Abstract
In the current study, we investigated the role of tribbles homolog 3 (TRIB3) in glucose-induced insulin resistance and whether the induction of TRIB3 by glucose is dependent on the nutrient-sensing hexosamine biosynthetic pathway (HBP) known to mediate glucose toxicity in diabetes. In diabetic rats, TRIB3 expression in skeletal muscle was increased after 10 days of hyperglycemia, and glycemia and muscle TRIB3 were both restored toward normal by insulin therapy. In L6 myocytes, the induction of TRIB3 by high glucose or glucosamine was reversible upon removal of these substrates. To assess the role of HBP in the induction of TRIB3, we demonstrated that the ability of high glucose to augment TRIB3 expression was prevented by azaserine, an inhibitor of glutamine: fructose-6-phosphate amidotransferase (GFAT), which is the rate-limiting enzyme in the HBP pathway. TRIB3 expression was also substantially stimulated by glucosamine, which bypasses GFAT, accompanied by a decrease in the insulin-stimulated glucose transport rate, and neither response was affected by azaserine. Further, knockdown of TRIB3 inhibited, and TRIB3 overexpression enhanced, the ability of both high glucose and glucosamine to induce insulin resistance. These data provide the mechanistic link between the HBP flux and insulin resistance and point to TRIB3 as a novel target for treatment of glucose-induced insulin resistance.
Insulin resistance is a major metabolic defect that helps establish and sustain hyperglycemia in type 2 diabetes mellitus (T2DM) and involves impaired insulin-stimulated glucose uptake into skeletal muscle (1,2). A component of insulin resistance in diabetic patients is induced by hyperglycemia itself (i.e., “glucose toxicity”) (3). Patients with metabolic syndrome and/or prediabetes are insulin-resistant; however, as glucose tolerance deteriorates into overt T2DM, the superimposition of hyperglycemia worsens overall insulin resistance. This latter component of insulin resistance is known as glucose-induced insulin resistance or glucose toxicity (3–6). Intensive therapy leading to euglycemia, whether by weight reduction (7), sulfonylureas (8,9), or insulin therapy (3), can reverse glucose-induced insulin resistance and put diabetes into remission, particularly in recently-diagnosed patients, and the increase in whole-body insulin sensitivity is paralleled by increased glucose transport rates in adipocytes (10) and skeletal muscle (7). Likewise, patients with type 1 DM (T1DM) in poor glycemic control exhibit insulin resistance, which can be reversed by intensified insulin therapy (11). Rats made diabetic by streptozotocin (STZ) exhibit a reduction in insulin-stimulated glucose transport in muscle and fat, which can be reversed by euglycemia induced by exogenous insulin or by promotion of glycosuria with phlorizin (12,13). Finally, multiple in vitro studies demonstrate direct effects of glucose to impair insulin-stimulated glucose transport in perfused target tissues (14) and cultured cell systems (15,16). Thus, a large body of data support the contention that glucose per se can induce desensitization of insulin's action to stimulate glucose uptake.
The mechanism by which glucose induces insulin resistance involves decreased activity of the glucose transport effector system and impaired translocation of intracellular GLUT4 glucose transporters to the cell surface in adipocytes and skeletal muscle (15,17,18). Furthermore, Marshall and colleagues (19–22) have shown that the ability of glucose to regulate its own uptake is dependent on its intracellular metabolism via the hexosamine biosynthetic pathway (HBP). The first and rate-limiting enzyme for this pathway is glutamine:fructose-6-phosphate (P) amidotransferase (GFAT), which converts fructose-6-P to glucosamine-6-P and the major end product, N-acetylglucosamine-6-P, serves as the substrate for N-linked and O-linked protein glycosylation. Studies have established that O-linked glycosylation, catalyzed by UDP–N-acetylglucosaminyl transferase (OGT), is linked to the induction of insulin resistance (23,24) and that this action involves effects on gene transcription (22). Involvement of hexosamine pathway products in glucose-induced insulin resistance is classically supported by two key observations: 1) azaserine, a glutamine antagonist that irreversibly inhibits GFAT, specifically blocks the ability of glucose to regulate glucose transport, and 2) glucosamine, which enters the hexosamine pathway distal to GFAT, can alone impair the glucose transport system with high potency in a manner that is not affected by azaserine.
Although glucose-induced insulin resistance involves augmented glucose metabolism via the hexosamine pathway, the actual identity of genes that diminish glucose transport system activity remain unknown despite more than a decade of study. We now demonstrate for the first time that glucose-induced insulin resistance is dependent on induction of TRIB3 in a process that requires HBP metabolism. Our interest in TRIB3 was first initiated when we used high-density cDNA microarrays (25,26) to identify TRIB3 as an upregulated gene in skeletal muscle from T2DM patients. Our subsequent work demonstrated that TRIB3 protein levels were elevated in skeletal muscle from hyperglycemic rodent models and from T2DM patients in a manner that was correlated with fasting glucose, that TRIB3 levels were regulated by media glucose concentrations in L6 cells, and that overexpression of TRIB3 blocked insulin-stimulated glucose transport rates (27). These data suggested that TRIB3 was a glucose-responsive gene that could mediate insulin resistance in muscle. The tribbles homolog 3 (TRIB3; also named TRB3, NIPK, and SIKP3) has been identified as an inhibitor of Akt activity by physically binding to its phosphorylation site. TRIB3 has also been reported to affect a number of functions, such as pancreatic β-cell survival and insulin secretory capacity, adipose and muscle cell differentiation, and endoplasmic reticulum (ER) stress (28,29). However, in addition to the Akt signaling pathway, studies show alternative pathways through which TRIB3 can exert effects on metabolism such as the peroxisome proliferator–activated receptor-γ signaling cascade (30). The current study demonstrates that TRIB3 is a mechanistic link between the HBP and glucose-induced insulin resistance.
RESEARCH DESIGN AND METHODS
All experimental procedures were approved by the University of Alabama at Birmingham Animal Care Committee, Birmingham, Alabama.
Diabetic rat model.
Hyperglycemia was induced by STZ (STZ; 50 mg/kg body weight i.p.). Five-week-old, male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were fed ad libitum a normal chow diet and randomized to three groups: control, diabetic, and diabetic + insulin treated (Humalog; 1.28 units/100 g i.p., twice daily). Blood glucose was measured using a Sirrus chemistry auto analyzer (Stanbio Laboratory, Boerne, TX). Tissue samples were snap-frozen in liquid nitrogen and stored at −80°C for later use.
Glucosamine injection.
Eight-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were fed a standard chow diet and housed in a 12-h light/dark cycle. After 1 week of accommodation, mice in the treatment group were injected in the tail vein with glucosamine (70 mg/kg/day) for 7 days, and control mice received injections with equal volumes of PBS buffer. Mice were killed, and tissue samples were snap-frozen in liquid nitrogen for later experiment.
