Abstract
The aim of this study was to use CO2 at sub-critical pressures as a tool to sinter 3D, macroporous, microsphere-based scaffolds for bone and cartilage Tissue Engineering Porous scaffolds composed of ~200 µm microspheres of either poly(lactic-co-glycolic acid) (PLGA) or polycaprolactone (PCL) were prepared using dense phase CO2 sintering, which were seeded with rat bone marrow mesenchymal stromal cells (rBMSCs), and exposed to either osteogenic (PLGA, PCL) or chondrogenic (PLGA) conditions for 6 weeks. Under osteogenic conditions, the PLGA constructs produced over an order of magnitude more calcium than the PCL constructs, whereas the PCL constructs had far superior mechanical and structural integrity (125 times stiffer than PLGA constructs) at week 6, along with twice the cell content of the PLGA constructs. Chondrogenic cell performance was limited in PLGA constructs, perhaps as a result of the polymer degradation rate being too high. The current study represents the first long-term culture of CO2-sintered microsphere-based scaffolds, and has established important thermodynamic differences in sintering between the selected formulations of PLGA and PCL, with the former requiring adjustment of pressure only, and the latter requiring the adjustment of both pressure and temperature. Based on more straightforward sintering conditions and more favorable cell performance, PLGA may be the material of choice for microspheres in a CO2 sintering application, although a different PLGA formulation with the encapsulation of growth factors, extracellular matrix-derived nanoparticles, and/or buffers in the microspheres may be advantageous for achieving a more superior cell performance than observed here.
Keywords: PLGA, PCL, Microspheres, Subcritical CO2, Sintering, Osteogenic Differentiation, Chondrogenic Differentiation
1.0 Introduction
In recent years, microsphere-based scaffolds have found a significant number of applications in the field of tissue engineering [1–4]. Microspheres offer the advantages of control over release rate of the encapsulated drug, degradation kinetics of the polymer and ease of fabrication [5]. Scaffold properties can be tailored by altering the microsphere design. Further control over the macromechanical and degradability properties of the scaffold can be achieved by selecting a suitable polymeric raw material. Poly(lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) are two polymers that have been extensively used for various applications such as in drug delivery, diagnostics and other applications of clinical and basic science research, including cardiovascular disease, cancer, vaccine and tissue engineering [6–18]. These polymers offer control over the degradation properties, which can be modified by altering one or more factors such as molecular weight, co-polymer ratio, tacticity, crystallinity, etc. [19, 20].
Techniques used to prepare microsphere-based scaffolds include solvent casting/particulate leaching [21], solvent/non-solvent treatment (using acetone and ethanol) [22], heat sintering [23, 24] and an anti-solvent sintering technique using ethanol [25]. Each of these methods has its own advantages, but they mostly make use of large amounts of organic solvents and/or exposure to elevated temperatures, which limits their use. CO2 technology provides an attractive alternative to these traditional methods of scaffold fabrication [26–30]. CO2 has been widely used since it has a relatively low critical temperature (Tc=31.1 °C and Pc=73.8 bar), which makes it suitable for processing thermo-sensitive compounds. It has the advantages of being inexpensive, non-toxic and non-flammable. The recovery of final products and removal of CO2 can be done easily with no residue left behind [31].
Ever since the use of CO2 for scaffold fabrication in tissue engineering was popularized in the mid-1990s as a tool for producing polymeric foam scaffolds, numerous dedicated studies have been successfully carried out that have leveraged CO2 as a swelling agent to impregnate scaffolds with drugs and other bioactive additives, and for encapsulation of plasmids within scaffolds for gene delivery. Moreover, in contrast to CO2 foaming, which typically relies on supercritical CO2 at very high pressures, CO2 at much lower pressures has also been used to sinter polymeric microspheres together in the presence of cells to create cell-seeded scaffolds in a single step.
