Abstract
The identification of diabetes-relevant islet autoantibodies is essential for predicting and preventing type 1 diabetes (T1D). The aim of the current study was to evaluate a newly developed electrochemiluminescence (ECL)-GAD antibody (GADA) assay and compare its sensitivity and disease relevance with standard radioassay. The assay was validated with serum samples from 227 newly diagnosed diabetic children; 68 prediabetic children who were prospectively followed to T1D; 130 nondiabetic children with confirmed islet autoantibodies to insulin, GAD65, IA-2, and/or ZnT8 longitudinally followed for 12 ± 3.7 years; and 181 age-matched, healthy, antibody-negative children. The ECL-GADA assay had a sensitivity similar to that of the standard GADA radioassay in children newly diagnosed with T1D, prediabetic children, and high-risk children with multiple positive islet autoantibodies. On the other hand, only 9 of 39 nondiabetic children with only a single islet autoantibody (GADA only) by radioassay were positive for ECL-GADA. GADA not detectable by ECL assay is shown to be of low affinity and likely not predictive of future diabetes. In conclusion, the new ECL assay identifies disease-relevant GADA by radioassay. It may help to improve the prediction and correct diagnosis of T1D among subjects positive only for GADA and no other islet autoantibodies.
Islet autoantibodies play an essential role in the prediction of type 1 diabetes (T1D) (1–3). These autoantibodies can appear as early as 6–9 months of age and usually precede clinical diabetes by years. Accurate detection of islet autoantibodies is crucial for finding the environmental factors that may trigger islet autoimmunity and promote progression to T1D.
In addition, islet autoantibodies are used extensively to stage diabetes risk and as the inclusion criteria for trials to prevent T1D. The risk of developing T1D is strongly associated with the number of islet autoantibodies among both first-degree relatives of patients with T1D and general population subjects. Children who have two or more persistent islet autoantibodies are at high risk; 70% of them will progress to diabetes in <10 years (4). In contrast, children with a single positive persistent autoantibody are at a much lower risk, with <10% progressing to diabetes in 10 years. Further stratification of these single autoantibodies for disease relevance by more-specific assays would greatly enhance staging of diabetes risk for clinical trials.
We recently developed an electrochemiluminescence (ECL) insulin autoantibody (IAA) assay (5,6) that has been shown to be more sensitive and more specific for detecting diabetes than the standard micro-IAA radioassay. In the current study, we evaluated a similar ECL-GAD antibody (GADA) assay and compared its sensitivity and disease relevance among GADA-positive children by the standard GADA radioassay.
RESEARCH DESIGN AND METHODS
From the children with newly diagnosed T1D at the Barbara Davis Center for Childhood Diabetes and studied within 2 weeks, we randomly selected 177 aged 0.9–17.8 years (median 9.2 years). Age-matched healthy children (n = 181) were selected as control subjects. From the Diabetes Autoimmunity Study in the Young (DAISY), we studied all 68 children who were prospectively followed up to 16.7 years (median 7.1 years) to clinical diabetes and all (n = 130) with persistent IAAs, GAD65, IA-2, and/or ZnT8. In addition, blindly coded Diabetes Autoantibody Standardization Program (DASP) samples from 50 patients with new-onset diabetes and 100 healthy control subjects were analyzed. Written informed consent was obtained from both participants and parents/guardians, and the study was approved by the Institutional Review Board of the University of Colorado.
ECL-GADA assay.