Cell culture.
Rat L6 myoblasts (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (5 mmol/L glucose; Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 100 units/mL penicillin, and 100 mg/mL streptomycin. Upon reaching 80% confluence, the culture medium was changed to DMEM with 2% horse serum (5 mmol/L glucose; Invitrogen) to induce differentiation and refreshed every 2 days. L6 myotubes were fully differentiated after 7 days and ready for experiments. 3T3-L1 cells were grown in DMEM containing 25 mmol/L glucose and 10% calf serum. Two days after full confluence, cells started differentiating in DMEM containing 25 mmol/L glucose, 0.5 mmol/L isobutyl methylxanthine, 1 μm dexamethasone, 10 μg/mL insulin, and 10% FBS for 3 days and then in DMEM containing 25 mmol/L glucose, 10 μg/mL insulin, and 10% FBS for 2 days. After which, cells were maintained in DMEM with 5 mmol/L glucose and 10% FBS until used for experiments on 10–14 days after initiation of the differentiation. For experiment, cells were cultured in 5 mmol/L glucose, 25 mmol/L glucose, or 25 mmol/L glucose with azaserine for 24 h.
Lentiviral-mediated overexpression and knockdown of TRIB3.
The wild-type TRIB3 gene was kindly provided by Dr. Kiss-Toth (University of Sheffield, Sheffield, U.K.). The lentivirus vector was constructed and packaged by ADV BioScience (Birmingham, AL). The stop codon in the TRIB3 cDNA was removed and c-Myc was attached to the COOH-terminal. The modified cDNA was ligated into lentivector (pHR-EF-IRES-Bla) at the BamHI and XhoI sites. DNA sequencing was used to ensure proper insertion. Lentiviral shRNA (sense: 5′-GAT CCA GGAAGA AAC CGT TGG AGT TTG TCA AGA GCA AAC TCC AAC GGT TTC TTC CTT TTT GG-3′; antisense: 5′-AAT TCC AAA AA GGA AGA AAC CGT TGG AGT TTG CTC TTG ACA AAC TCC AAC GGT TTC TTC CTG-3′) locates at rat TRIB3 cDNA position 212 (NM_144755). To establish stably transfected cell lines, procedures were performed as previously described (27).
Glucose transport assays.
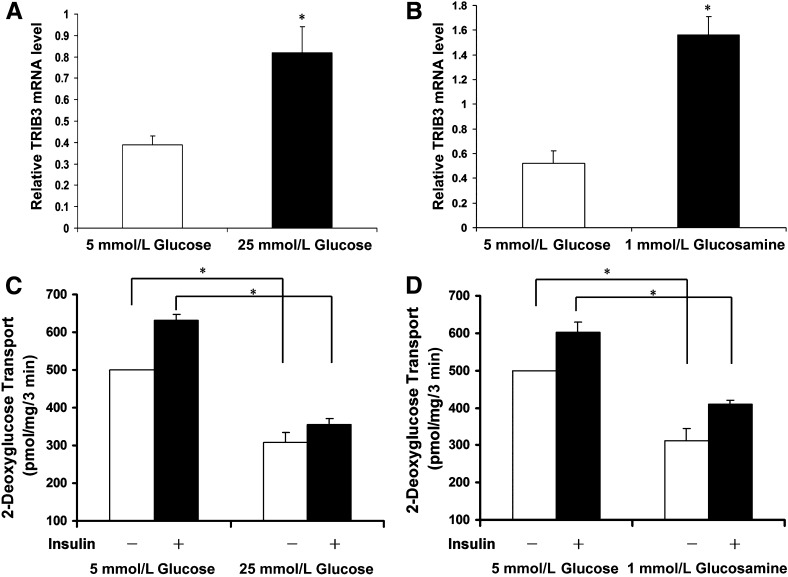
Glucose transport rates were measured in L6 muscle cells as previously described (31). Briefly, the same numbers of L6 cells were plated in each well. Cells were grown and differentiated into mytotubes as described above. Insulin resistance was induced by culturing L6 myotubes in 25 mmol/L high glucose or 5 mmol/L glucose medium with 1 mmol/L glucosamine for 18 to 24 h. Myotubes were incubated in serum-free DMEM for 3 h, followed by 45-min incubation in the absence or presence of 100 nmol/L insulin stimulation. Basal and the maximally insulin-stimulated 2-d-glucose transport rates were determined and normalized to total protein quantity, as described (31). Percent decrement of glucose transport of myotubes cultured in 25 mmol/L glucose or 1 mmol/L glucosamine compared with controls in 5 mmol/L glucose was calculated as an indicator of glucose-/glucosamine-induced insulin resistance.
RNA isolation and analysis.
RNeasy columns (Qiagen, Valencia, CA) were used to isolate total RNA from L6 cells, and total RNA from tissue samples was extracted using Trizol reagent (Invitrogen). cDNA was synthesized by VILO kit (Invitrogen) following the manufacturer’s instructions. StepOnePlus 96-well machine (Applied Biosystems, Foster City, CA) was applied for real-time quantitative PCR analysis. PCR products were detected using Sybr Green and normalized to 18S ribosomal RNA using the following sequences: rat 18S, 5′-GGAGGATGAGGTGGAGCGAGT-3′ (5′ primer) and 5′-GCCTCTCCAGGTCCTCACGC-3′ (3′ primer); rat TRIB3, 5′-AGAGTCCTGGAACGGGTATC-3′ (5′ primer), 5′- AGTTGCGTCGATTTGTCTTC-3′ (3′ primer).
Protein isolation and immunoblot.
Proteins were extracted from tissues or cells in lysis buffer. The Bicinchoninic Acid kit (Sigma-Aldrich, St. Louis, MO) was used for quantifying protein concentrations. Membranes were incubated with anti-TRIB3 antibody (Calbiochem or Santa Cruz Biotechnology) or anti–O-N-Acetylglucosamine (GlcNAc) antibody (CTD110.6 mouse mAb, Cell Signaling) and followed by anti-rabbit or anti-mouse immunoglobulin G (Santa Cruz Biotechnology). Images were captured by using enhanced chemiluminescence (Pierce) on a ChemiDoc XRS imager (BioRad), and Image Laboratory software (BioRad) was used for quantification.
Statistical analysis.
Experimental results are shown as the mean ± SD. Statistical analyses were conducted using the unpaired Student t test assuming unequal variance, unless otherwise indicated. Significance was defined as P < 0.05 or P < 0.01.
RESULTS
Increased TRIB3 expression parallels with upregulated protein O-GlcNAc modification levels.
