The Diabetes Control and Complications Trial (DCCT) (1) and its observational follow-up, the Epidemiology of Diabetes Interventions and Complications (EDIC) Study (2), are celebrating the 30th anniversary since the start of the DCCT and 20th since the reporting of the DCCT primary results (3). During the past three decades, our understanding of the relationship between metabolic control and complications and the treatment of type 1 diabetes (T1D) has been transformed by the results of DCCT/EDIC. Most importantly, the long-term prospects for patients have dramatically improved with the adoption of intensive therapy designed to achieve near-normal glycemia as the standard of care of T1D. In this Perspective, we present an overview of the major scientific advances provided by the DCCT/EDIC Research Group, the resulting changes in therapy that have improved long-term outcomes in patients with T1D worldwide, and the challenges that remain.
Diabetes Control and Complications Trial (1983–1993)
Background and rationale.
After the introduction of insulin therapy in 1922, type 1 diabetes (T1D) was transformed from a uniformly fatal disease to a chronic degenerative one (4). During the 1930–1960s, the development of chronic complications affecting the eyes, kidneys, peripheral and autonomic nervous system, and a substantially increased risk of cardiovascular disease (CVD) were observed in patients who had survived >20 years with the disease (5). The origin of these newly discovered complications was debated vigorously, and theories to explain them abounded (4,6). The debate led to two opposing philosophies of diabetes treatment: one in which treatment to achieve glucose concentrations as low as possible was endorsed and another in which glycemic levels were thought to be inconsequential, at least with regard to the pathogenesis of long-term complications (7,8). Although the debate regarding the so-called glucose hypothesis was vigorous, it was largely academic, since objective means of measuring long-term glycemia and of achieving near-normal glycemia did not exist. However, the introduction and refinement in the late 1970s of glycated hemoglobin assays, self-monitoring of blood glucose (SMBG) meters, continuous subcutaneous insulin infusion (CSII) pumps, and multiple-daily injection (MDI) regimens, as well as the means of objectively measuring diabetes complications, provided all of the components necessary to test the glucose hypothesis.
DCCT planning.
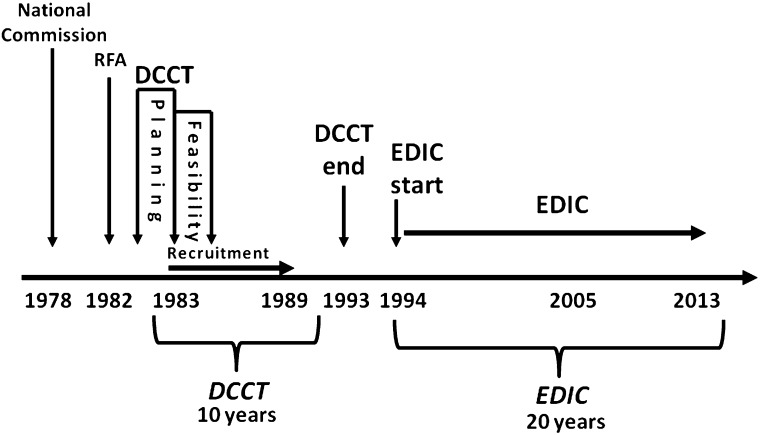
In 1975, the National Commission on Diabetes, established by the National Diabetes Mellitus Research and Education Act (PL 93-354), recommended that the National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases (NIADDK, which became NIDDK in 1986) and the National Heart, Lung, and Blood Institute initiate and support a clinical trial to assess the effects of glucose control on the development of microvascular and macrovascular complications of T1D. An external advisory committee to the National Institutes of Health studied the possibility of such a project, and a request for research proposals was issued in 1981. Twenty-one clinical centers were selected, and their principal investigators, study coordinators, dietitians, and behaviorists, in collaboration with statisticians from the Coordinating Center at The George Washington University Biostatistics Center, a bioethicist, NIADDK project scientists, and other relevant consultants, planned the study between 1982 and 1983 (Fig. 1). The clinical trial was named the Diabetes Control and Complications Trial (1).
FIG. 1.
Study time line of the DCCT/EDIC Study. RFA, research funding announcement.
The consensus protocol addressed two major clinical questions:
Primary prevention: Will an intensive treatment program designed to achieve glycemic control as close to the nondiabetic range as safely possible prevent or delay the appearance of early background retinopathy?
Secondary intervention: Will such an intervention prevent the progression of early retinopathy to more advanced forms of retinopathy?
While retinopathy was the primary outcome, nephropathy and neuropathy were important secondary outcomes, as were CVD and quality of life (1). With the recognition that severe hypoglycemia was the most frequent serious adverse effect of intensive treatment (9), cognitive function became another important safety outcome.
The study was designed as a parallel-arm randomized clinical trial (1). For practical reasons, the study treatments could not be masked, but complications outcomes were masked unless specific therapeutic interventions, such as laser therapy, were required. The two treatment arms were called “standard” (conventional) and “experimental” (intensive), and all diabetes care was provided by the DCCT clinic teams. The clinical goals of conventional therapy (CONV) were absence of symptoms of hyperglycemia and avoidance of severe or frequent episodes of hypoglycemia. Severe hypoglycemia was defined as an episode requiring treatment assistance from another person. CONV, which reflected diabetes care practices in the early 1980s, included one or two daily injections of single or mixed insulin, daily urine or SMBG testing, and diabetes education. CONV remained unchanged unless the quarterly obtained hemoglobin A1c (HbA1c) was >13.11% (119.8 mmol/mol). This safety alert represented 2 SD above the mean for T1D patients cared for in the clinics of the participating DCCT institutions.
Intensive therapy (INT) had the same clinical goals as CONV but with superimposed glycemic targets. INT included MDI with at least three injections of insulin per day or treatment with CSII, with dose adjustment guided by four or more SMBG tests per day, meal size and content, and anticipated exercise. The daily goals of INT included premeal glucose concentrations between 70 and 120 mg/dL (3.89–6.67 mmol/L), postmeal (90–120 min) concentrations <180 mg/dL (10 mmol/L), and a weekly 3:00 a.m. SMBG concentration that was >65 mg/dL (3.61 mmol/L), directed at detecting and preventing nocturnal hypoglycemia. The overall goal of INT was to achieve and maintain HbA1c levels <6.05% (42.6 mmol/mol). This goal was the mean ± 2 SD of a nondiabetic 13- to 39-year-old population that was recruited by the clinical centers and tested by the central laboratory with the same method used in the study (10). INT was implemented by a team expert in such therapy that included diabetologists, nurses, dietitians, and behavioralists. Hospitalization was used to initiate INT with the goal of lowering HbA1c levels as quickly as safely possible.
The eligibility criteria shown in Table 1 were used in order to test the primary prevention and secondary intervention hypotheses. Retinopathy was measured every 6 months with seven-field stereoscopic fundus photography. The development or progression of a three-step or greater change from baseline, based on the Airlie House modified Early Treatment of Diabetic Retinopathy Study (ETDRS) scale (11), was the primary outcome. Kidney function (albumin excretion and creatinine clearance) was tested annually with a 4-h timed collection. Neuropathy was assessed clinically by a board-certified neurologist, nerve conduction studies, and cardiac autonomic function tests.
TABLE 1.
