12, P = 0.26, n = 86; interaction term P = 0.02). For a subject with a BMI >29 kg/m2, a 100-kcal decrease from the predicted AFT value corresponded to an average 0.3% increase in body weight per year (0.4 kg/year). Similar results were obtained in sensitivity analyses done to test the robustness of the statistical model. These included 1) using the clinical BMI cutoff for defining obesity (BMI ≥30 kg/m2); 2) replacing %BF with FM or WC in the baseline model for AFT residuals; 3) a subset analysis using only subjects with normal glucose regulation, and 4) using the WC inflection point (instead of BMI) to categorize subjects (Fig. 4B). AFT was inversely related to rate of percent weight change in subjects with a WC above the sex-dependent threshold (ρ = −0.22, P = 0.003, n = 189) but not in subjects below the threshold (ρ = 0.10, P = 0.32, n = 101; interaction term P = 0.02).
FIG. 4.
Lower values of AFT predict the rate of weight change in obese subjects and in individuals with a higher WC. Differing relationships between AFT (adjusted for age, sex, ethnicity, %BF, FFM, and FPG) and rate of percent body weight change in 204 obese subjects with a BMI ≥29 kg/m2 (A, right) vs. 86 nonobese subjects with a BMI <29 kg/m2 (A, left), as well as in 189 subjects with a WC above the sex-dependent threshold of ≥103 cm for men and ≥95 cm for women (B, right) compared with 101 subjects below the threshold (B, left). The median follow-up time is 6.6 years. Rate of weight change on the y-axis is calculated as the difference between follow-up and initial weight, normalized to initial weight and to follow-up time (i.e., rate of percent weight change), and is reported on a safe-logarithmic scale. A relatively linear rate of weight gain over time has been previously described in longitudinal studies of Native Americans (23). hrs, hours.
In the 193 Native Americans with follow-up data for body composition measures, the relationship between AFT and the rate of FM change was again different between subjects with a baseline BMI above or below 29 kg/m2 (interaction term P = 0.04). AFT was not associated with the rate of FFM change (ρ = 0.07, P = 0.30) in either group.
DISCUSSION
We investigated the concept of AFT as a component of 24-h EE and its role in body weight regulation in a cohort of 509 healthy subjects. AFT represents ∼10% of 24-h EE and is inversely related to age and glucose tolerance. AFT explains as much deviation from expected 24-h enbal as SLEEP. AFT was not clinically important in subjects with a BMI ≤29 kg/m2. However, in subjects with a BMI >29 kg/m2, AFT was inversely related to body adiposity, and further, lower-than-expected values of AFT were predictive of long-term weight gain in these subjects in longitudinal analyses.
AFT represents the non–activity-related increase in EE beyond the minimum requirements observed during sleep. Theoretically, it comprises the energy cost of being awake and the energy expended for eating, digesting, and storing macronutrients, two obligatory conditions required for survival. CoA and TEF both contribute to human body thermogenesis, the former being the energy necessary to perform normal awake functions, whereas the latter is the energy expended in response to food intake. On average, TEF is a function of consumed macronutrients, whereas CoA is more likely to be composed of internal, individual-specific factors such as genotype and efficiency of cellular processes. These two components of EE are heavily intertwined because obtaining, consuming, and digesting food compose a large portion of the waking hours. Feeding happens uniquely during the awake state and involves not only a mechanical digestive phase but also the sensory response to the anticipation of food intake represented, in part, by the cephalic phase (30); therefore, it is not only difficult but possibly overly simplistic to distinguish between these two contributions during normal living.
The advantage of using a whole-room indirect calorimeter to assess the components of EE is that EE is measured continuously over 24 h. This overcomes the problem with the shorter duration of ventilated hood experiments in which the rise of EE after food intake may not be fully captured. The TEF can last up to 6 h, and the response to more than one meal may overlap (8,31,32). Hence, to fully understand the impact of TEF in everyday life, it is appropriate to measure EE during consumption of multiple meals until EE returns to baseline after a night of fasting. In addition, because CoA and TEF are so intricately intertwined, a 24-h EE measure allows for measurement of AFT, which may be of greater biological relevance. A previous study estimated an EE variable using a calculation similar to the one we used, calling it TEF (16). However, CoA was also included within this calculation, such that the EE variable was actually AFT (16), but the CV was higher (48%) compared with ours (24%), which may be partly explained by differences in food intake and weight in duplicate measurements. In that study (16), AFT was not associated with weight change in longitudinal analysis; however, whether there were differences between obese and nonobese subjects was not reported. The effect of AFT solely in subjects with higher BMIs might explain the difference between our findings compared with other studies. We detected a nonlinear relationship between AFT and BMI by spline analysis, with an inflection point at 29 kg/m2, with similar results if the clinical threshold for obesity (BMI = 30 kg/m2) is used instead.