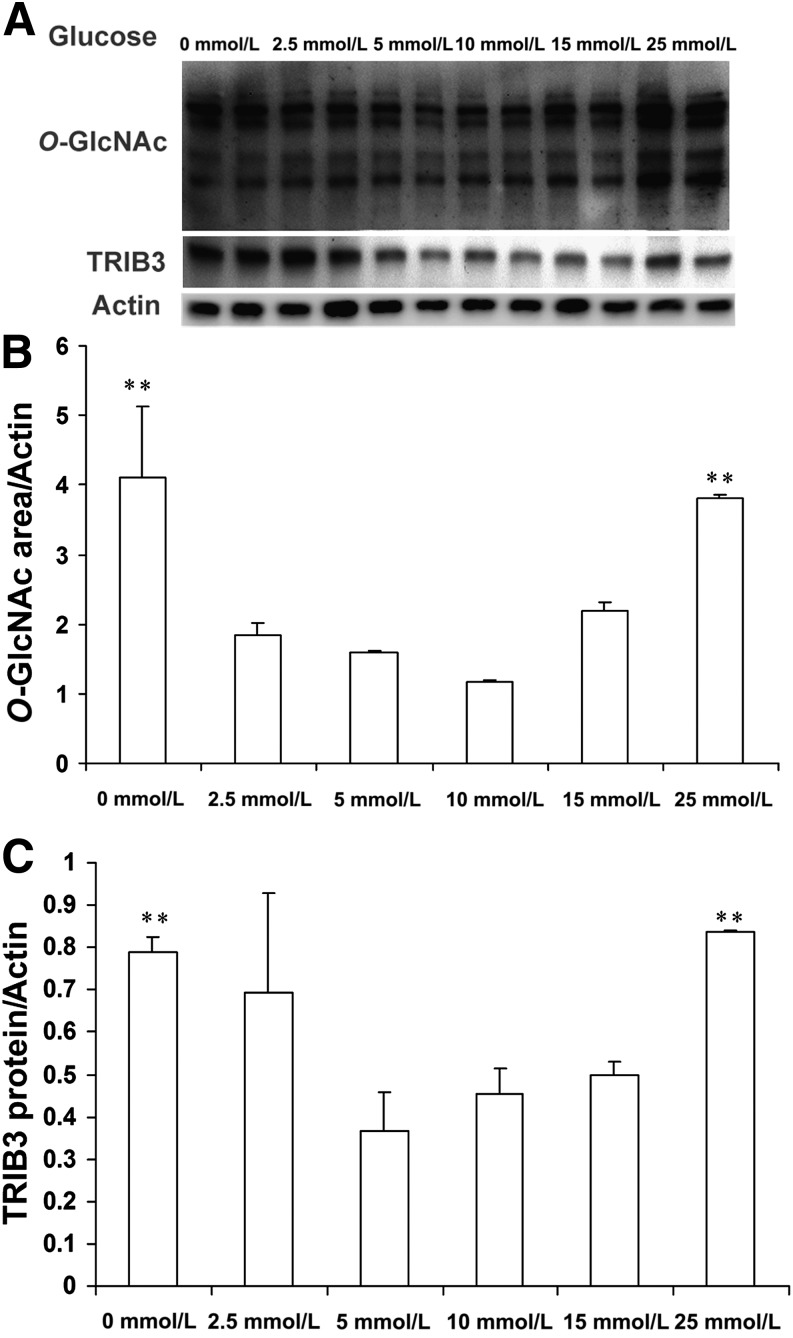
Increased protein O-GlcNAc modifications are classically observed under high glucose/hyperglycemia conditions (19–22); however, recent studies have shown upregulated protein O-GlcNAcylation levels in cancer cells and cardiomyocytes under glucose deprivation (32–34). In the current study, we observed upregulated protein O-GlcNAc modification levels under glucose deprivation (0 mmol/L) and high glucose (25 mmol/L) conditions in cultured L6 muscle cells (Fig. 1). Consistent with our previous finding (27), TRIB3 expression was significantly induced by glucose deprivation and by high glucose (Fig. 1). This paralleled upregulation of TRIB3 expression and protein O-GlcNAcylation levels suggests that the HBP could participate in the regulation of TRIB3 by glucose.
FIG. 1.
Induction of TRIB3 is accompanied with upregulation of protein O-glycosylation levels. A: L6 myotubes were cultured in medium containing 0, 2.5, 5, 10, 15, and 25 mmol/L glucose for 24 h. Representative Western blot films of protein O-GlcNAc modification and TRIB3 protein expression are shown. B: Quantitative analysis of protein O-GlcNAc modification area. C: Quantitative analysis of TRIB3 protein. Results are expressed as means ± SD, n = 3–6, and were calculated from three independent experiments. **P < 0.01.
TRIB3 expression is induced by high glucose and glucosamine in a reversible manner.
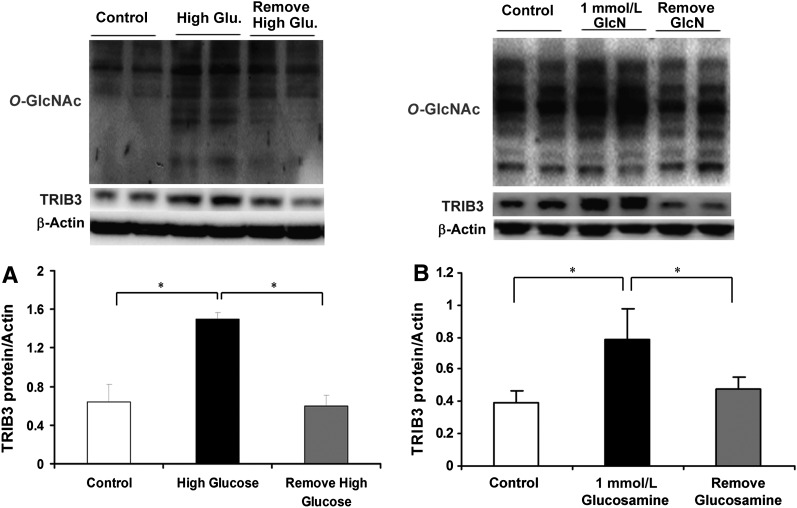
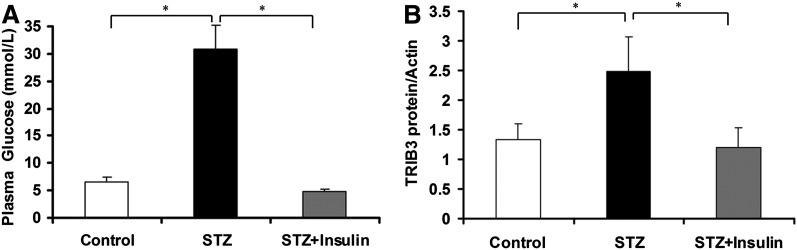
We previously reported that TRIB3 expression was upregulated in skeletal muscle from hyperglycemic T2DM patients and insulin-resistant rodent models and was induced by exposure to high glucose in L6 myotubes (27). In Fig. 2A, we have confirmed that TRIB3 expression is augmented in L6 myotubes treated with 25 mmol/L glucose DMEM medium for 24 h (∼2.3-fold, P < 0.05; Fig. 2A) compared with cells incubated with 5 mmol/L glucose. We now further demonstrate that the increase in TRIB3 protein induced by exposure to 25 mmol/L glucose is restored to baseline over the subsequent 24 h in the presence of 5 mmol/L glucose DMEM medium (P < 0.05; Fig. 2A). Similarly, TRIB3 protein was induced in L6 cells treated with 1 mmol/L glucosamine, and this effect was also reversed upon removal of glucosamine because TRIB3 levels returned toward baseline (Fig. 2B). Consistently, this induction and restoration of TRIB3 expression was associated with upregulation and downregulation of protein O-GlcNAcylation levels (Fig. 2). To assess whether the glucose-responsive expression of TRIB3 was reversible in vivo, we induced diabetes in rats by administering moderate-dose STZ, and observed an approximately twofold increase in TRIB3 protein levels in skeletal muscle (P < 0.05). As shown in Fig. 3, restoration of euglycemia using exogenous insulin injections was accompanied by a reduction in muscle TRIB3 to baseline levels. These results indicate that the induction of TRIB3 in muscle by high glucose can be reversed in the presence of lower glucose concentrations in vitro and in vivo.