Eligibility criteria of primary prevention and secondary intervention cohorts
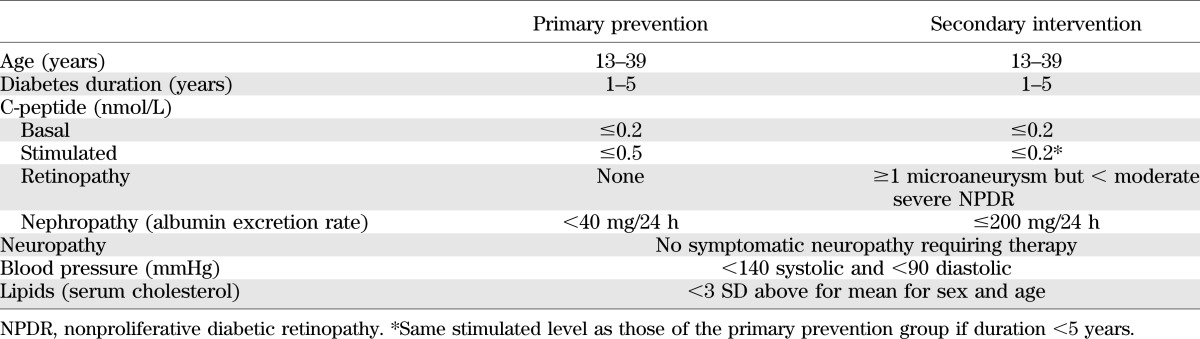
The power calculations required 700 subjects (350 in each treatment arm) for each of the primary prevention and secondary intervention cohorts in order to detect a 33% difference in the onset of retinopathy in the former and progression of retinopathy in the latter cohort.
The study.
Recruitment began in 1983. A 1-year feasibility phase (Fig. 1) with 278 subjects, including 87 adolescents, was conducted to determine whether recruitment could be successfully initiated, and random assignment and protocol implementation were performed with adequate separation of glycemia (12). During the feasibility phase, emphasis was placed on the recruitment of adolescents, who were considered more challenging than adults with regard to recruitment and management. Patients already using CSII were excluded, and those expressing a strong preference for one or the other treatment assignment were considered inappropriate candidates. With the successful completion of the feasibility phase (12), recruitment for the full-scale trial began in 1986 and 8 centers were added, bringing the total to 29 centers (Supplementary Data). The selection of the DCCT study cohort was based on a careful assessment of each person’s anticipated adherence prior to enrollment, including a 2-week run-in period requiring SMBG four times daily and comprehensive record keeping and passing a multiple choice test examining participant’s understanding of the purpose of the trial, its design, and the principle of randomized treatment assignment. Recruitment ended in 1989 with a total of 1,441 participants.
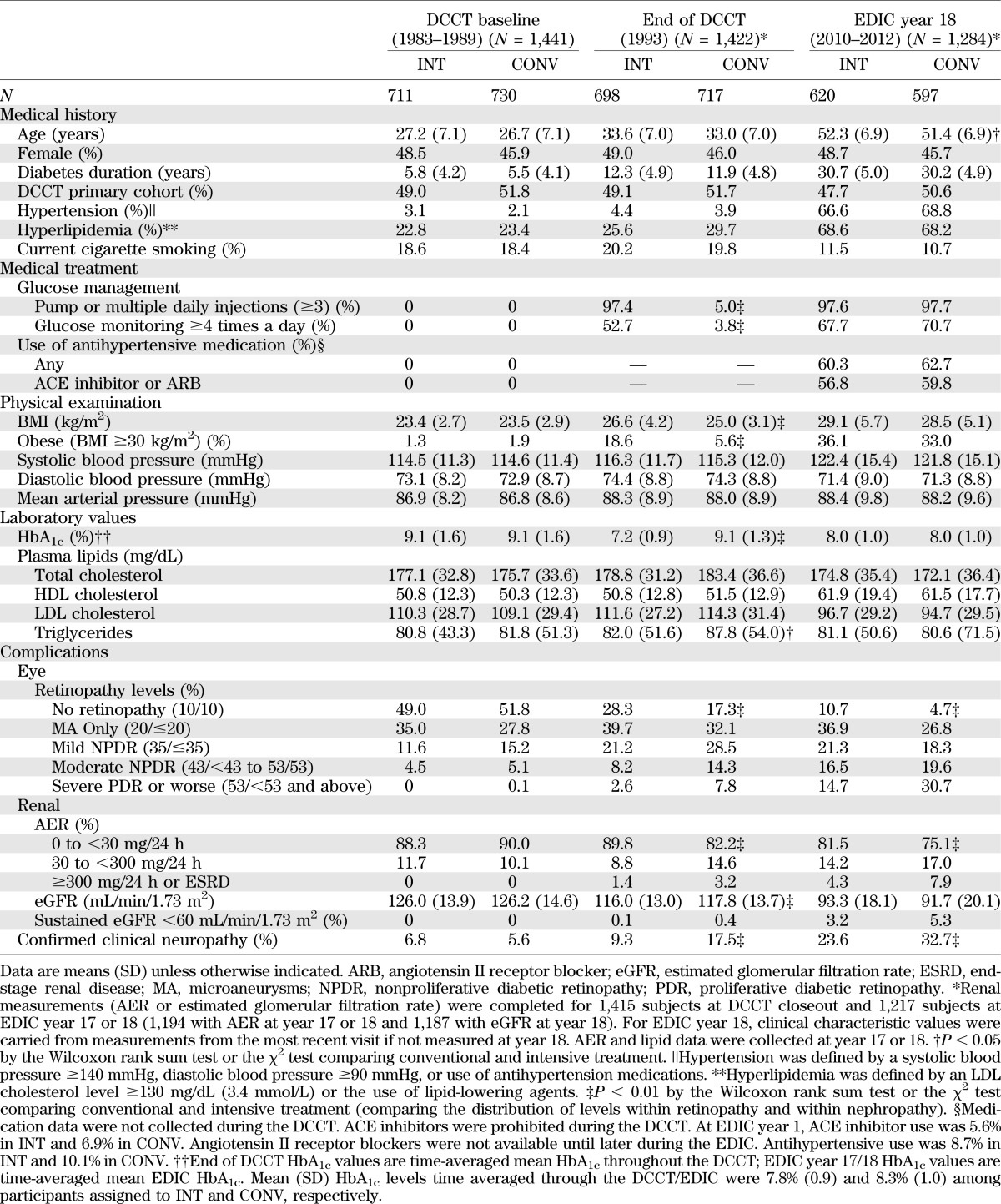
At baseline, the DCCT cohort had either no or relatively early diabetes complications and was generally healthy (Table 2). The cohort included a total of 196 adolescents, aged 13–17 years (13). The mean age of the entire cohort was 27 years, and the mean T1D duration was 2.6 and 8.7 years in the primary and secondary cohorts, respectively (3).
TABLE 2.