Energy intake was found to be the major determinant of AFT. This is likely due to the known linear relationship between TEF and caloric intake (10,32). When expressed as a percentage of intake, AFT was also normalized to body size because the diet given in the chamber was calculated according to body size (25). Several studies have demonstrated an inverse relationship between TEF and age (33,34), and a previous study reported CoA is also inversely related to age (19). Lower values of AFT with increasing age may be related to a reduced sympathetic nervous system response. The mitochondrial dysfunction that occurs with aging may also explain some of the age-associated diminished thermogenic response (35,36). The inverse relationship between AFT and FPG is likely due to the decreased thermogenesis in response to food intake previously observed with insulin resistance (9). However, the differences in the relationship between AFT and weight change observed between subjects with a BMI above or below 29 kg/m2 in our larger group were verified in the subset of subjects with normal glucose regulation, indicating that this finding is independent of the effects of insulin resistance. The finding that age and FPG were both independent determinants of AFT is consistent with a previous study (33).
A thermogenic defect has been proposed as a possible cause of future weight gain (37). In our study, AFT explained additional variation in the deviation from expected 24-h enbal, even after accounting for SLEEP and SPA, indicating a role of AFT in daily energy balance and, possibly, weight regulation. Our longitudinal results show that a negative deviation from the predicted AFT was associated with an increase in body weight per year, but only in subjects with a BMI >29 kg/m2. The relationship between AFT and future body weight only in subjects with a greater amount of adiposity may partly explain the reported relationship between relative deviations from expected 24-h EE and future weight change (2). In addition, ethnic differences in body habitus may account for the conflicting results between studies evaluating the relationship between 24-h EE and risk of weight gain. For example, a positive relationship between EE variance and future weight gain was observed in lean Nigerian adults (38), whereas we recently confirmed a negative association in an overweight Native American population (2).
The unexplained variance of AFT predicted weight change, but only in individuals with WC greater than the data-derived inflection point. Although the underlying mechanisms of AFT’s role in weight regulation are not clearly established, these findings support the hypothesis that individuals with central adiposity have a reduced ability to transfer the heat generated after food consumption across the abdominal wall compared with lean individuals. In a study assessing heat exchange with a glucose load, overall heat loss throughout the study was lower in obese females compared with lean control subjects, and the obese subjects (5) had a lower TEF after the glucose load. It has also been demonstrated (12) that artificial body insulation can reduce heat transfer across the abdominal wall after food consumption in lean subjects. These findings support the idea that lean individuals generate a higher TEF in order to maintain core body temperature in contrast to obese individuals who have limited postprandial heat loss due to the insulating properties of central adiposity. Body heat loss in obese subjects may be reduced partly due to the insulating properties of adipose tissue, such that there is a attenuated capacity for heat loss into the environment as well as a decreased need to generate heat with cooler temperatures (39). Support for this theory can be found as early as 1902 in a study demonstrating that the minimum metabolism of a dog was reached at a lower temperature when the dog was obese than when it was emaciated (40).
Our study population has a high prevalence of Pima Indians, whose body distribution differs from Caucasians (41). Although the prevalence of obesity and type 2 diabetes is higher in Native American populations, in general, findings from this study population have been replicated in other study populations (42). AFT is an estimate derived from 24-h EE measures but demonstrated reasonable reproducibility in replicate measures. In addition, the analysis of a large cohort of subjects with long-term longitudinal data allowed us to identify its determinants and to test whether AFT plays a role in future body weight regulation. Although the explained variance of AFT was relatively low, all the associations are concordant with prior literature on TEF and CoA, and results were consistent in the cross-sectional and longitudinal analyses. Our longitudinal results indicate that although AFT may not be an important contributor to weight maintenance in lean individuals, once abdominal adiposity develops, a relative reduction in AFT increases the risk of further weight gain and may be a potential impediment to weight loss attempts. Although it is unknown whether the lower AFT caused obesity to develop or whether the excess adiposity led to blunting of the AFT in the cross-sectional analysis, we demonstrate in the longitudinal analysis that a lower baseline AFT (regardless of whether it was due to excess adiposity or other factors) predicts future weight gain. We, therefore, hypothesize that once AFT is blunted in subjects with a BMI >29 kg/m2, there is an even greater predisposition to further weight gain and worsening of obesity.