FIG. 2.
TRIB3 is induced by high glucose and glucosamine (GlcN) in a reversible manner in cultured L6 myotubes. Representative Western blot films of protein O-GlcNAcylation levels and TRIB3 are shown. A: L6 myotubes were cultured in 5 mmol/L glucose (control), 25 mmol/L glucose for 24 h (high glucose), or 25 mmol/L glucose for 24 h, followed by 5 mmol/L glucose for 24 h (high glucose removed). B: L6 myotubes were cultured in 5 mmol/L glucose (control), 1 mmol/L GlcN for 24 h, or 1 mmol/L GlcN for 24 h, followed by 5 mmol/L glucose for 24 h (GlcN removed). Results are expressed as means ± SD, n = 3–6; experiment was repeated at least three times. *P < 0.05.
FIG. 3.
Increased TRIB3 expression in hyperglycemia was reversed by insulin treatment in STZ-induced diabetic rats. A: Plasma glucose levels of control, STZ-diabetic rats (STZ), and STZ-diabetic rats treated with insulin (STZ+Insulin). B: Protein expression of TRIB3 in skeletal muscle of control, STZ-diabetic rats (STZ), and STZ-diabetic rats treated with insulin (STZ+Insulin). Hyperglycemia was confirmed after 5 days of STZ injection, and insulin was administrated twice (7:00 a.m. and 7:00 p.m.) daily successively for 7 days. Results are expressed as means ± SD, n = 6. *P < 0.05.
TRIB3 expression is induced by HBP metabolism in vitro and in vivo.
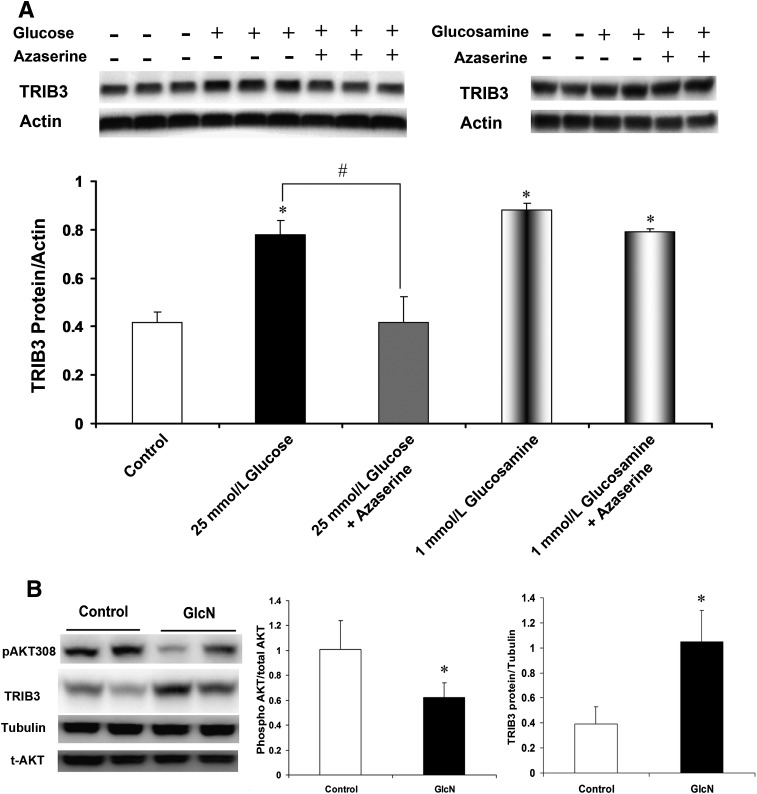
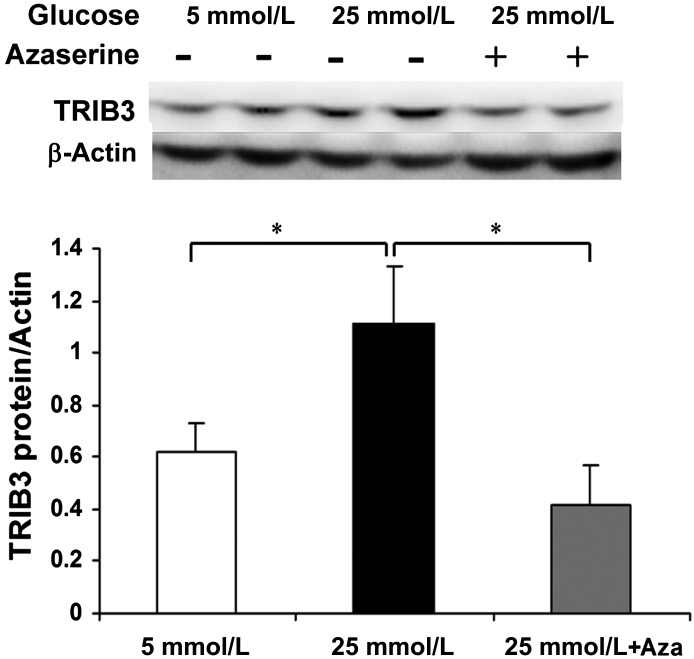
On the basis of previous reports that glucose-induced insulin resistance required glucose metabolism via the HBP (16–22), we hypothesized that induction of TRIB3 by glucose was also dependent on the HBP. To test this idea, we cultured L6 myotubes in high-glucose medium with and without azaserine, an inhibitor of GFAT, the rate-limiting enzyme for glucose metabolism via the HBP. On one hand, as shown in Fig. 4A, azaserine prevented the induction of TRIB3 by 25 mmol/L glucose. On the other hand, glucosamine, which enters the HBP distal to GFAT, increased TRIB3 protein levels in the muscle cells to the same extent as 25 mmol/L glucose; however, azaserine was not able to block induction of TRIB3 by glucosamine. The increase in TRIB3 induced by glucose and glucosamine was accompanied by reductions in basal and insulin-stimulated glucose transport activity, as shown in Fig. 5, consistent with the functional consequences that HBP metabolism exerts on the glucose transport effector system (16–22). Because we had earlier shown that glucosamine infusion causes insulin resistance in rats (17,18), we injected mice daily with glucosamine and observed a 2.6-fold increase in skeletal muscle TRIB3 protein and a 64.7% decrease in phosphorylation of Akt at Thr308 (both P < 0.05) compared with control rats injected with saline (Fig. 4B). We further examined the role of the HBP and TRIB3 induction in another insulin target cell, namely, 3T3-L1 adipocytes. In fully differentiated adipocytes, TRIB3 protein expression was also significantly induced by high glucose, and consistent with the findings in L6 muscle cells, this induction of TRIB3 was also blocked by azaserine (Fig. 6). These results showing effects of glucose and glucosamine, with the ability of azaserine to block the action of glucose but not glucosamine, fulfill the classic criteria for demonstrating that the effects are dependent on HBP metabolism.