The format of ECL-GADA assay was adapted from ECL-IAA assay (5) but without acid treatment of serum. The serum samples were diluted five times with PBS and mixed at a 1:1 ratio with SULFO-TAG–labeled GAD65 protein 32 ng/mL (Diamyd Medical, Pittsburgh, PA) and biotin-labeled GAD65 protein 1,000 ng/mL in PBS containing 5% BSA for overnight incubation at 4°C. On the same day, the streptavidin-coated plate (Meso Scale Diagnostics [MSD], Gaithersburg, MD) was blocked with 3% Block A (MSD) overnight at 4°C. On the second day, the plate was washed three times with PBS with 0.05% Twin-20 buffer, and 30 µL overnight incubates were added per well. After 1 h of incubation at room temperature on a plate shaker set at low speed, the plate was washed again three times with PBS with 0.05% Twin-20 buffer followed by the addition of 150 µL 2× reader buffer and then counted on an Imager 2400 counter (MSD). The results were expressed as an index against our internal standard positive control of GAD65 monoclonal antibody (Abcam, Cambridge, MA). The assay cutoff index of 0.023 was set at the 99.5th percentile of 181 healthy control subjects, and the interassay coefficient of variance was 8.8% (n = 10) for the sample, with an index value of ∼1.0 and 14.6% for the sample near the index 99.5th percentile cutoff.
GADA radioassay.
The GADA radioassay was performed with National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) harmonized standard methods (7), and the upper limit of normal (20 DK units/mL) was established as the 98th percentile from the receiver operating characteristic curves in 500 healthy control subjects and 50 patients with new-onset diabetes. The interassay coefficient of variance for the sample with an index value of ∼1.0 was 8.3% (n = 20).
GADA competition assay.
The GADA competition assay format was identical to that of the GADA radioassay. Each serum was run in six separate wells: one without competition and five with competition by adding unlabeled GAD65 in five different concentrations from 4.6 × 10−7 to 4.6 × 10−11 (mol/L) into the incubating serum with labeled GAD65.
Statistical analysis.
Correlation analysis, Wilcoxon rank sum test, or Fisher exact test were performed with Prism version 4.0 software (GraphPad Software Inc., San Diego, CA). A two-tailed P value with an α level for significance was set at 0.05.
RESULTS
Similar sensitivity of the ECL-GADA assay and the standard GADA radioassay.
Among 177 newly diagnosed and 68 prediabetic children (total 245 children), 187 of 245 (76%) were GADA positive by the new ECL-GADA assay compared with 175 (71%) by GADA radioassay. Figure 1 shows the comparison of GADA positivity and levels between the ECL-GADA assay and the GADA radioassay. With the specificity set at the 99.5th percentile of 181 healthy control subjects for the ECL-GADA assay and the 98.0th percentile of 500 healthy control subjects for the GADA radioassay, the sensitivity of the ECL-GADA assay (75% [133 of 177]) was similar to that of the GADA radioassay (67% [119 of 177]) in newly diagnosed diabetic children (Fig. 1A). The sensitivity was also similar for ECL-GADA (79% [54 of 68]) and GADA radioassay (82% [56 of 68]) in prediabetic children (Fig. 1B). The levels of GADA were correlated between the two assays (R2 = 0.607 and 0.599 among new-onset and prediabetic children, respectively, both P < 0.0001). A few discordant samples showed low levels of GADA. In the blinded set of 50 DASP participants (Fig. 1C), the ECL-GADA assay had 76% sensitivity vs. 66% for the GADA radioassay, with the same specificity of 99% among 100 healthy control subjects for both assays. GADA levels from both assays correlated (R2 = 0.354, P < 0.0001) in the DASP set as well.
FIG. 1.
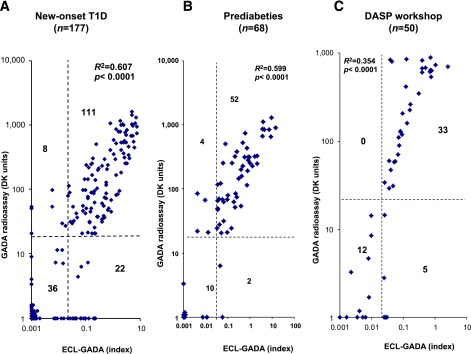
GADA levels from ECL-GADA assay compared with the standard NIDDK harmonized GADA radioassay. The dashed lines represent the assay cutoffs. The two assays were correlated (P < 0.0001) for all three groups, with both assays set at 99% specificity. A: Newly diagnosed children with T1D (n = 177) at the Barbara Davis Center. B: Prediabetic DAISY participants (n = 68). C: Newly diagnosed DASP workshop participants (n = 50).