Clinical characteristics of DCCT/EDIC participants at DCCT baseline, DCCT closeout, and EDIC year 18
DCCT major findings
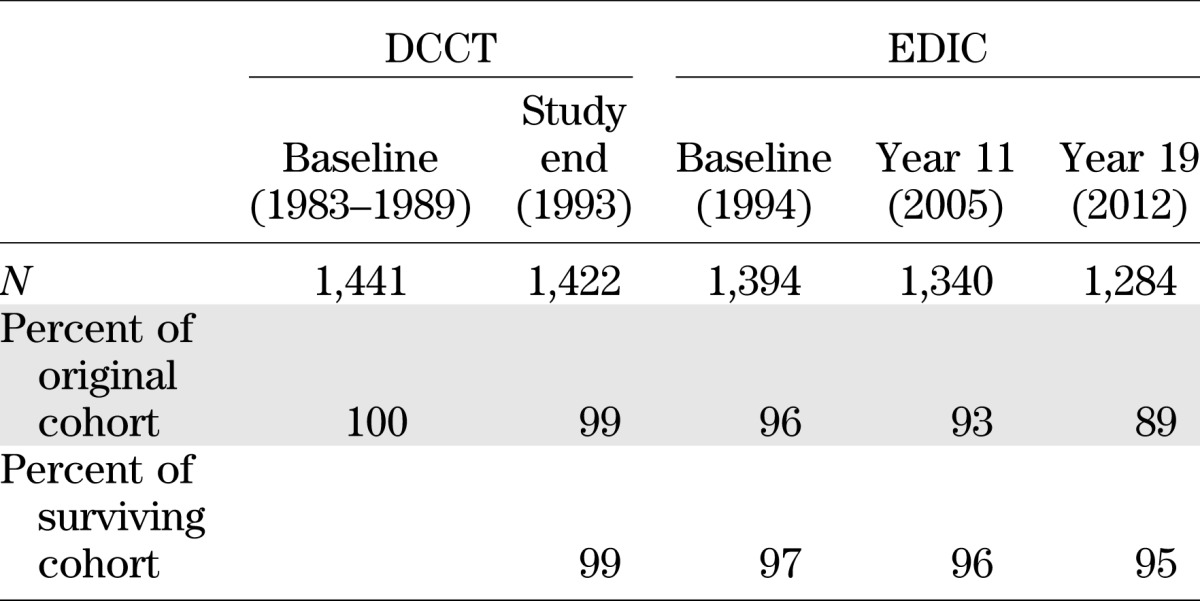
More than 99% of the original DCCT cohort remained in the trial over the mean 6.5 years (range 3–10 years) (Table 3), and >97% of time in the study was spent in assigned therapy (3). The majority of the “crossover” time in the CONV group was owing to the protocol-mandated institution of intensive treatment with preparation for and during pregnancy. After delivery, the CONV mothers returned to their assigned treatment. The study staff aimed to include participants as full partners in the study, and the frequent contact and provision of all diabetes care and supplies free of charge contributed to the extraordinary level of retention and adherence. Of the 1,441 subjects who were randomized, 11 died during the DCCT. Only 8 of 1,430 survivors failed to participate in the final closeout assessments.
TABLE 3.
Retention of DCCT/EDIC cohort over time
Glycemia.
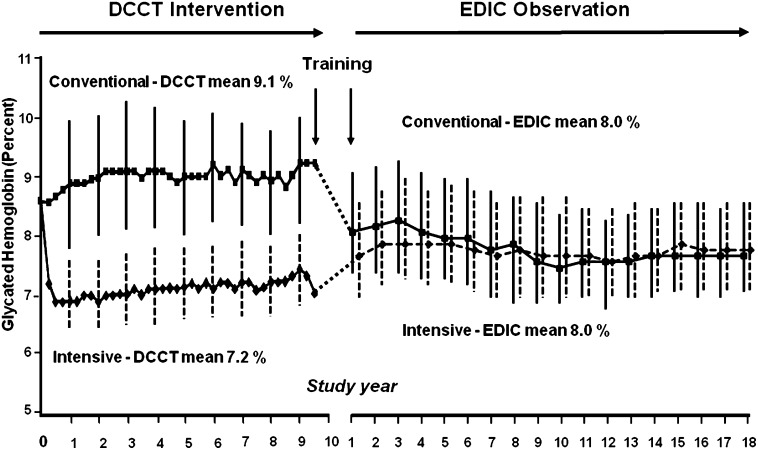
As a test of the “glucose hypothesis,” it was critical that glycemic separation be achieved between the CONV and INT treatment groups. The aggressive initiation of INT achieved a large fall in HbA1c levels by 3–6 months, with a mean nadir of 6.9% at 1 year and stable maintenance of this level with a 2% separation in HbA1c between INT and CONV over the course of the DCCT (Fig. 2). The adolescents achieved HbA1c levels ~1% higher than their adult counterparts in both CONV and INT (13).
FIG. 2.
Median HbA1c from DCCT through EDIC year 19. HbA1c was measured quarterly in CONV (dashed line) and INT (solid line) during DCCT and annually during EDIC.
Microvascular outcomes.
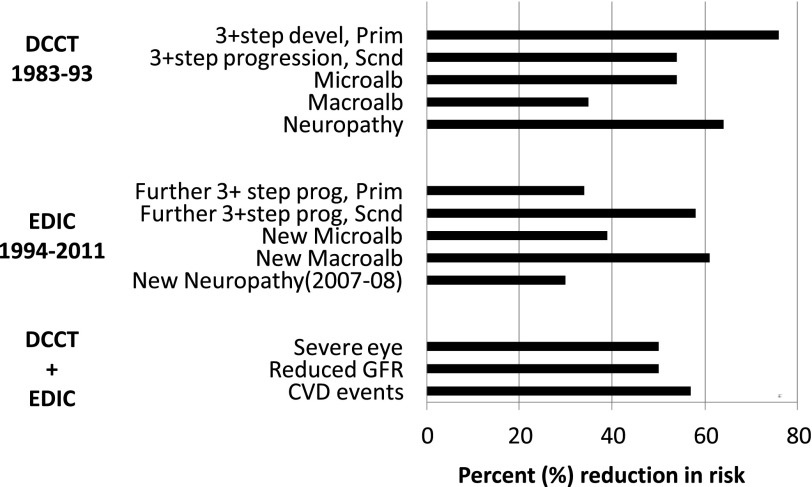
In 1993, the Data Safety Monitoring Board recommended that the DCCT be stopped 1 year ahead of schedule because the major study questions had been answered. The salutary effects of INT for primary prevention and secondary intervention of retinopathy, nephropathy, and neuropathy were consistent, significant, and clinically meaningful (3) (Fig. 3). These results, first presented at the 1993 American Diabetes Association (ADA) Scientific Sessions, demonstrated a dramatic improvement in the early objective measures of microvascular disease and neuropathy and provided the best evidence supporting the glucose hypothesis (13–19). The DCCT findings established a new paradigm for the management of T1D. INT as practiced in the DCCT was quickly advocated as the standard of care (20).
FIG. 3.
Intensive treatment reduction (%) in cumulative incidence of complications during DCCT, during EDIC (further progression, adjusting for level of complications at DCCT end), and during DCCT/EDIC combined. CVD events include nonfatal myocardial infarction or stroke or fatal CVD events; Macroalb, macroalbuminuria >300 mg/24 h; Microalb, microalbuminuria ≥40 mg/24 h; Neuropathy, clinical neuropathy consisting of the presence of signs/symptoms consistent with peripheral neuropathy and presence of either abnormal electrophysiologic findings of peripheral neuropathy or abnormal autonomic function testing (see ref. 17); severe eye, defined as proliferative diabetic retinopathy, clinically significant macular edema, laser surgery, ocular surgery (including cataract extraction, vitrectomy, glaucoma-related, other cornea–related, posterior capsulotomy, and enucleation), and blindness; Prim, primary prevention cohort; Reduced GFR, reduced glomerular filtration rate <60 mL/min/1.73 m2; Scnd, secondary intervention cohort.