FIG. 4.
Activation of HBP is required for induction of TRIB3 by glucose. A: In L6 myotubes, inhibition of HBP by azaserine (40 μmol/L) blocked the induction of TRIB3 expression by 25 mmol/L glucose, without affecting the induction of TRIB3 expression by 1 mmol/L glucosamine (GlcN). Culture medium with 25 mmol/L high glucose or 1 mmol/L glucosamine was added with 40 μmol/L azaserine for 24 h. B: Representative analysis of TRIB3 expression and AKT phosphorylation in skeletal muscle of mice injected with saline or glucosamine (70 mg/kg/day) for 7 days. t-AKT, total AKT. *P < 0.05 vs. control; #P < 0.05 vs. indicated group.
FIG. 5.
Induction of TRIB3 by high glucose and glucosamine is accompanied with decreased glucose transport rate. TRIB3 mRNA levels of L6 myotubes cultured in medium with or without presence of 25 mmol/L high glucose (A) or 1 mmol/L glucosamine (B). C: Basal and insulin-stimulated glucose rate of L6 myotubes cultured in 5 mmol/L or 25 mmol/L glucose medium for 24 h. D: Basal and insulin-stimulated glucose rate of L6 myotubes cultured in 5 mmol/L glucose medium, with or without presence of 1 mmol/L glucosamine, for 24 h. Results were from three to six repeated experiments and are expressed as means ± SD. *P < 0.05.
FIG. 6.
GFAT inhibitor, azaserine (Aza), blocked induction of TRIB3 protein by high glucose in 3T3-L1 adipocytes. Fully differentiated 3T3-L1 adipocytes were incubated in 5 mmol/L or 25 mmol/L high glucose, with or without azaserine (40 μmol/L), for 24 h. Representative Western blot film is shown. Protein bands were quantified by Image Laboratory software (BioRad). Results (n = 6) are expressed as means ± SD. *P < 0.05.
Induction of insulin resistance by glucose and glucosamine is dependent on TRIB3.
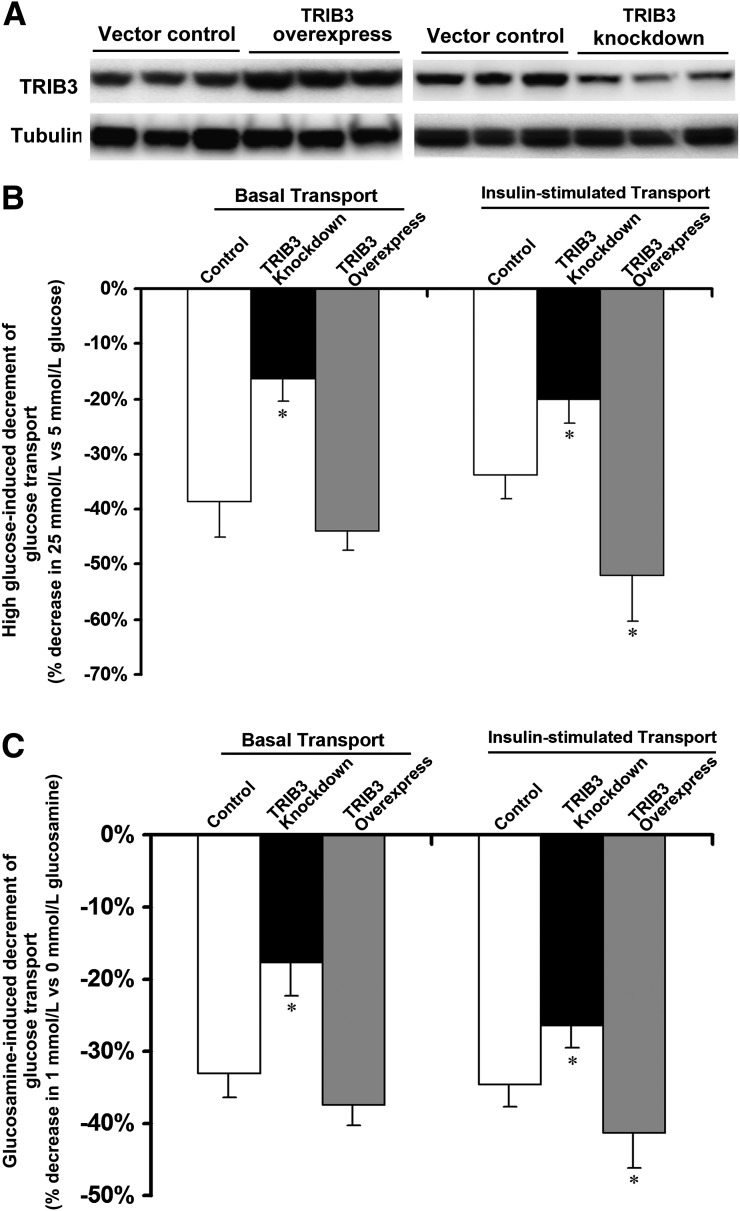
We previously showed that TRIB3 knockdown in L6 muscle cells led to significant increase in basal and insulin-stimulated glucose uptake rates along with enhanced phosphorylation of insulin receptor substrate-1 (IRS-1) at Tyr612 and insulin-stimulated phosphorylation of Akt on Ser473. To determine whether TRIB3 is necessary for glucose-induced insulin resistance, we treated control and TRIB3-knockdown muscle cells with high glucose and glucosamine. Figure 7 demonstrates that incubation of control cells for 24 h in 25 mmol/L high glucose (Fig. 7B) or in 1 mmol/L glucosamine (Fig. 7C) led to significant decrements in basal and insulin-stimulated glucose transport rates. Moreover, in TRIB3-knockdown cells, the ability of glucose and glucosamine to decrease basal and insulin-stimulated glucose transport rates was reduced by ∼50%. These results demonstrate that glucose-induced insulin resistance is at least partially dependent on TRIB3.