Discrimination of disease-relevant GADA.
In the DAISY study, 130 nondiabetic children were persistently islet autoantibody positive for IAA, GADA, IA-2 antigen, and/or ZnT8A without yet progressing to diabetes. Of these, 47 were multiple autoantibody positive, and 83 were single autoantibody positive, including 39 single GADA-positive children. The single and multiple autoantibody–positive children did not differ by age (mean 13.7 ± 4.0 vs. 14.5 ± 4.3 years, respectively) or by follow-up duration (12.3 ± 3.7 vs. 12.4 ± 3.8 years, respectively). Of the 39 children with a single GADA autoantibody on multiple visits with years of follow-up, only 9 were ECL-GADA positive (Fig. 2). In contrast, almost all children positive for two or more autoantibodies showed congruent results by GADA radioassay and ECL assay (41 of 43, P < 0.0001) (Fig. 3). In addition, 48 of 130 nondiabetic children were GADA negative by radioassay but persistently positive for other islet autoantibodies, and only 1 of 44 children with a single autoantibody vs. 3 of 4 with multiple autoantibodies (P < 0.001) was found to be positive by ECL-GADA assay.
FIG. 2.
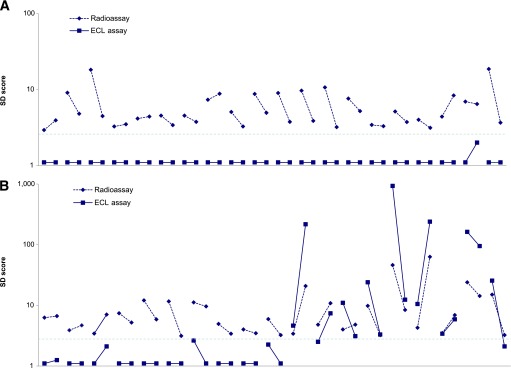
A: GADA levels from ECL-GADA assay compared with the standard NIDDK harmonized GADA radioassay in all 39 DAISY participants who were persistently positive for single islet autoantibody (GADA only) on multiple clinic visits with years of follow-up. B: ECL-GADA results were consistently negative in all but 9 participants. The horizontal line represents the 3-SD score, which is close to the cutoffs for both assays.
FIG. 3.
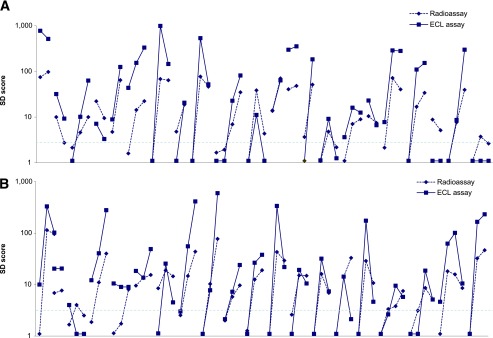
A: GADA levels from ECL-GADA assay compared with the standard NIDDK harmonized GADA radioassay in all 43 DAISY participants who were persistently positive for multiple islet autoantibodies. B: GADA levels on ECL-GADA assay and GADA radioassay correlated closely. The horizontal line represents the 3-SD score, which is close to the cutoffs for both assays.
Autoantibodies detected by the ECL-GADA assay are of high affinity.