Building on these advantages and based on our previous evaluations of utilizing the plasticizing properties of CO2 to produce scaffolds using monodisperse microspheres [32, 33], the goal of the present study was to evaluate the performance of rat bone marrow mesenchymal stromal cells (rBMSCs) on these scaffolds over a 6 week period, in particular under either osteogenic or chondrogenic conditions. An additional purpose of this study was to examine the sintering of microspheres of either PLGA or PCL, with the former requiring adjustment of pressure only, and the latter requiring the adjustment of both pressure and temperature.
2.0 Materials and Methods
2.1 Preparation of Microspheres
Poly(D,L-lactic acid-co-glycolic acid) copolymer (PLGA) having a lactide:glycolide content of 50:50, molecular weight (MW) ~42,000–44,000 Da, degradation time of < 3 months and an intrinsic viscosity of 0.37 dL/g was purchased from Lakeshore Biomaterials (Birmingham, AL). Poly(ε-caprolactone) (PCL) having an intrinsic viscosity of 1–1.3 dL/g and MW of ~110,000–125,000 Da with a degradation time of > 24 months was purchased from LACTEL Absorbable Polymers (Birmingham, AL). Poly(vinyl alcohol) (PVA; 88% hydrolyzed, MW~25,000 Da) was purchased from Polysciences, Inc. (Warrington, PA). Dichloromethane (DCM; HPLC grade) was obtained from Fisher Scientific (Pittsburgh, PA).
Uniform PLGA and PCL microspheres were prepared as developed and described in our previous work [25, 34, 35]. The particles were then lyophilized for 48 hrs and stored at −20 °C. A 10% polymer solution for PCL and 20% polymer solution for PLGA were used to prepare the microspheres.
2.2 Scaffold Fabrication
Scaffolds were fabricated by exposure to sub-critical levels of CO2 in a custom-designed stainless steel vessel having a pressure safety rating of 60 bar. Specific amounts of microspheres (80 mg of PLGA microspheres or 60 mg of PCL microspheres) were loaded into a Teflon mold and exposed to CO2. Based on parameters selected following preliminary studies, PLGA microspheres were exposed to a CO2 absolute pressure of 364 psi (~25 bar) at room temperature for a period of 1 hour followed by depressurization at 0.101 psi/s for 1 hour (Back pressure regulator (ABPR), Waters Technologies Corp, Milford, MA). In contrast, PCL scaffolds were prepared by exposure to an absolute pressure of 690 psi (47.6 bar) at 45 °C for a period of 4 hours followed by depressurization at ~0.2 psi/s for 1 hour, parameters that were identified to be suitable for PCL scaffold fabrication in our preliminary studies. The target was to obtain a comparable degree of sintering between PLGA and PCL as evidenced by porosity, SEM imaging and integrity over 1 week in PBS at 37°C. Scaffolds were 7.8 ± 0.2 mm in diameter and 1.7 ± 0.7 mm in height for PCL constructs, and 8.3 ± 0.3 mm in diameter and 1.8 ± 0.5 mm in height for PLGA constructs. The porosities of the cylindrical scaffolds were calculated as:
| (1.0) |
where ρapp is the apparent density of the scaffold, given by ρapp = 4 m/πd2h, ρ is the density of the stock PLGA or PCL, m is mass of the cylindrical scaffolds, h is thickness and d is the diameter. As per other studies, both PLGA [36, 37] and PCL [38] are expected to have high hydrophobicity that would facilitate cellular adhesion.
2.3 Scanning Electron Microscopy (SEM)
Scaffolds in culture were fixed in glutaraldehyde followed by dehydration in ethanol. Critical point drying of both PLGA and PCL scaffolds was done by dissolving in hexamethyldisilazane (HMDS) for 30 min followed by coating with a gold/palladium target. The imaging was performed using a Leo 1550 field emission scanning electron microscope at an accelerating voltage of 5 kV under a high vacuum.