Risk factors and pathogenetic mechanisms for microvascular outcomes.
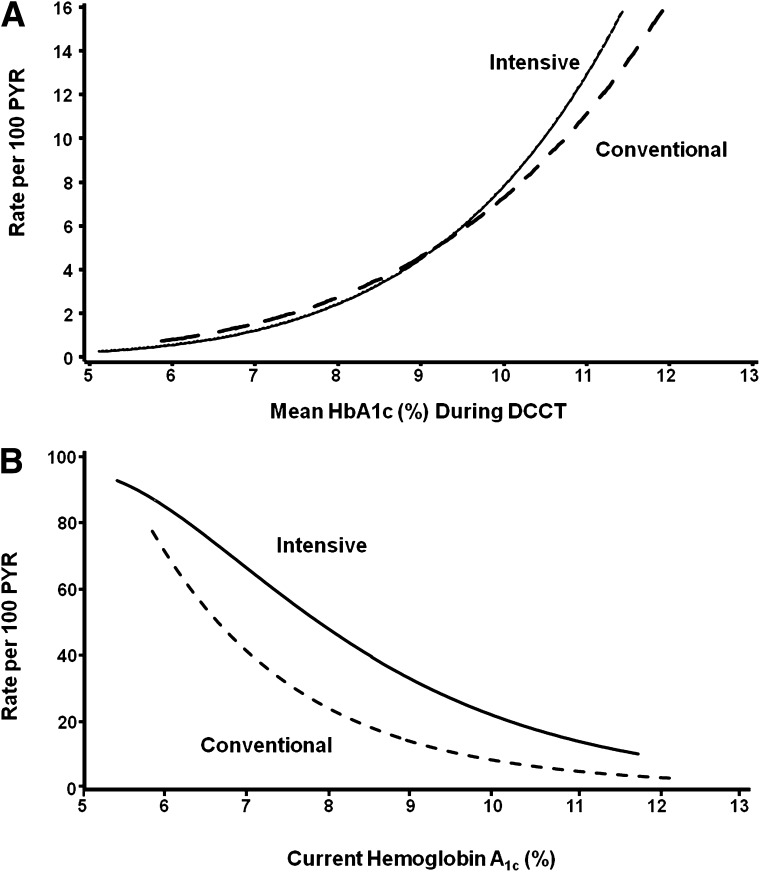
The risk of progression of microvascular complications was strongly related to the mean HbA1c during the DCCT, with similar risk relationships demonstrated within the two treatment groups (21,22) (Fig. 4). For example, for every 10% reduction in HbA1c (e.g., 10 to 9 or 9 to 8.1) the risk of retinopathy progression was reduced on average by 44%, of microalbuminuria (or worse) by 25%, of macroalbuminuria or worse by 44%, and of confirmed clinical neuropathy by 30% (21). There were no thresholds or break points in these risk relationships. The difference in mean HbA1c between the INT and CONV groups explained >96% of the statistical difference between the treatment groups in risk of retinopathy onset or progression and 98 and 92% for nephropathy and neuropathy, respectively (22). Furthermore, no other factor among a multitude examined, including lipid concentrations or blood pressure levels, contributed to the differences in complications between INT and CONV.
DCCT other findings
Cardiovascular.
CVD is an important, although nonspecific, complication of T1D; however, the DCCT population was generally too young and too healthy, with subjects with prior CVD or hypertension or dyslipidemia excluded during screening, to observe enough major CVD cases and events during the DCCT (24 events in 12 subjects) for reliable analysis (23). Nonetheless, there was a tantalizing, albeit nonsignificant (P = 0.059), difference in CVD events between the INT (3 events in 3 subjects) and CONV (21 events in 9 subjects) treatment groups.
β-Cell preservation.
During the selection of the DCCT cohort, patients with persistent, albeit low-level, insulin secretion, measured as a stimulated C-peptide concentration >0.2 but ≤0.5 nmol/L 90 min after a mixed-meal challenge, were allowed into the study if their diabetes duration at baseline was <5 years (Table 1). Three hundred and three subjects fulfilled this criterion, of whom 165 were randomly assigned to CONV and 138 to INT (24). Although the C-peptide concentrations declined progressively during the first 6 years of the DCCT, with only a handful of subjects retaining measureable C-peptide concentrations, INT slowed the rate of loss of C-peptide responsiveness by ~50% (24). The clinical benefits of persistent C-peptide secretion included significantly lower HbA1c levels with lower insulin doses, fewer hypoglycemic episodes, and significantly less retinopathy. The clinically important effects of C-peptide preservation further emphasized the application of INT early in the course of diabetes and currently serve as the main rationale for β-cell preservation studies.
Quality of life.
A diabetes-specific quality of life (DQOL) measure was developed to assess the effects of DCCT interventions (25). It tested satisfaction, impact, diabetes worry, and social/vocational worry. Approximately 20% of participants in the INT and CONV treatment groups had significant decreases in their DQOL by DCCT closeout; however, despite the rigors of INT and associated hypoglycemia, the difference in QOL between INT and CONV groups was nonsignificant (26).
Neurocognitive function.
Repeated severe hypoglycemic events, especially with coma or seizure, raised concern that INT might adversely affect long-term cognitive ability. Conversely, a putative benefit of INT regarding the accelerated cognitive decline associated with diabetes was postulated. At DCCT closeout, there were no substantive or significant differences in cognitive function between the treatment groups, despite the threefold increased frequency of severe hypoglycemia with INT (27). Moreover, the cumulative number of severe hypoglycemic events had no influence on cognitive test results, which were within the range recorded for a large sample of healthy persons without diabetes. For example, subjects with more than one episode of coma or seizure per year had no significant differences in the cognitive tests compared with subjects with one or fewer episodes per year (27). The testing assessed eight domains of neurocognitive function. INT did have a modest, significant (P = 0.004) beneficial impact on motor speed compared with CONV. Thus, at least within the limited exposure period of DCCT, INT and associated hypoglycemia did not impair cognitive function and may have had a limited benefit. Similar findings were noted in the adolescent DCCT population.
Adverse effects of intensive treatment
Hypoglycemia.
The major adverse outcome associated with INT as implemented during DCCT was hypoglycemia. Severe hypoglycemia was defined as an event requiring the assistance of others to treat and was associated with blood glucose <50 mg/dL or prompt recovery after administration of intravenous glucose, glucagon, or oral carbohydrate. Of all events, 55% occurred during sleep and ~30% were characterized by coma or seizure (9). Risk factors for severe episodes included prior hypoglycemia (odds ratio 2.5), longer duration of T1D, and a lower recent HbA1c level (Fig. 4).
FIG. 4.
A: Relationship of current updated mean HbA1c levels with three-step progression of retinopathy. B: Relationship of current HbA1c levels measured every 3 months during DCCT with occurrence of severe hypoglycemia, defined as episodes of hypoglycemia requiring assistance for treatment. PYR, patient-years.
During the DCCT, severe hypoglycemia event rates were approximately threefold higher in INT (62 per 100 patient-years) compared with CONV (19 per 100 patient-years) (3,28). Fifty percent of INT subjects experienced multiple events compared with 21% of CONV subjects.
Weight gain.