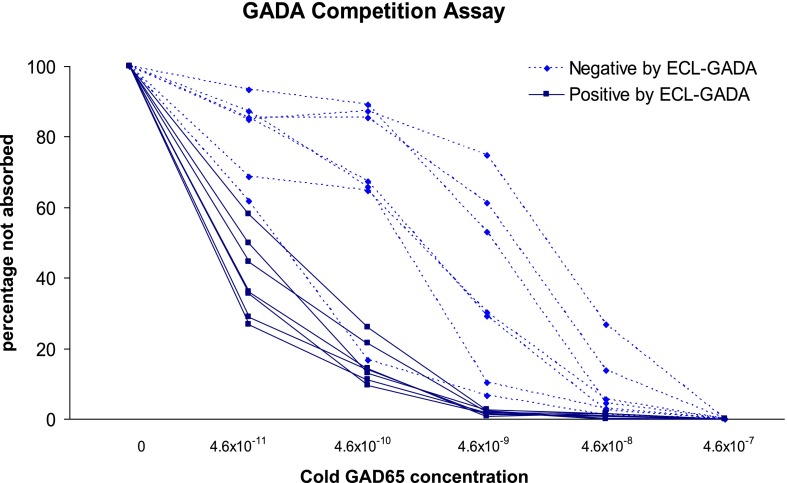
Fourteen GADA-positive samples by radioassay were randomly selected for the serial competition assay: seven positive by ECL-GADA assay (six multiple autoantibodies and one single GADA) and seven negative by ECL-GADA assay (all single GADA). Radioassay GADA levels were comparable between ECL-GADA positive (median 126 DK units, range 105–269 DK units) and ECL-GADA negative (median 125 DK units, range 94–235 DK units, P = 0.58). The results of the competition assay are illustrated in Fig. 4. Parallel to the finding from the ECL-IAA assay (5), GADA not detectable by ECL assay required a 10- to 100-fold higher concentration of unlabeled GAD65 protein (10−9 to 10−10 [mol/L]) for 50% inhibition of binding of GADA to labeled GAD65 protein for seven samples negative by ECL-GADA assay than for seven samples (10−11 [mol/L]) positive by ECL-GADA assay.
FIG. 4.
Sera with positive GADA by radioassay from 14 subjects (7 ECL-GADA positive and 7 ECL-GADA negative) were incubated with different concentrations of unlabeled GAD65 protein and analyzed with standard NIDDK harmonized GADA radioassay. GADA negative by ECL-GADA assay (dotted line), compared with GADA positive by ECL-GADA assay (solid line), required higher concentrations of GAD65 protein for 50% maximal inhibition, which is consistent with low affinity. Results are expressed as percentage of signal not absorbed.
DISCUSSION
This article reports a major improvement in the diabetes-relevant GADA measurement by a new ECL-based assay among GADA-positive children by the standard NIDDK harmonized GADA radioassay. The specificity of GADA radioassay (98.0th percentile) was based on a much larger number of control subjects and was slightly less than the ECL-GADA assay (99.5th percentiles), and both assays showed identical specificity in DASP workshop participants. The current findings are consistent with our previous finding (5) that an ECL-IAA assay is able to detect disease-relevant, high-affinity autoantibodies while ignoring a low-affinity signal that did not predict the development of multiple islet autoantibodies and progression to diabetes. This advance is likely to have important implications for population screening programs, cohort studies searching for environmental triggers, and clinical trials to prevent T1D. In the near future, applying these new ECL assays to adolescent and adult patients with late autoimmune diabetes in adults and hybrid or undetermined-type diabetes will also be helpful for improving clinical diagnosis.
In DAISY, TrialNet Pathway to Prevention, TEDDY (The Environmental Determinants of Diabetes in the Young), and other major programs, 30–60% of autoantibody positivity is attributable to a single islet autoantibody, with the majority of these single antibodies being GADA or IAA. Patients who express only a single islet autoantibody are at a low risk for developing T1D compared with those with multiple autoantibodies. Relatives expressing only IAA in the DPT-1 (Diabetes Prevention Trial of Type 1 Diabetes) study essentially did not progress to diabetes (8). The antibody affinity study by competition assay we used is very similar to previous reports (9–12), although it is an indirect measurement. Our previous (5) and present studies demonstrate that most of the single IAA or GADA detected only by radioassay were low-affinity antibodies and failed to produce a signal with the bivalent ECL assay. A few laboratories previously reported that both IAA and GADA affinity in multiple antibody–positive children was significantly higher than in children who remained single autoantibody positive or became autoantibody negative (9–11). We hypothesized (5) that most of these single antibodies may result from immunization with a cross-reactive molecule, whereas higher-affinity IAA or GADA result from immunization with an islet autoantigen itself. Both forms of antibody can be competed with native antigen molecule and thus are not biochemically false positive; however, in terms of biologic relevance, antibodies detected with only the radioassay appear not to predict diabetes.