FIG. 7.
Knockdown of TRIB3 inhibited, and TRIB3 overexpression enhanced, the ability of both high glucose and glucosamine to induce insulin resistance. A: Representative Western blot film shows efficacy of TRIB3 overexpression and TRIB3 knockdown in L6 myotubes. Incubation for 24 h in high glucose (25 mmol/L) (B) or glucosamine (1 mmol/L) (C) significantly reduced basal and insulin glucose transport. TRIB3 knockdown inhibited and TRIB3 overexpression enhanced the ability of high glucose (B) and glucosamine (C) to induce insulin resistance in L6 myotubes. Development of insulin resistance induced by high glucose or glucosamine was calculated as the percentage of decrement of glucose transport of cells cultured in 25 mmol/L high glucose or 1 mmol/L glucosamine compared with cells cultured in 5 mmol/L glucose medium. Results were from three to six independent experiments (n = 3–6 for each group). *P < 0.05 vs. control.
DISCUSSION
In the current study, we show that the ability of high glucose to diminish glucose transport system activity in muscle cells is dependent on induction of TRIB3 and glucose metabolism via the HBP. When combined with our previous observations, the novel current results provide critical information to indicate that induction of TRIB3 via the HBP is relevant to the pathophysiology of glucose toxicity in diabetes. In prior studies, we have shown that TRIB3 expression is significantly increased in skeletal muscle biopsy specimens from patients with T2DM compared with insulin-sensitive individuals and is positively correlated with fasting blood glucose levels (27). Also, elevated muscle TRIB3 expression was observed in multiple hyperglycemic rodent models, including STZ-induced diabetic rats, Zucker diabetic fatty rats, and db/db mice (27). In vitro, incubation of cultured L6 myocytes in the presence of high glucose, under conditions that reduce basal and insulin-stimulated glucose transport rates, increased TRIB3 mRNA and protein expression (27). Finally, stable overexpression of TRIB3 in L6 muscle cells, using a lentiviral expression vector, led to suppression of insulin-stimulated glucose uptake and GLUT4 translocation to the cell surface as well as insulin-stimulated Akt phosphorylation (27). These previous studies established that TRIB3 is a glucose-responsive gene in muscle and can reduce basal and insulin-stimulated glucose transport activity together with insulin-stimulated GLUT4 translocation and Akt phosphorylation.
Our previous data notwithstanding, whether TRIB3 induction was responsible for glucose-induced insulin resistance was still not clear. The current data address this issue by addressing three critical aspects that pertain to the pathophysiology of glucose toxicity: First, we found that the induction of TRIB3 is reversible under conditions that restore full activity of the glucose transport effector system. This was the case in vitro after removal of high glucose or glucosamine from the medium and in vivo when STZ-induced diabetic mice were made euglycemic by treatment with exogenous insulin. These results are consistent with studies in poorly controlled patients with T2DM or T1DM, demonstrating that there is a component of insulin resistance that can be reversed by several weeks of intensive insulin treatment (3–14).
The second criterion was that TRIB3 induction in response to high glucose should involve increased glucose metabolism via the HBP, which has been shown to mediate glucose toxicity in cell models and in vivo systems including diabetic patients (19–22). The current data demonstrate that the induction of TRIB3 fulfills the classic criteria (16–22) for participation of the HBP, namely, that 1) azaserine, an antagonist of GFAT, the rate-limiting enzyme for the pathway, blocked the ability of glucose to augment TRIB3 expression and that 2) glucosamine, which enters the hexosamine pathway distal to GFAT, can alone impair the glucose transport system with high potency in a manner that is not affected by azaserine.
The third aspect of the current data is that short hairpin RNA–mediated suppression of TRIB3 in L6 myocytes blocked the ability of high glucose and glucosamine to diminish basal and insulin-stimulated glucose transport rates by ∼50% compared with that observed in control cells. Furthermore, TRIB3 overexpression increased the ability of high glucose and glucosamine to decrease insulin-stimulated transport without modulating effects on basal transport activity.
Therefore, the current data indicate that glucose-induced insulin resistance is at least partly dependent on induction of TRIB3, which in turn is dependent on glucose metabolism via the HBP. Thus, TRIB3 is a missing link between HBP metabolism and diminished activity of the glucose transport effector system under conditions of glucose toxicity.
The pathophysiological role of TRIB3 in mediating glucose toxicity with chronic hyperglycemia should not be confused with the short-term physiological role of TRIB3, which regulates nutrient metabolism during feeding and fasting (35). We found that short-term fasting in rats enhanced whole-body insulin sensitivity concomitant with decrements in TRIB3 mRNA in muscle and increments of TRIB3 in adipose tissue when compared with nonfasted controls. On the other hand, rats fed a Western diet for 7 days became insulin-resistant concomitant with increments in TRIB3 in muscle and a decrease in adipose. Furthermore, TRIB3 upregulation impaired insulin sensitivity at the cell level, whereas TRIB3 downregulation led to an increase in basal and insulin-stimulated glucose transport rates. Thus, TRIB3 influenced tissue glucose uptake oppositely in muscle and fat according to conditions of nutrient deprivation and excess (35). With nutrient excess, TRIB3 upregulation limits excessive uptake of glucose into muscle, whereas the decrease in TRIB3 expression in adipose tissue leads to the increase in glucose uptake needed for glycerol/triglyceride synthesis, thereby, redirecting fuel from muscle to adipose tissue for storage. The opposite scenario accompanies nutrient deprivation. Fasting reduces muscle TRIB3 levels, resulting in increased glucose transport in a setting where glycogen stores are depleted. When the next meal is eventually consumed and insulin levels rise, these changes would facilitate the restoration of muscle glycogen stores. In adipose tissue, fasting increases TRIB3 and impairs glucose uptake under conditions when fat is geared for lipolysis as opposed to lipogenesis. These physiological roles for TRIB3 to acutely regulate fuel metabolism in fat and muscle during feeding and fasting are in contradistinction to the pathophysiological role in diabetes, where chronic hyperglycemia results in persistent upregulation of muscle TRIB3, thus contributing to glucose-induced insulin resistance.
