Insulin resistance is the primary abnormality associated with obesity, type 2 diabetes, and hypertension (1,2). Interestingly, these disease processes also share a common underlying impairment of vascular endothelial function. For many years the endothelium was thought of as nothing more than a benign barrier between blood and extracellular space. But intensive investigations demonstrate a myriad of actions by the endothelium, including regulation of blood flow (3,4), vascular tone (5), vascular permeability (6), and the immune response (7). The capillary endothelium regulates blood flow via the release of several vasoactive agents, in particular nitric oxide (NO) (8). Baron (4) first demonstrated that insulin can stimulate the release of endothelial NO, thereby regulating blood flow and its own delivery. Insulin has multiple hemodynamic actions to enhance its ability to increase glucose uptake in skeletal muscle (4, 9–11). The three major methods include: 1) increasing blood flow to the tissue by modulation of endothelial factors; 2) increasing the number of capillaries that dilate within the tissue (increasing microvascular volume), which allows for an increase in total permeable surface area for insulin and glucose to enter the space that bathes the active myocytes (i.e., the interstitial space); and 3) increasing permeability of the capillaries, thus allowing more glucose and insulin to pass from the blood to the interstitial space. In insulin-resistant states, these hemodynamic actions of insulin are impaired, presumably due to reduced NO bioavailability (12).
The seminal study by Bergman and colleagues (13) with intravenous insulin infusion in a canine model showed that the rate at which glucose uptake increased exhibited a “hand in glove” relationship with the rate of rising lymph insulin. The strong correlation observed between glucose uptake and lymph insulin did not exist with plasma insulin levels, suggesting that interstitial insulin, as measured by lymph, directed glucose uptake. Thus, it was the rate of transport across the endothelial barrier that determined the rate of insulin action. These findings have since been corroborated by other investigators using microdialysis to directly measure interstitial fluid in human (14) and rat (15) studies. Moreover, bypassing the capillary endothelium and administering insulin directly into the interstitial space has been shown to prevent any delays in insulin action that occurs in normal physiology (16), supporting the concept of transendothelial transport (TET) as a rate-limiting step for insulin action.
Capillary endothelial cells are the direct interface for metabolic processes and are therefore closely involved in TET. However, the mechanism of insulin transport across the capillary endothelium is debatable. In a study conducted in bovine aortic endothelial cells, King and Johnson (17) reported that the transport of insulin across a cell monolayer is saturable and unidirectional, consistent with receptor-mediated transport. However, in vivo animal studies support the notion that insulin transport is not saturable and occurs via passive diffusion (18). Thus, insulin transport may consist of a saturable, receptor-mediated process under physiological conditions, but under supraphysiological conditions, additional methods may be recruited to allow insulin to cross the endothelial barrier, such as insulin-like growth factor. This idea is supported by an in vivo study conducted in humans demonstrating saturable insulin transport kinetics under physiological insulin ranges (30–300 pmol/L) from the same group whose current findings are featured in Wang et al. (19). Nonetheless, TET of insulin is a crucial step for insulin-mediated glucose uptake.
Given the critical role of the endothelium as the gatekeeper of insulin transport into interstitial fluid, the vascular endothelium is positioned as a prime candidate to affect insulin action. Yet, despite overwhelming evidence implicating impaired endothelial function in the pathogenesis of insulin resistance, the potential relationship is poorly understood. In this issue, Wang et al. (19) used bovine endothelial aortic cells to provide evidence from a robust series of experiments that demonstrate: 1) NO directly promotes endothelial cell insulin uptake and transport as measured by uptake of fluorescein isothiocyanate (FITC)-labeled insulin and accumulation of [125I] TyrA14 insulin in Transwell plates, respectively; 2) NO donors such as sodium nitroprusside and l-arginine enhance insulin uptake while treatment with the NO synthase inhibitor l-NG-nitro-l-arginine methyl ester (l-NAME) prevents uptake; 3) NO donor sodium nitroprusside promotes insulin transport, which was determined to be independent of endothelial NO synthase (eNOS) activity; 4) NO increases insulin transport by enhancing protein S-nitrosylation, including protein-tyrosine phosphatase 1B (PTB1B); and 5) exogenous NO restored insulin signaling and uptake in rat aortic endothelial cells from animals rendered insulin resistant with high-fat feeding.