A prospective follow-up study observed that the development of islet autoantibodies often happens sequentially (13); that is, the majority of subjects are initially single antibody positive. Thus, differentiation of high- from low-risk islet autoantibodies at that stage could be important in identifying truly high-risk subjects for prevention trials. The present study shows that individuals positive for only GADA by radioassay but negative by ECL assay remained ECL negative for years, even though they had multiple positive follow-up tests by radioassay. We did not see individuals who after being IAA (5,6) or GADA positive only by radioassay develop a second islet autoantibody or diabetes. On the other hand, in prediabetic or high-risk subjects with multiple islet autoantibodies, ECL assay always detected IAA (6) or GADA from the very beginning. Siljander et al. (12) found that there was no affinity maturation observed over time from seroconversion to T1D diagnosis. Thus, the new ECL assays may make it possible to correctly identify high-risk IAA (6) and GADA on the first positive sample. The major strength of this study was availability of well-characterized, prospectively collected DAISY samples. However, it would be ideal to confirm these results in additional cohorts with long-term follow-up (e.g., DPT-1, TrialNet Pathway to Prevention, TEDDY). Although we provide evidence that the disease-relevant ECL-GADA assay is of high affinity, full confirmation awaits a separate study. In conclusion, the ECL-GADA assay is, similarly to the ECL-IAA assay, able to differentiate high-risk islet autoantibodies from low-risk or nondisease-relevant antibodies at the earliest stage of screening.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grant DK-32083, DAISY grant DK-32493, Diabetes Endocrinology Research Center grant P30-DK-57516, Clinical and Translational Science Award 5ULIRR025780, Immune Tolerance Network grant AI15416, JDRF grant 11-2005-15, the Children’s Diabetes Foundation, and the Brehm Coalition.
No potential conflicts of interest relevant to this article were reported.
L.Y. researched data and wrote the manuscript. D.M., K.M.G., F.D., and L.J. researched data and reviewed the manuscript. A.K.S. reviewed and edited the manuscript. M.R. designed the DAISY study, researched data, and reviewed and edited the manuscript. G.S.E. researched data. L.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, Illinois, 21–25 June 2013.
Footnotes
See accompanying commentary, p. 4009.
Deceased.
REFERENCES
- 1.Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45:926–933 [DOI] [PubMed] [Google Scholar]
- 2.Bingley PJ, Bonifacio E, Williams AJK, Genovese S, Bottazzo GF, Gale EAM. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 [DOI] [PubMed] [Google Scholar]
- 3.Greenbaum CJ, Sears KL, Kahn SE, Palmer JP. Relationship of beta-cell function and autoantibodies to progression and nonprogression of subclinical type 1 diabetes: a follow-up of the Seattle Family Study. Diabetes 1999;48:170–175 [DOI] [PubMed] [Google Scholar]
- 4.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu L, Miao D, Scrimgeour L, Johnson K, Rewers M, Eisenbarth GS. Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes 2012;61:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Dong F, Miao D, Fouts AR, Wenzlau JM, Steck AK. Proinsulin/insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care 2013;36:2266–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases Consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orban T, Sosenko JM, Cuthbertson D, et al. Diabetes Prevention Trial-Type 1 Study Group Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest 2004;114:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayr A, Schlosser M, Grober N, et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 2007;56:1527–1533 [DOI] [PubMed] [Google Scholar]
- 11.Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol 2012;167:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siljander H, Härkönen T, Hermann R, et al. Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes Metab Res Rev 2009;25:615–622 [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]