Remaining questions not fully addressed by the current study pertain to the mechanisms by which HBP metabolism leads to the increase in TRIB3 expression and the explanation of how TRIB3 mediates desensitization of the glucose transport effector system. Regarding the former question, increased flux through the hexosamine pathway rapidly elevates UDP-N-acetylglucosamine levels (23,24) providing substrate for both N-linked and O-linked protein glycosylation. O-GlcNAcylation has been reported as a common post-translational modification regulating activity for many important cytosolic and nuclear proteins (36), including the transcription factors Sp1 (37), c-Myc (38), cAMP-responsive element–binding protein (39), Stat 5 (39), pancreatic duodenal homeobox-1 (40), and glycogen synthase (41). Studies have established that O-linked glycosylation is linked to the induction of insulin resistance (19,23,24) and that this action depends on changes in gene transcription (22). Increased protein O-GlcNAc modifications are classically observed under high glucose/hyperglycemia conditions (19–22); however, recent studies have shown upregulated protein O-GlcNAcylation levels under glucose deprivation in cancer cells and cardiomyocytes (32–34). In the current study, we found that protein O-GlcNAcylation levels were also paradoxically increased under no/low-glucose and high-glucose conditions in L6 myotubes. In addition, the upregulated protein O-GlcNAcylation levels were accompanied with induction of TRIB3 protein levels, which strongly indicates that O-GlcNAcylation could be the mechanism by which HBP metabolism regulates TRIB3 gene expression. To date, despite its clinical importance, the actual proteins being modified by O-glycosylation and the identity of regulated genes involved in glucose-induced insulin resistance have remained a mystery for 2 decades. The current data indicate that O-glycosylation involves proteins that produce induction of TRIB3 as a key gene mediating glucose-induced insulin resistance. In fact, the TRIB3 promoter region has three classes of binding sites that are compelling candidates for glucose regulation (42–45), including Sp1, E-Box, and upstream stimulatory factor binding sites. Sp1 was the first transcription factor shown to be regulated by O-glycosylation (46), and the regulation of transforming growth factor-β1 (47), plasminogen activator inhibitor 1 (48), and acetyl-CoA carboxylase (49) are regulated by high glucose and/or glucosamine via post-translational O-linked glycosylation of Sp1. In any event, further studies are needed to address the mechanisms by which HBP metabolism results in induction of TRIB3.
The second question involves TRIB3 effects on insulin action and glucose transport. We (27,49) and others (50) have shown that TRIB3 interacts with Akt and blocks its phosphorylation and activation in response to insulin. However, TRIB3 may exert its influence via other potential mechanisms. For example, TRIB3 acts like a scaffolding protein and interacts with multiple cytosol and nuclear proteins, including several transcription factors (28), protein kinases (50), and other proteins (29), which lead to multiple biological effects, including modulation of stress response, cell viability, and glucose or lipid metabolism.
In summary, our findings include:
1) The induction of TRIB3 is accompanied with upregulation of protein O-GlcNAcylation levels under both glucose deprivation and high glucose conditions.
2) The induction of TRIB3 by high glucose and glucosamine in L6 myocytes is reversible upon removal of these substrates.
3) STZ-induced diabetes in rats is associated with increased expression of TRIB3 in skeletal muscle, and TRIB3 levels are restored to baseline by euglycemia achieved with exogenous insulin therapy.
4) TRIB3 induction under conditions of glucose toxicity involves increased HBP metabolism.
5) Knockdown of TRIB3 in L6 muscle cells significantly prevented, and TRIB3 overexpression enhanced, the development of insulin resistance induced by high glucose or glucosamine.
When considered in light of our previous data showing that muscle TRIB3 levels are increased in T2DM patients and that TRIB3 diminishes activity of the insulin-responsive glucose transport system, the current study substantiates the role of TRIB3 as a mechanistic link between the HBP metabolism and glucose-induced insulin resistance. The data identify TRIB3 is as novel potential target for treatment of glucose toxicity in diabetes.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (DK-038765 and DK-083562) and by the Merit Review program of the Department of Veterans Affairs. We also acknowledge support from core facilities of the University of Alabama (UAB) Diabetes Research Center (P60 DK-079626).
W.T.G. has served on advisory boards for Novo Nordisk, Eisai, Daiichi Sankyo, Liposcience, Vivus, Janssen, Boehringer Ingelheim, and Bristol-Myers Squibb/AstraZeneca; speaker's list for Merck, Eisai, and Amylin; sponsored research funded by Amylin, Merck, and Weight Watchers. No other potential conflicts of interest relevant to this article were reported.
W.Z. designed the study, performed experiments, analyzed data, and wrote, reviewed, and edited the manuscript. J.L., L.T., and Q.L. helped with animal experiments and glucose transport experiments. Y.F. and W.T.G. designed the study, interpreted data, and reviewed the manuscript. W.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. John C. Chatham (UAB Division of Molecular and Cellular Pathology) and Dr. Andrew James Paterson (UAB Division of Endocrinology, Diabetes and Metabolism) for technical assistance.
Parts of this study were an oral presentation at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
REFERENCES
- 1.DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care 1992;15:318–368 [DOI] [PubMed] [Google Scholar]
- 2.Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med 1998;105:331–345 [DOI] [PubMed] [Google Scholar]
- 3.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes 1985;34:222–234 [DOI] [PubMed] [Google Scholar]
- 4.Garvey W, Birnbaum M. Insulin resistance and disease. In Bailliere's clinical endocrinology and metabolism Ferrannini E, Ed. London, U.K., Bailliere Tindall, 1994, p. 785–873 [Google Scholar]
- 5.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 1990;13:610–630 [DOI] [PubMed] [Google Scholar]
- 6.Unger RH, Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia 1985;28:119–121 [DOI] [PubMed] [Google Scholar]
- 7.Friedman JE, Dohm GL, Leggett-Frazier N, et al. Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest 1992;89:701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield MS, Doberne L, Rosenthal M, Schulz B, Widstrom A, Reaven GM. Effect of sulfonylurea treatment on in vivo insulin secretion and action in patients with non-insulin-dependent diabetes mellitus. Diabetes 1982;31:307–312 [DOI] [PubMed] [Google Scholar]
- 9.Kolterman OG, Gray RS, Shapiro G, Scarlett JA, Griffin J, Olefsky JM. The acute and chronic effects of sulfonylurea therapy in type II diabetic subjects. Diabetes 1984;33:346–354 [DOI] [PubMed] [Google Scholar]
- 10.Scarlett JA, Kolterman OG, Ciaraldi TP, Kao M, Olefsky JM. Insulin treatment reverses the postreceptor defect in adipocyte 3-O-methylglucose transport in type II diabetes mellitus. J Clin Endocrinol Metab 1983;56:1195–1201 [DOI] [PubMed] [Google Scholar]
- 11.Yki-Järvinen H, Helve E, Koivisto VA. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 1987;36:892–896 [DOI] [PubMed] [Google Scholar]