The INT group gained 4.6 kg more than the CONV group during DCCT, with BMI increasing 1.5 kg/m2 in men and 1.8 kg/m2 in women (29). When analyzed by quartiles of weight gain in the INT group, the highest quartile compared with the lowest three quartiles exhibited a greater BMI (31 vs. 24 kg/m2) and higher blood pressure (120/77 vs. 113/73 mmHg), LDL cholesterol (122 vs. 106 mg/dL), and triglyceride (88 vs. 70 mg/dL) concentrations, with no significant difference in HDL cholesterol levels (30). In the aggregate, these differences raise concern that INT subjects who gain the most weight might have a higher CVD risk, which is under investigation in EDIC.
EDIC Study (1994–)
Rationale and design.
At the end of the DCCT, the salutary effects of INT on early-stage microvascular complications had been demonstrated; however, the relatively brief duration of diabetes and presence at baseline of no or only minimal-to-moderate complications meant that the differential effects of INT on more advanced complications, including cardiovascular disease, could not be studied. The major goal of EDIC was to follow the DCCT cohort for the time required to determine whether the original DCCT therapies would have a longer-term effect on more advanced stages of diabetes microvascular complications and their clinical sequelae and on CVD (2).
At the end of the DCCT, as a consequence of the salutary effects of INT versus CONV, all of the CONV group participants were trained in INT by DCCT staff, although hospitalization to implement such therapy was not performed. In addition, since EDIC was envisioned as observational in nature, the diabetes care of all participants was subsequently transferred to their own care providers, with ∼50% initially maintaining clinical care at the institution that housed their DCCT clinic. EDIC evaluations were performed annually with methodologies that were largely identical as in DCCT, and extensive efforts were made to ensure consistency of these methods over time (2,31). Ninety-six percent (N = 1,394) of the surviving DCCT cohort elected to continue their participation in the observational follow-up (Table 3).
Major EDIC findings
By design, the differences in treatment of glycemia that had been created during DCCT dissipated rapidly during EDIC (Fig. 2). In addition, the return of the subjects’ diabetes care to their own care providers, with access to care and diabetes supplies influenced by insurance status, probably also contributed to the effacement of the differences in HbA1c levels between the former DCCT INT and CONV groups. The HbA1c level at DCCT end rose by ∼0.8 in the INT group and fell by ∼1.0 in the CONV group. By year 5 of EDIC, the HbA1c levels were no longer statistically different, and the mean levels over the past 20 years of EDIC have been similar.
Metabolic memory.
Recognizing the large effect that the differences in HbA1c between the two treatment groups had on the differences in the risks of complications during DCCT, it would be reasonable to expect that the separation in rates of complications would disappear when the HbA1c differences disappeared during EDIC. However, within the first 4 years of EDIC, it became clear that the separation in the incidence of complications between the former treatment groups was widening. Compared with former CONV-treated subjects, former INT treatment subjects showed a dramatic 70% reduction in the risk of further progression of retinopathy from the level present at the close of the DCCT (32). The durable effect of the earlier separation in glycemia during DCCT on microvascular complications during EDIC was referred to as “metabolic memory” (Fig. 3). Metabolic memory was shown to apply to nephropathy and neuropathy (32–38), and these long-term benefits of DCCT INT versus CONV have persisted to date.
Advanced microvascular complications.
The continued observation during EDIC allowed the examination of longer-term effects of DCCT INT and CONV. The differential effects of the two interventions on the relatively early stages of complications during DCCT expanded substantially during EDIC, owing in part to metabolic memory. Not only was a greater effect demonstrated on three-step change in retinopathy during EDIC, but beneficial effects on macular edema and proliferative retinopathy (39) and glomerular filtration rate (40) became evident with longer-term follow-up (Fig. 3). By 2012 (EDIC year 19), the risk of severe retinal outcomes, such as complication-related ocular surgeries, had been reduced by 48% with former DCCT INT versus CONV (41). In addition to a substantial reduction in incidence of albuminuria, more severe renal dysfunction, as measured by decreases in glomerular filtration rate, was reduced by ∼50% in the former INT compared with CONV group (40). The results of the DCCT on early-stage complications had translated into lower rates of severe complications during EDIC.
Atherosclerosis and CVD.
Although EDIC demonstrated salutary effects of INT compared with CONV on the progression of carotid intima-medial thickness over time (42–44) and on coronary calcification (45), the improvements in these two measures of atherosclerosis did not directly address whether CVD events would be reduced with prior INT. EDIC specified a priori that analysis of major CVD events by DCCT treatment group would only be conducted after 50 cases had been observed in the original CONV group. This case-based analytic strategy provided 85% power to detect a putative 50% difference between the treatment groups. The CVD landmark was reached after an average of 18 years of follow-up in DCCT and EDIC combined (46). INT reduced the risk of the primary CVD outcome (including major nonfatal and fatal CVD events, angina, or revascularization) by 42% and of fatal and nonfatal myocardial infarction and stroke by 58% (Fig. 3).
As with microvascular complications, 97% of the difference in risk with INT versus CONV was explained by the difference in the DCCT mean HbA1c. Although the difference in risk for CVD between treatment groups was partially explained by onset of albuminuria during DCCT or EDIC, the difference between treatment groups remained significant after adjusting for albuminuria; moreover, the occurrence of albuminuria was a function of the differences in the DCCT HbA1c (46). Further analyses to examine the role of traditional and nontraditional CVD risk factors will be conducted when 100 CVD cases have been observed in the original CONV group. That landmark is approaching.
Other outcomes.
Other outcomes associated with hyperglycemia, including manifestations of autonomic neuropathy such as bladder and sexual dysfunction and cardiac autonomic neuropathy, were reduced by INT (47–49).
Hypoglycemia and cognitive function.
During DCCT, the frequency of severe hypoglycemia was related to HbA1c level achieved. In the presence of higher HbA1c levels during EDIC, it is not surprising that the overall frequency of severe hypoglycemia, defined identically as during DCCT, decreased by approximately one-third. Moreover, with the equalization of diabetes treatment and HbA1c levels between the original INT and CONV groups during EDIC, the average frequency of severe hypoglycemia is now similar (39.7 and 35.1/100 patient-years, respectively).
The comprehensive evaluation of eight domains of cognitive function during DCCT did not reveal any pernicious effects of INT, or of the accompanying increased occurrence of hypoglycemia, on any domain of cognitive function (27). Repeat testing was performed at EDIC year 12, ~18 years after the initiation of DCCT therapy, to determine whether prior hypoglycemia might cause even longer-term adverse effects (50). There were no significant differences in any of the eight domains between the original treatment groups or between those who had experienced frequent severe hypoglycemia episodes and those who had not (50). Similar findings were noted in the subcohort that entered the DCCT as adolescents (51).
Mechanisms and risk factors for microvascular disease.
While a dominant role for hyperglycemia in the development and progression of complications can no longer be questioned, the mechanistic connection(s) between hyperglycemia and complications remains to be elucidated. Moreover, metabolic memory needs to be explained. Genetics and other DCCT/EDIC ancillary studies have offered possible explanations for these related mechanistic issues.
The DCCT conducted a family study including 217 DCCT probands and 241 first-degree family members with either type 1 or type 2 diabetes in order to explore potential genetic effects on the risk for developing complications (52). The study demonstrated familial clustering of complications, including retinopathy and nephropathy. Subsequent studies have examined DNA samples collected from the entire DCCT cohort, using candidate gene and genome-wide association scanning to identify genetic risk factors for complications. These studies have identified or confirmed several loci that appear to confer risk for the development of retinopathy (53), nephropathy (54,55), and erectile dysfunction (56).