An exciting finding from the current study demonstrates that l-NAME completely blocks insulin uptake, suggesting that endothelial insulin transport only occurs in the presence of NO. This finding is consistent with a recent in vivo study in which the IRS2 gene was selectively deleted from vascular endothelial cells. The study showed that knockout of IRS2 caused inhibition of eNOS activation and almost completely inhibits insulin’s transendothelial delivery to the interstitium within 60 min after initiating the insulin clamp (20).
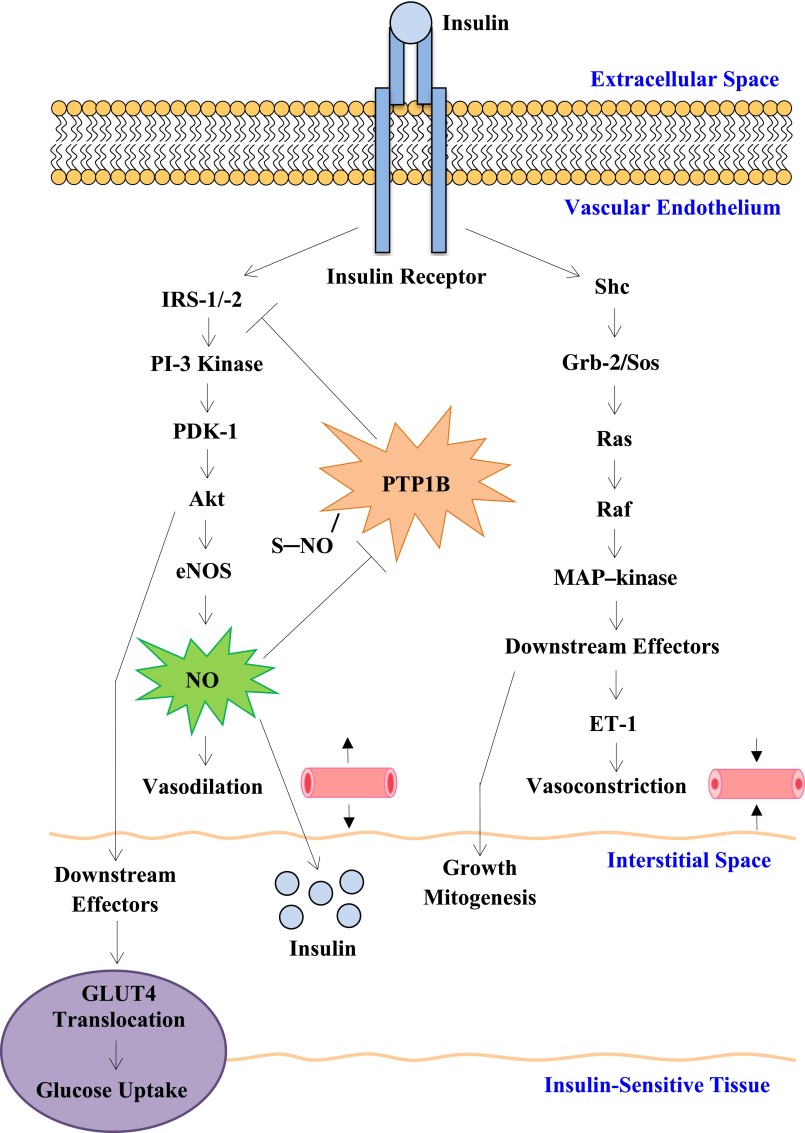
Wang et al. (19) have provided mechanistic insight into the development of insulin resistance in the face of impaired endothelial function. The current study, as well as earlier studies from Wang and colleagues (21–23), provide key information in enhancing our understanding of how changes in endothelial function can affect insulin transport and insulin sensitivity. Although it is known that the many components of insulin resistance may have adverse effects on endothelial function, the intriguing question still remains: Is impairment of the capillary endothelium truly integral to the insulin-resistant state? Limitations of the current study beg for additional investigation in insulin-resistant in vivo models, such as the NO-deficient animal model. Additionally, NO’s effects are shown to be mediated through the PI3K pathway. However, there are two insulin signaling pathways and how the mitogen-activated protein kinase pathway, mediator of the potent vasoconstrictor endothelin, plays a role in the current findings is unknown (Fig. 1). Although these two pathways are known to have opposing effects and are considered relatively separate, studies suggest cross talk between the two branches (24). Resolution of these issues will have important implications for our understanding of the origins of vascular insulin resistance and ultimately its prevention and treatment.