- 12.Karnieli E, Armoni M, Cohen P, Kanter Y, Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes 1987;36:925–931 [DOI] [PubMed] [Google Scholar]
- 13.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 1987;79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter EA, Hansen BF, Hansen SA. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J 1988;252:733–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvey WT, Olefsky JM, Matthaei S, Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem 1987;262:189–197 [PubMed] [Google Scholar]
- 16.Bailey CJ, Turner SL. Glucosamine-induced insulin resistance in L6 muscle cells. Diabetes Obes Metab 2004;6:293–298 [DOI] [PubMed] [Google Scholar]
- 17.Baron AD, Zhu JS, Zhu JH, Weldon H, Maianu L, Garvey WT. Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle. Implications for glucose toxicity. J Clin Invest 1995;96:2792–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooksey RC, Hebert LF, Jr, Zhu JH, Wofford P, Garvey WT, McClain DA. Mechanism of hexosamine-induced insulin resistance in transgenic mice overexpressing glutamine:fructose-6-phosphate amidotransferase: decreased glucose transporter GLUT4 translocation and reversal by treatment with thiazolidinedione. Endocrinology 1999;140:1151–1157 [DOI] [PubMed] [Google Scholar]
- 19.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 1991;266:4706–4712 [PubMed] [Google Scholar]
- 20.Marshall S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Sci STKE 2006;2006:re7. [DOI] [PubMed] [Google Scholar]
- 21.Traxinger RR, Marshall S. Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine. Role of hexosamine biosynthesis in enzyme regulation. J Biol Chem 1991;266:10148–10154 [PubMed] [Google Scholar]
- 22.Marshall S, Bacote V, Traxinger RR. Complete inhibition of glucose-induced desensitization of the glucose transport system by inhibitors of mRNA synthesis. Evidence for rapid turnover of glutamine:fructose-6-phosphate amidotransferase. J Biol Chem 1991;266:10155–10161 [PubMed] [Google Scholar]
- 23.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 2002;99:5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arias EB, Kim J, Cartee GD. Prolonged incubation in PUGNAc results in increased protein O-linked glycosylation and insulin resistance in rat skeletal muscle. Diabetes 2004;53:921–930 [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Wang J, Cui X, et al. The effect of insulin on expression of genes and biochemical pathways in human skeletal muscle. Endocrine 2007;31:5–17 [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Patki A, Lara-Castro C, et al. Genes and biochemical pathways in human skeletal muscle affecting resting energy expenditure and fuel partitioning. J Appl Physiol 2011;110:746–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, Wu X, Franklin JL, et al. Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am J Physiol Endocrinol Metab 2010;298:E565–E576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liew CW, Bochenski J, Kawamori D, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest 2010;120:2876–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prudente S, Sesti G, Pandolfi A, Andreozzi F, Consoli A, Trischitta V. The mammalian tribbles homolog TRIB3, glucose homeostasis, and cardiovascular diseases. Endocr Rev 2012;33:526–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi Y, Ohoka N, Hayashi H, Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPARγ transcriptional activity. J Lipid Res 2008;49:880–892 [DOI] [PubMed] [Google Scholar]
- 31.Mayor P, Maianu L, Garvey WT. Glucose and insulin chronically regulate insulin action via different mechanisms in BC3H1 myocytes. Effects on glucose transporter gene expression. Diabetes 1992;41:274–285 [DOI] [PubMed] [Google Scholar]
- 32.Taylor RP, Parker GJ, Hazel MW, et al. Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J Biol Chem 2008;283:6050–6057 [DOI] [PubMed] [Google Scholar]
- 33.Zou L, Zhu-Mauldin X, Marchase RB, et al. Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent. J Biol Chem 2012;287:34419–34431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor RP, Geisler TS, Chambers JH, McClain DA. Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux. J Biol Chem 2009;284:3425–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Zhang W, Chuang GC, et al. Role of TRIB3 in regulation of insulin sensitivity and nutrient metabolism during short-term fasting and nutrient excess. Am J Physiol Endocrinol Metab 2012;303:E908–E916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 2001;291:2376–2378 [DOI] [PubMed] [Google Scholar]
- 37.Yang X, Su K, Roos MD, Chang Q, Paterson AJ, Kudlow JE. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A 2001;98:6611–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamemura K, Hayes BK, Comer FI, Hart GW. Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins: alternative glycosylation/phosphorylation of THR-58, a known mutational hot spot of c-Myc in lymphomas, is regulated by mitogens. J Biol Chem 2002;277:19229–19235 [DOI] [PubMed] [Google Scholar]
- 39.Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, Groner B. The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem 2004;279:3563–3572 [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 β-cells. Arch Biochem Biophys 2003;415:155–163 [DOI] [PubMed] [Google Scholar]
- 41.Parker GJ, Lund KC, Taylor RP, McClain DA. Insulin resistance of glycogen synthase mediated by O-linked N-acetylglucosamine. J Biol Chem 2003;278:10022–10027 [DOI] [PubMed] [Google Scholar]
- 42.Travers MT, Vallance AJ, Gourlay HT, et al. Promoter I of the ovine acetyl-CoA carboxylase-α gene: an E-box motif at -114 in the proximal promoter binds upstream stimulatory factor (USF)-1 and USF-2 and acts as an insulin-response sequence in differentiating adipocytes. Biochem J 2001;359:273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sul HS, Latasa MJ, Moon Y, Kim KH. Regulation of the fatty acid synthase promoter by insulin. J Nutr 2000;130(Suppl.):315S–320S [DOI] [PubMed] [Google Scholar]
- 44.Sander M, Griffen SC, Huang J, German MS. A novel glucose-responsive element in the human insulin gene functions uniquely in primary cultured islets. Proc Natl Acad Sci U S A 1998;95:11572–11577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portois L, Maget B, Tastenoy M, Perret J, Svoboda M. Identification of a glucose response element in the promoter of the rat glucagon receptor gene. J Biol Chem 1999;274:8181–8190 [DOI] [PubMed] [Google Scholar]
- 46.Jokela TA, Makkonen KM, Oikari S, et al. Cellular content of UDP-N-acetylhexosamines controls hyaluronan synthase 2 expression and correlates with O-linked N-acetylglucosamine modification of transcription factors YY1 and SP1. J Biol Chem 2011;286:33632–33640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClain DA, Paterson AJ, Roos MD, Wei X, Kudlow JE. Glucose and glucosamine regulate growth factor gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A 1992;89:8150–8154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldberg HJ, Scholey J, Fantus IG. Glucosamine activates the plasminogen activator inhibitor 1 gene promoter through Sp1 DNA binding sites in glomerular mesangial cells. Diabetes 2000;49:863–871 [DOI] [PubMed] [Google Scholar]
- 49.Daniel S, Zhang S, DePaoli-Roach AA, Kim KH. Dephosphorylation of Sp1 by protein phosphatase 1 is involved in the glucose-mediated activation of the acetyl-CoA carboxylase gene. J Biol Chem 1996;271:14692–14697 [DOI] [PubMed] [Google Scholar]
- 50.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003;300:1574–1577 [DOI] [PubMed] [Google Scholar]