As with the glycation of hemoglobin in circulating erythrocytes, tissue proteins also undergo glycation. After nonenzymatic glycation of amino groups in collagen and subsequent reactions and rearrangements, advanced glycation end products (AGEs), such as pentosidine, glucosepane, and carboxymethyllysine, are produced. These act to cross-link proteins, potentially altering their structure and function. Human collagen has a very long half-life, e.g., 15 years for dermal collagen, and is a major component of basement membrane that is altered in diabetic tissues.
Three DCCT/EDIC observations support a role for AGEs in the pathogenesis of complications. In a cross-sectional study of 216 DCCT participants who had skin punch biopsies near the time of DCCT closeout, a panel of AGEs in collagen and alteration of collagen solubility were correlated with the presence of retinopathy, nephropathy, and neuropathy independent of HbA1c (57). The AGEs proved to be predictive risk factors for the development and progression of these complications over the ensuing 10 years of EDIC, again independent of HbA1c (58). Finally, AGE levels correlated with DCCT mean HbA1c and were significantly lower in collagen obtained from the INT compared with that from the CONV group, demonstrating that AGE formation is modifiable by glycemic treatment. In concert, these observations suggest a role for AGEs in the metabolic memory phenomenon.
Another mechanism to explain metabolic memory could be epigenetic, with exposure to hyperglycemia modifying long-term gene expression. Pilot studies examining alterations of chromatin histone and DNA in DCCT/EDIC subjects are ongoing.
Worldwide implementation of INT and its metabolic consequences and effects on morbidity
Improved insulin formulations and regimens, and laboratory and technological advances, have all contributed to adoption of intensive diabetes management since the end of the DCCT in 1993. At the time that the DCCT results were announced, community estimates indicated that ~21% of individuals with T1D were using a single type of insulin, 8% of individuals with T1D were using a single daily injection, 13% were using MDI strategies, and <1% were using CSII (59,60). For those using more than one injection, a twice-daily split mix (regular/NPH) was the most commonly used regimen (61). Although urine glucose monitoring was being phased out in the late 1980s, as more convenient, accurate, and less costly SMBG devices were introduced, only ∼45% of individuals with T1D reported performing SMBG at least once daily (60,61). In 1989, 7% of a sample of individuals with T1D reported having an HbA1c test within the past 6 months, 57% had never heard of the test, and the mean HbA1c in the community was reported as >10% (60,61). Prior to the development of the ADA Standards of Medical Care in 1989, no evidence-based standards for medical care of diabetes existed. The ADA standards before the end of the DCCT recommended glucose monitoring without specifying urine or blood or the frequency of testing (62). Assessment of glycemic control using HbA1c was not well established, and laboratory measurements of this parameter were not standardized.
The results of the DCCT delivered the simple message that glucose control matters. As a result, individuals with diabetes and the health care community were challenged to reevaluate the prevailing views and diabetes care practices. The DCCT endorsed intensive insulin therapy using three or more daily insulin injections or CSII, frequent daily SMBG, and quarterly assessment of HbA1c, with the overall goal being to achieve glycemic control as close to the nondiabetic range as safely possible for people with T1D. Multidisciplinary team care as practiced in the DCCT (63,64), use of SMBG results to make daily proactive and reactive dose adjustments, and frequent communication between the patient and the patient’s health care team were advocated and quickly incorporated into consensus recommendations along with DCCT-established glycemic targets (20). Flexible nutrition management and compensatory adjustments were emphasized to promote adoption of this more rigorous treatment regimen into daily life, with emphasis changed from adjusting meals and lifestyle to match insulin to the manipulation of insulin to match lifestyle. The National Glycohemoglobin Standardization Program, established by DCCT investigators, standardized glycohemoglobin assays so that the results were “DCCT-aligned” (65). The evidence from the DCCT also provided the justification to accelerate the development of new pharmacologic and technologic tools to manage diabetes.
In the wake of the DCCT, have diabetes care practices and glycemic control changed? Has the T1D population benefited from the adoption of INT with lower morbidity and mortality? The dearth of national registries and longitudinal follow-up of populations with T1D, especially in the U.S., makes it difficult to answer these questions definitively; however, the Epidemiology of Diabetes Complications study, a long-term T1D community-based study in Pittsburgh, demonstrated decreases in HbA1c from ∼9.0% in the 1980s to 8.3% in 2006 (39). Other studies using T1D registries, many of them in Scandinavia, have shown reductions in HbA1c in children and adolescents from ∼9 to ∼8% in the decade that followed the DCCT (66–69). The HbA1c levels achieved parallel the results in the DCCT adolescents (14). Unfortunately, almost no longitudinal studies have examined the changes in HbA1c levels since the DCCT in adults with T1D. The recent (2012) cross-sectional analysis of 22,502 self-enrolled persons with T1D ≥1 year from 67 U.S. clinical centers in the T1D Exchange Clinic Registry (70) showed a mean HbA1c of 7.6% in those older than 26 years of age. The T1D Exchange Clinic Registry suggests that more work needs to be done. Although mean HbA1c was 8.3% in the entire group and CSII was used by ∼50%, only 20–25% of teens and 20–35% of adults achieved the ADA targets of 7.5 and 7.0%, respectively.
The impact of changes in therapy and glycemia on long-term complications is also difficult to assess with little reliable longitudinal population-based data. However, the risk of developing severe microangiopathy appears to be falling substantially, as has been demonstrated in the EDIC follow-up of the DCCT (39,40,46). A longitudinal study in Denmark has shown reductions in clinical albuminuria, a direct antecedent of falling glomerular filtration rate, and in proliferative retinopathy after 20 years of diabetes duration from ∼30 to ∼13% between the 1970s and the post-DCCT era (71). The incidence of treatment for end-stage renal disease has also decreased by ∼4.3% per year between 1990 and 2006, based on analyses of the U.S. Renal Data System (72). An Australian longitudinal study showed a progressive decline in retinopathy detected with fundus photography in temporal cohorts after 20 years from ∼50% in the pre-DCCT era cohort to 12% in the post-DCCT era cohort (73). Mortality rates have also fallen, at least in some populations, in the post-DCCT era (74). Of course, the improvement in long-term outcomes of type 1 diabetes cannot be solely attributed to glycemic control, as contemporaneous improvements in blood pressure and lipid control have also occurred. However, the use of INT and lower levels of glycemia correlate closely with the improvements in outcomes (71,73,75).
Unanswered questions and challenges for the future
Thirty years ago, physicians and their patients were faced with two divergent roads of diabetes management: the path of conventional treatment, largely predicated on reducing symptomatic hyperglycemia with simple regimens, and the less traveled road of intensive treatment aimed at near-normal glycemia with more physiologic insulin replacement. The results of DCCT/EDIC and other studies of control and complications in T1D, including the Stockholm Diabetes, Oslo, and Steno Studies (76–78), have shown that intensive diabetes management substantially and consistently reduces the occurrence and progression of diabetes and its complications. As a consequence, INT is now the standard-of-care therapy for people with T1D. Recent recommendations have emphasized the need to individualize metabolic goals based on anticipated benefits, depending on the stage of disease and complications, balanced against risk for hypoglycemia.
