Abstract
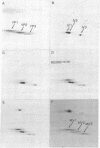
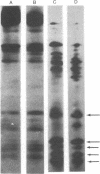
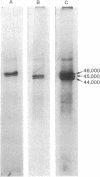
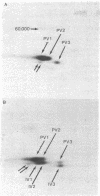
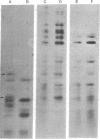
Synthesis of the three vitellogenin polypeptides (molecular weights of 44,000, 45,000, and 46,000) of Drosophila melanogaster has been analyzed in vivo and in a cell-free system. After labeling periods in vivo, the three vitellogenin polypeptides were made as the principal synthetic products of the female fat body. During a short (0.5 min) labeling period, they were identified as discrete species on two-dimensional gels. Two of the polypeptides have molecular weights of 45,000 and a third has a molecular weight of 44,000. After longer labeling periods (5-45 min) the three mature vitellogenins appeared. Both immunoprecipitation and peptide mapping confirmed that the species labeled at 0.5 min are immature forms of the vitellogenin polypeptides. In vitro translation of poly(A)-RNA from female fat body indicated another processing step in vitellogenin synthesis. Three polypeptides were obtained that were identified as precursors of the vitellogenins on the basis of immunoprecipitation and peptide mapping. Two of the translation products have a molecular weight of 46,000 and the third has a molecular weight of 45,000. Because the vitellogenins are secreted proteins, we interpret the higher molecular weight of the in vitro translation products as being due to signal peptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Waring G. L., Mahowald A. P. Mass isolation of pole cells from Drosophila melanogaster. Dev Biol. 1977 Apr;56(2):372–381. doi: 10.1016/0012-1606(77)90277-9. [DOI] [PubMed] [Google Scholar]
- Beadle G. W., Ephrussi B. The Differentiation of Eye Pigments in Drosophila as Studied by Transplantation. Genetics. 1936 May;21(3):225–247. doi: 10.1093/genetics/21.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink E. W., Wallace R. A. Precursor-product relationship between amphibian vitellogenin and the yolk proteins, lipovitellin and phosvitin. J Biol Chem. 1974 May 10;249(9):2897–2903. [PubMed] [Google Scholar]
- Bieger W., Seybold J., Kern H. F. Studies on intracellular transport of secretory proteins in the rat exocrine pancreas. V. Kinetic studies on accelerated transport following caerulein infusion in vivo. Cell Tissue Res. 1976 Jul 26;170(2):203–219. doi: 10.1007/BF00224299. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bownes M., Hames B. D. Analysis of the yolk proteins in Drosophila melanogaster. Translation in a cell free system and peptide analysis. FEBS Lett. 1978 Dec 15;96(2):327–330. doi: 10.1016/0014-5793(78)80428-1. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Strahlendorf P. W., Wyatt G. R. Vitellin and vitellogenin from locusts (Locusta migratoria). Properties and post-translational modification in the fat body. J Biol Chem. 1978 Aug 10;253(15):5325–5331. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., Eldridge J. D., Goldberger R. F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J Biol Chem. 1975 Dec 10;250(23):9060–9066. [PubMed] [Google Scholar]
- Gordon J. I., Deeley R. G., Burns A. T., Paterson B. M., Christmann J. L., Goldberger R. F. In vitro translation of avian vitellogenin messenger RNA. J Biol Chem. 1977 Nov 25;252(22):8320–8327. [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koeppe J., Ofengand J. Juvenile hormone-induced biosynthesis of vitellogenin in Leucophaea maderae. Arch Biochem Biophys. 1976 Mar;173(1):100–113. doi: 10.1016/0003-9861(76)90239-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Quantitation of parameters that determine the rate of ovalbumin synthesis. Cell. 1975 Mar;4(3):189–189. doi: 10.1016/0092-8674(75)90167-1. [DOI] [PubMed] [Google Scholar]
- Paul M., Goldsmith M. R., Hunsley J. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. Production of eggshell proteins by silkmoth follicular cells. J Cell Biol. 1972 Dec;55(3):653–680. doi: 10.1083/jcb.55.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Postlethwait J. H., Kaschnitz R. The synthesis of Drosophila melanogaster vitellogenins in vivo, in culture, and in a cell-free translation system. FEBS Lett. 1978 Nov 15;95(2):247–251. doi: 10.1016/0014-5793(78)81004-7. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Baker H. J. Purification and characterization of Xenopus laevis vitellogenin messenger RNA. J Biol Chem. 1977 Aug 10;252(15):5244–5250. [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Tata J. R. The expression of the vitellogenin gene. Cell. 1976 Sep;9(1):1–14. doi: 10.1016/0092-8674(76)90047-7. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Allis C. D., Mahowald A. P. Isolation of polar granules and the identification of polar granule-specific protein. Dev Biol. 1978 Sep;66(1):197–206. doi: 10.1016/0012-1606(78)90284-1. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Mahowald A. P. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 1979 Mar;16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Warren T. G., Mahowald A. P. Isolation and partial chemical characterization of the three major yolk polypeptides from Drosophila melanogaster. Dev Biol. 1979 Jan;68(1):130–139. doi: 10.1016/0012-1606(79)90248-3. [DOI] [PubMed] [Google Scholar]
- Wyatt G. R., Pan M. L. Insect plasma proteins. Annu Rev Biochem. 1978;47:779–817. doi: 10.1146/annurev.bi.47.070178.004023. [DOI] [PubMed] [Google Scholar]