FIG. 1.
Simplified schematic of insulin transduction pathways. PI-3 kinase pathway regulates NO production in the vascular endothelium and GLUT4 translocation in insulin-sensitive tissues, i.e., skeletal muscle. The mitogen-activated protein (MAP) kinase pathway regulates endothelin (ET-1) production in the vascular endothelium and downstream effects of growth and mitogenesis. The study by Wang et al. (19) implicates NO as a critical mediator of transendothelial insulin transport into the interstitial space, consequently promoting glucose uptake. NO purportedly increases insulin transport via protein S-nitrosylation (PTB1B). Potential role or cross talk with the MAP kinase pathway in mediating insulin transport remains open for investigation.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. Jang Youn at the Keck School of Medicine of University of Southern California for his critical review of the document. Additionally, the author thanks Natasha Soares for producing the scientific illustration used in this commentary and LaShanni Butler for her technical assistance with references, both at Keck School of Medicine.
Footnotes
See accompanying original article, p. 4030.
REFERENCES
- 1.Bergman RN, Kim SP, Hsu IR, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med 2007;120(Suppl. 1):S3–S8; discussion S29–S32 [DOI] [PubMed] [Google Scholar]
- 2.Gill H, Mugo M, Whaley-Connell A, Stump C, Sowers JR. The key role of insulin resistance in the cardiometabolic syndrome. Am J Med Sci 2005;330:290–294 [DOI] [PubMed] [Google Scholar]
- 3.James DE, Burleigh KM, Storlien LH, Bennett SP, Kraegen EW. Heterogeneity of insulin action in muscle: influence of blood flow. Am J Physiol 1986;251:E422–E430 [DOI] [PubMed] [Google Scholar]
- 4.Baron AD. Hemodynamic actions of insulin. Am J Physiol Endocrinol Metab 1994;267(2 Pt 1):E187–E202 [DOI] [PubMed] [Google Scholar]
- 5.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet 1989;2:997–1000 [DOI] [PubMed] [Google Scholar]
- 6.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol 2002;39:225–237 [DOI] [PubMed] [Google Scholar]
- 7.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol 1993;11:767–804 [DOI] [PubMed] [Google Scholar]
- 8.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 2007;293:E1250–E1255 [DOI] [PubMed] [Google Scholar]
- 10.Bonadonna RC, Saccomani MP, Del Prato S, Bonora E, DeFronzo RA, Cobelli C. Role of tissue-specific blood flow and tissue recruitment in insulin-mediated glucose uptake of human skeletal muscle. Circulation 1998;98:234–241 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Vincent MA, Richards SM, et al. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 2004;53:447–453 [DOI] [PubMed] [Google Scholar]
- 12.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 2006;113:1708–1714 [DOI] [PubMed] [Google Scholar]
- 13.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 1989;84:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol 1987;253:E228–E231 [DOI] [PubMed] [Google Scholar]
- 15.Holmäng A, Müller M, Andersson OK, Lönnroth P. Minimal influence of blood flow on interstitial glucose and lactate-normal and insulin-resistant muscle. Am J Physiol 1998;274:E446–E452 [DOI] [PubMed] [Google Scholar]
- 16.Chiu JD, Richey JM, Harrison LN, et al. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes 2008;57:828–835 [DOI] [PubMed] [Google Scholar]
- 17.King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science 1985;227:1583–1586 [DOI] [PubMed] [Google Scholar]
- 18.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 1996;97:1497–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes 2013;62:4030–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab 2011;13:294–307 [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 2008;57:540–547 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Wang AX, Barrett EJ. Insulin-induced endothelial cell cortical actin filament remodeling: A requirement for trans-endothelial insulin transport. Mol Endocrinol 2012;26:1327–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Wang AX, Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab 2011;300:E134–E144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 2006;7:85–96 [DOI] [PubMed] [Google Scholar]