Despite the unequivocal benefit provided by INT and the advances in the tools for diabetes care and self-management developed in the post-DCCT era, the majority of patients still do not achieve target HbA1c levels. Health care provider and patient and societal barriers to care have hindered translation of INT from the clinical trial setting to clinical practice. Limited time for clinic staff to interact and follow up with patients is a modern-day medical practice challenge. Behavioral stress and fatigue related to the rigors associated with INT, including the need to administer a complicated regimen of multiple insulin doses titrated to carbohydrate intake and activity, and frequent SMBG testing, can lead to dose omissions and incomplete adherence. Lack of social support, limited access to care, and economic constraints often impede patients from reaching diabetes management goals. Coincident common psychiatric conditions such as depression can also adversely affect adherence and predispose to other consequences, such as eating disorders characterized by insulin dose omissions to prevent weight gain. These barriers require a multidisciplinary approach including specialists in behavioral medicine, dietitians, and social workers, who may not be routinely available across clinical centers. Current diabetes management must identify and address these individual and system-wide barriers to enable more patients to travel safely and successfully down the path of intensive treatment.
Finally, studies have consistently identified hypoglycemia, a risk inherent in current day INT, as a major barrier to implementation of INT (79). The fear of recurrent hypoglycemia, with potentially severe consequences, and the desire to avoid awkward social situations may inhibit adherence to the prescribed regimen. Advances in technology-assisted daily management of diabetes, especially real-time continuous glucose monitoring (CGM), may facilitate early patient awareness of impending hypoglycemia. In adults, CGM has been beneficial in helping reach HbA1c targets and limiting hypoglycemia (80). Yet, adolescent participants have struggled with consistent use of the sensors and have not uniformly improved their glycemic control (80). While the utility and clinical role of CGM remain to be defined, current results support careful individual application of these tools.
Novel educational approaches through peer support or Web-based programming may increase patient independence in diabetes management decisions and extend support services outside of time-limited clinic visits. Increasing use of social media among patients, such as text messaging treatment plan changes, are avenues under investigation to build patient endurance for intensive management. Until automated insulin delivery in closed-loop systems becomes a clinical reality, interventions to improve adherence and minimize hypoglycemia are required.
In addition to the individual and societal barriers, physiologic barriers to the achievement of near-normal glycemia remain. The spectrum of insulins now available is far larger than what was available during the DCCT, including very-rapid-acting insulins with more physiologic profiles that are more convenient and may reduce risk of hypoglycemia. However, subcutaneous administration of insulin will always be nonphysiologic, and the currently available very-rapid-acting drugs are still relatively sluggish compared with profiles of endogenous insulin secretion. Improvements in insulin and insulin delivery are still necessary.
Although hyperglycemia appears to be the major modifiable risk factor for long-term diabetes complications, explaining almost all of the difference in outcomes between the two DCCT treatment groups, it explains only a modest fraction of overall risk for diabetes complications. Identifying all of the causes of long-term complications, the biologic pathways linking hyperglycemia to complications, and the mechanism of metabolic memory remain critical issues that must be understood to improve our ability to reduce the development and progression of complications across patients of all ages and stages of disease. Identification of reliable biomarkers of diabetes complications is also necessary to focus our efforts and potentially provide alternate routes to reduce complications. The ongoing DCCT/EDIC is continuing to study these important topics.
Summary
The results of the DCCT catapulted the expectations for optimal diabetes care to a new level. The EDIC has reinforced these expectations by demonstrating the long-lasting benefits of INT on severe diabetes complications. Individuals with diabetes, health care providers and systems, and professional organizations all recognize the importance of glycemic control in preventing long-term complications, but achievement of optimal glycemic control remains elusive to many. Continued efforts to simplify daily management and provide access to sufficient education and support to facilitate successful self-management are needed.
Prevention of diabetes remains the ideal. However, in the meantime, reducing the onset and progression of long-term complications is critical to preserve QOL and long-term health in persons with type 1 diabetes. The DCCT/EDIC has shown the way to accomplish these goals.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
All members of the writing group contributed to the writing of the manuscript including D.M.N., M.B., P.C., S.G., R.G.-K., J.M.L., G.L., and B.L.
The authors thank Mary Hawkins (The George Washington University) for technical assistance and editorial compliance.
Footnotes
Clinical trial reg. nos. NCT00360815 and NCT00360893, clinicaltrials.gov.
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1093/-/DC1.
*A current listing of the DCCT/EDIC Research Group can be found in the Supplementary Data online.
See accompanying Perspective, p. 3963.
REFERENCES
- 1.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 2.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med 1993;328:1676–1685 [DOI] [PubMed] [Google Scholar]
- 5.Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of thirty-one. I. Survival, causes of death, and complications. Diabetologia 1978;14:363–370 [DOI] [PubMed] [Google Scholar]
- 6.Siperstein MD, Foster DW, Knowles HC, Jr, Levine R, Madison LL, Roth J. Control of blood glucose and diabetic vascular disease. N Engl J Med 1977;296:1060–1063 [DOI] [PubMed] [Google Scholar]
- 7.Boyd JD, Jackson RL, Allen JH. Avoidance of degenerative lesions in diabetes mellitus. JAMA 1942;118:694 [Google Scholar]
- 8.Dolger H. Clinical evaluation of vascular damage in diabetes mellitus. J Am Med Assoc 1947;134:1289–1291 [DOI] [PubMed] [Google Scholar]
- 9.The DCCT Research Group Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med 1991;90:450–459 [PubMed] [Google Scholar]
- 10.The DCCT Research Group Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem 1987;33:2267–2271 [PubMed] [Google Scholar]
- 11.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991;98(Suppl.):786–806 [PubMed] [Google Scholar]
- 12.The DCCT Research Group Diabetes Control and Complications Trial (DCCT): results of feasibility study. Diabetes Care 1987;10:1–19 [DOI] [PubMed] [Google Scholar]
- 13.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Control and Complications Trial Research Group Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology 1995;102:647–661 [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol 1995;113:36–51 [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications (DCCT) Research Group Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int 1995;47:1703–1720 [DOI] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med 1995;122:561–568 [DOI] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol 1995;38:869–880 [DOI] [PubMed] [Google Scholar]
- 19.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia 1998;41:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association Implications of the Diabetes Control and Complications Trial. Diabetes Care 1993;16:1517–1520 [DOI] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes 1995;44:968–983 [PubMed] [Google Scholar]
- 22.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN, DCCT/EDIC Research Group Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial—revisited. Diabetes 2008;57:995–1001 [DOI] [PubMed] [Google Scholar]
- 23.The Diabetes Control and Complications Trial Research Group Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995;75:894–903 [DOI] [PubMed] [Google Scholar]
- 24.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 25.The DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988;11:725–732 [DOI] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complications Trial Research Group Influence of intensive diabetes treatment on quality-of-life outcomes in the diabetes control and complications trial. Diabetes Care 1996;19:195–203 [DOI] [PubMed] [Google Scholar]
- 27.The Diabetes Control and Complications Trial Research Group Effects of intensive diabetes therapy on neuropsychological function in adults in the Diabetes Control and Complications Trial. Ann Intern Med 1996;124:379–388 [DOI] [PubMed] [Google Scholar]