In conclusion, this study describes the combined role of CoA and TEF, which we have termed awake and fed thermogenesis, in body weight regulation. AFT is related to glucose tolerance and age and explains variance in deviations from daily energy balance. AFT is negatively correlated with body adiposity in individuals with a BMI >29 kg/m2 and a WC of 103 cm in men or 95 cm in women. A relative reduction in this thermogenic component of 24-h EE favors further weight gain in individuals with abdominal adiposity, indicating that a decreased ability to transfer heat to the external environment once obesity develops may predispose to further weight gain. Although weight gain does not occur without an excess of food intake, a decreased rate of thermogenesis may play a significant role in energy requirements and, thus, the maintenance of, and risk for, further adiposity.
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
No potential conflicts of interest relevant to this article were reported.
P.P. wrote the manuscript and analyzed data. J.K., C.B., and M.S.T. contributed to discussion and reviewed and edited the manuscript. All authors critically revised the draft and approved the final manuscript. P.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank the nursing, clinical, and dietary staffs, and the laboratory technicians of the clinical research center for their valuable assistance and care of the volunteers.
Footnotes
Clinical trial reg. no. NCT00340132, clinicaltrials.gov.
REFERENCES
- 1.Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest 1986;78:1568–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab 2013;98:E703–E707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Secor SM. Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 2009;179:1–56 [DOI] [PubMed] [Google Scholar]
- 4.Bessard T, Schutz Y, Jéquier E. Energy expenditure and postprandial thermogenesis in obese women before and after weight loss. Am J Clin Nutr 1983;38:680–693 [DOI] [PubMed] [Google Scholar]
- 5.Pittet P, Chappuis P, Acheson K, De Techtermann F, Jéquier E. Thermic effect of glucose in obese subjects studied by direct and indirect calorimetry. Br J Nutr 1976;35:281–292 [DOI] [PubMed] [Google Scholar]
- 6.Schutz Y, Bessard T, Jéquier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. Am J Clin Nutr 1984;40:542–552 [DOI] [PubMed] [Google Scholar]
- 7.Segal KR, Gutin B, Nyman AM, Pi-Sunyer FX. Thermic effect of food at rest, during exercise, and after exercise in lean and obese men of similar body weight. J Clin Invest 1985;76:1107–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz RS, Ravussin E, Massari M, O’Connell M, Robbins DC. The thermic effect of carbohydrate versus fat feeding in man. Metabolism 1985;34:285–293 [DOI] [PubMed] [Google Scholar]
- 9.Ravussin E, Acheson KJ, Vernet O, Danforth E, Jéquier E. Evidence that insulin resistance is responsible for the decreased thermic effect of glucose in human obesity. J Clin Invest 1985;76:1268–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Alessio DA, Kavle EC, Mozzoli MA, et al. Thermic effect of food in lean and obese men. J Clin Invest 1988;81:1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felber JP, Meyer HU, Curchod B, et al. Glucose storage and oxidation in different degrees of human obesity measured by continuous indirect calorimetry. Diabetologia 1981;20:39–44 [DOI] [PubMed] [Google Scholar]
- 12.Brundin T, Thörne A, Wahren J. Heat leakage across the abdominal wall and meal-induced thermogenesis in normal-weight and obese subjects. Metabolism 1992;41:49–55 [DOI] [PubMed] [Google Scholar]
- 13.Schutz Y, Golay A, Felber JP, Jéquier E. Decreased glucose-induced thermogenesis after weight loss in obese subjects: a predisposing factor for relapse of obesity? Am J Clin Nutr 1984;39:380–387 [DOI] [PubMed] [Google Scholar]
- 14.Thörne A, Hallberg D, Wahren J. Meal-induced thermogenesis in obese patients before and after weight reduction. Clin Physiol 1989;9:481–498 [DOI] [PubMed] [Google Scholar]
- 15.Thörne A, Näslund I, Wahren J. Meal-induced thermogenesis in previously obese patients. Clin Physiol 1990;10:99–109 [DOI] [PubMed] [Google Scholar]