- 28.The Diabetes Control and Complications Trial Research Group Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997;46:271–286 [PubMed] [Google Scholar]
- 29.The Diabetes Control and Complications Trial Research Group Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care 1995;18:1415–1427 [DOI] [PubMed] [Google Scholar]
- 30.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA 1998;280:140–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steffes M, Cleary P, Goldstein D, et al. Hemoglobin A1c measurements over nearly two decades: sustaining comparable values throughout the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. Clin Chem 2005;51:753–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachin J, Genuth S, Nathan D, Davis M, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the Diabetes Control and Complications Trial. Arch Ophthalmol 2008;126:1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White NH, Sun W, Cleary PA, et al. DCCT-EDIC Research Group Effect of prior intensive therapy in type 1 diabetes on 10-year progression of retinopathy in the DCCT/EDIC: comparison of adults and adolescents. Diabetes 2010;59:1244–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pop-Busui R, Low PA, Waberski BH, et al. DCCT/EDIC Research Group Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albers JW, Herman WH, Pop-Busui R, et al. Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010;33:1090–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pop-Busui R, Herman WH, Feldman EL, et al. DCCT/EDIC Research Group DCCT and EDIC studies in type 1 diabetes: lessons for diabetic neuropathy regarding metabolic memory and natural history. Curr Diab Rep 2010;10:276–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Boer IH, Sun W, Cleary PA, et al. DCCT/EDIC Research Group Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 2011;365:2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aiello LP, Sun W, Cleary P, et al.; DCCT/EDIC Research Group. Intensive diabetes therapy reduces ocular surgeries in patients with type 1 diabetes: twenty-eight year followup of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study (Abstract). ARVO 2013;A4024
- 42.Zinman B, Cleary P, O’Leary D, Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Effect of intensive diabetes treatment on carotid artery wall thickness in the epidemiology of diabetes interventions and complications. Diabetes 1999;48:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nathan DM, Lachin JM, Cleary P, et al. Diabetes Control and Complications Trial. Epidemiology of Diabetes Interventions and Complications Research Group Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polak JF, Backlund JY, Cleary PA, et al. DCCT/EDIC Research Group Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes 2011;60:607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleary PA, Orchard TJ, Genuth S, et al. DCCT/EDIC Research Group The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2006;55:3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan DM, Cleary PA, Backlund JY, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wessells H, Penson DF, Cleary P, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive glycemic therapy on erectile function in men with type 1 diabetes. J Urol 2011;185:1828–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarma AV, Kanaya A, Nyberg LM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Risk factors for urinary incontinence among women with type 1 diabetes: findings from the epidemiology of diabetes interventions and complications study. Urology 2009;73:1203–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enzlin P, Rosen R, Wiegel M, et al. DCCT/EDIC Research Group Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/ EDIC study cohort. Diabetes Care 2009;32:780–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobson AM, Musen G, Ryan CM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musen G, Jacobson AM, Ryan CM, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care 2008;31:1933–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The Diabetes Control and Complications Trial Research Group Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes 1997;46:1829–1839 [PubMed] [Google Scholar]
- 53.Al-Kateb H, Mirea L, Xie X, et al. DCCT/EDIC Research Group Multiple variants in vascular endothelial growth factor (VEGFA) are risk factors for time to severe retinopathy in type 1 diabetes: the DCCT/EDIC genetics study. Diabetes 2007;56:2161–2168 [DOI] [PubMed] [Google Scholar]
- 54.Boright AP, Paterson AD, Mirea L, et al. DCCT/EDIC Research Group Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes: the DCCT/EDIC Genetics Study. Diabetes 2005;54:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Kateb H, Boright AP, Mirea L, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 2008;57:218–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hotaling JM, Waggott DR, Goldberg J, et al. DCCT/EDIC Research Group Pilot genome-wide association search identifies potential loci for risk of erectile dysfunction in type 1 diabetes using the DCCT/EDIC study cohort. J Urol 2012;188:514–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999;48:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Genuth S, Sun W, Cleary P, et al. DCCT Skin Collagen Ancillary Study Group Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005;54:3103–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hiss RG, Anderson RM, Hess GE, Stepien CJ, Davis WK. Community diabetes care. A 10-year perspective. Diabetes Care 1994;17:1124–1134 [DOI] [PubMed] [Google Scholar]
- 60.Harris MI. Medical care for patients with diabetes. Epidemiologic aspects. Ann Intern Med 1996;124:117–122 [DOI] [PubMed] [Google Scholar]
- 61.American Diabetes Association. Therapy for Diabetes Mellitus and Related Disorders. Alexandria, VA, American Diabetes Association, 1991, p. 127–146 [Google Scholar]
- 62.American Diabetes Association Standards of medical care in diabetes–1993. Diabetes Care 1993;16(Suppl. 1):S10–S13 [PubMed] [Google Scholar]
- 63.Ahern J, Grove N, Strand T, et al. The DCCT Research Group The impact of the Trial Coordinator in the Diabetes Control and Complications Trial (DCCT). Diabetes Educ 1993;19:509–512 [DOI] [PubMed] [Google Scholar]
- 64.Delahanty L, Simkins SW, Camelon K, The DCCT Research Group Expanded role of the dietitian in the Diabetes Control and Complications Trial: implications for clinical practice. J Am Diet Assoc 1993;93:758–764, 767 [DOI] [PubMed] [Google Scholar]
- 65.Little RR, Rohlfing CL, Sacks DB, National Glycohemoglobin Standardization Program (NGSP) Steering Committee Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem 2011;57:205–214 [DOI] [PubMed] [Google Scholar]
- 66.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care 2004;27:2293–2298 [DOI] [PubMed] [Google Scholar]
- 67.Gerstl EM, Rabl W, Rosenbauer J, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr 2008;167:447–453 [DOI] [PubMed] [Google Scholar]
- 68.Rosenbauer J, Dost A, Karges B, et al. DPV Initiative and the German BMBF Competence Network Diabetes Mellitus Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care 2012;35:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Svensson J, Johannesen J, Mortensen HB, Nordly S, Danish Childhood Diabetes Registry Improved metabolic outcome in a Danish diabetic paediatric population aged 0-18 yr: results from a nationwide continuous Registration. Pediatr Diabetes 2009;10:461–467 [DOI] [PubMed] [Google Scholar]
- 70.Wood JR, Miller KM, Maahs DM, et al.; T1D Exchange Clinic Network. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013;36:2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003;26:1258–1264 [DOI] [PubMed] [Google Scholar]
- 72.Burrows NR, Li Y, Geiss LS. Incidence of treatment for end-stage renal disease among individuals with diabetes in the U.S. continues to decline. Diabetes Care 2010;33:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Downie E, Craig ME, Hing S, Cusumano J, Chan AKF, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care 2011;34:2368–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harjutsalo V, Forsblom C, Groop P-H. Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ 2011;343:d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nordwall M, Arnqvist HJ, Bojestig M, Ludvigsson J. Good glycemic control remains crucial in prevention of late diabetic complications—the Linköping Diabetes Complications Study. Pediatr Diabetes 2009;10:168–176 [DOI] [PubMed] [Google Scholar]
- 76.Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet 1986;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 77.Dahl-Jørgensen K, Brinchmann-Hansen O, Hanssen KF, et al. Effect of near normoglycaemia for two years on progression of early diabetic retinopathy, nephropathy, and neuropathy: the Oslo study. Br Med J (Clin Res Ed) 1986;293:1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichard P, Nilsson BY, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304–309 [DOI] [PubMed] [Google Scholar]
- 79.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamborlane WV, Beck RW, Bode BW, et al. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.