- 16.Tataranni PA, Larson DE, Snitker S, Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. Am J Clin Nutr 1995;61:1013–1019 [DOI] [PubMed] [Google Scholar]
- 17.Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord 1999;23:287–292 [DOI] [PubMed]
- 18.Schulz LO, Nyomba BL, Alger S, Anderson TE, Ravussin E. Effect of endurance training on sedentary energy expenditure measured in a respiratory chamber. Am J Physiol 1991;260:E257–E261 [DOI] [PubMed] [Google Scholar]
- 19.Fontvieille AM, Ferraro RT, Rising R, Larson DE, Ravussin E. Energy cost of arousal: effect of sex, race and obesity. Int J Obes Relat Metab Disord 1993;17:705–709 [PubMed]
- 20.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 21.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report National Institutes of Health Obes Res 1998;6(Suppl. 2):51S–209S [PubMed] [Google Scholar]
- 22.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452 [PubMed] [Google Scholar]
- 23.Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr 1991;53(Suppl.):1543S–1551S [DOI] [PubMed] [Google Scholar]
- 24.Pannacciulli N, Salbe AD, Ortega E, Venti CA, Bogardus C, Krakoff J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am J Clin Nutr 2007;86:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott WG, Howard BV, Christin L, et al. Short-term energy balance: relationship with protein, carbohydrate, and fat balances. Am J Physiol 1988;255:E332–E337 [DOI] [PubMed] [Google Scholar]
- 26.Schutz Y, Ravussin E, Diethelm R, Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. Int J Obes 1982;6:23–28 [PubMed] [Google Scholar]
- 27.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995;62:730–734 [DOI] [PubMed] [Google Scholar]
- 28.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–836 [Google Scholar]
- 29.Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001;54:343–349 [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc J, Cabanac M. Cephalic postprandial thermogenesis in human subjects. Physiol Behav 1989;46:479–482 [DOI] [PubMed] [Google Scholar]
- 31.Reed GW, Hill JO. Measuring the thermic effect of food. Am J Clin Nutr 1996;63:164–169 [DOI] [PubMed] [Google Scholar]
- 32.Kinabo JL, Durnin JV. Thermic effect of food in man: effect of meal composition, and energy content. Br J Nutr 1990;64:37–44 [DOI] [PubMed] [Google Scholar]
- 33.Golay A, Schutz Y, Meyer HU, et al. Glucose-induced thermogenesis in nondiabetic and diabetic obese subjects. Diabetes 1982;31:1023–1028 [DOI] [PubMed] [Google Scholar]
- 34.Golay A, Schutz Y, Broquet C, Moeri R, Felber JP, Jéquier E. Decreased thermogenic response to an oral glucose load in older subjects. J Am Geriatr Soc 1983;31:144–148 [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Vina J. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med 2012;50:1287–1295 [DOI] [PubMed]
- 36.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol 2012;13:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jéquier E, Schutz Y. Energy expenditure in obesity and diabetes. Diabetes Metab Rev 1988;4:583–593 [DOI] [PubMed] [Google Scholar]
- 38.Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr 2006;83:1076–1081 [DOI] [PubMed] [Google Scholar]
- 39.Jéquier E, Gygax PH, Pittet P, Vannotti A. Increased thermal body insulation: relationship to the development of obesity. J Appl Physiol 1974;36:674–678 [DOI] [PubMed] [Google Scholar]
- 40.Lusk G. The Elements of the Science of Nutrition. Sligo, Ireland, HardPress Publishing, 2012 [Google Scholar]
- 41.Tulloch-Reid MK, Williams DE, Looker HC, Hanson RL, Knowler WC. Do measures of body fat distribution provide information on the risk of type 2 diabetes in addition to measures of general obesity? Comparison of anthropometric predictors of type 2 diabetes in Pima Indians. Diabetes Care 2003;26:2556–2561 [DOI] [PubMed] [Google Scholar]
- 42.Buscemi S, Verga S, Caimi G, Cerasola G. Low relative resting metabolic rate and body weight gain in adult Caucasian Italians. Int J Obes (Lond) 2005;29:287–291 [DOI] [PubMed] [Google Scholar]