2.4 Cell Seeding of PLGA and PCL Scaffolds
Rat bone marrow stem cells (rBMSCs) were obtained from the femurs of fifteen young male Sprague-Dawley rats (176–200 g, SASCO) following a University of Kansas approved IACUC protocol (175-08). Briefly, all rats were euthanized by exposure to CO2 for 5 minutes followed by removal of the leg bones. The femur was then separated from the tibia and all excess muscle was removed. The marrow cavity was then flushed out of the femur using a syringe filled with 1% Antibiotic-Antifungal/PBS solution. All cells obtained were plated for expansion in monolayer up to P1. The ‘plain rBMSC medium’ was composed of Alpha MEM and 1% Penicillin-Streptomycin (both from Invitrogen Life Technologies, Carlsbad, CA), and 10% fetal bovine serum qualified (FBS; Gemini, West Sacramento, CA) and was changed every 2–3 days. After the second passage (P2), the cell solution was re-suspended at the density of 1 million cells per mL of the freezing medium (Cell Culture Freezing Media DMSO, Fisher Scientific). The cell suspensions were transferred to cryotubes and stored at −80 °C overnight in Mr. Frosty freezing containers (Nalgene, Rochester, NY), and then transferred to a liquid nitrogen cryogenic storage tank at −196 °C for future use.
Scaffolds were sterilized by ethylene oxide prior to seeding. After sterilization, the scaffolds were air dried in a fume hood for 1 day and placed in a 24-well plate under sterile conditions. Frozen rBMSCs were then thawed and expanded to P4, then seeded at a density of 107 cells/mL of scaffold. 55.2 µL (50% of the scaffold volume, which approximately corresponds to the pore volume [25]) of the cell suspension was placed directly on top of the scaffolds, allowing cells to penetrate into the scaffold via capillary action. The cells were then allowed to attach for 3 hours and the scaffolds were denoted as “week 0” at that time. 1.5 mL of the respective culture media was then added to all scaffolds and they were cultured statically.
There were four groups in the study, all of which included rBMSCs. The first group consisted of a PLGA control that was cultured in the aforementioned plain rBMSC medium (no growth factors). Two groups were cultured in osteogenic medium, with either PLGA or PCL scaffolds. These PLGA and PCL osteogenic groups were cultured in medium consisting of Dulbecco’s modified Eagle medium (DMEM-LG; Invitrogen, Carlsbad, CA), 1% penicillin-streptomycin, 10% fetal bovine serum qualified, 50 µg/mL L-ascorbic acid (Sigma), 10 nM 1α,25 dihydroxyvitamin D3 (Biomol International, Plymouth Meeting, PA), 10 mM β-glycerophosphate (disodium salt, pentahydrate; Calbiochem, San Diego, CA), 0.1 µM dexamethasone and 50 ng/mL BMP-2 (Peprotech, Inc., Rocky Hill, NJ). The fourth and final group consisted of PLGA scaffolds cultured in chondrogenic medium, which was composed of DMEM-high glucose (Invitrogen), 1X insulin-transferrin-selenium (ITS)-premix (BD Biosciences, San Jose, CA), 50 µg/mL L-ascorbic acid (Sigma), 1% penicillin-streptomycin, 40 µg/mL L-proline, 100 µM sodium pyruvate (Fisher Scientific, Pittsburgh, PA), 1% non-essential amino acids (NEAA) (Invitrogen) and 10 ng/mL transforming growth factor (TGF)-β3. Media were replaced for all constructs every 48 hrs. After 3 weeks in culture, 15 mM HEPES buffer (Fisher Scientific) was added to the medium of all groups.
2.5 Biochemical Analyses
At weeks 0, 3 and 6, constructs (n=4) were analyzed for matrix production using biochemical assays. The constructs were digested by adding 1.2 mL of papain solution comprising of 125 µg/mL papain (from papaya latex, Sigma), 5 mM N-acetyl cysteine, 5 mM ethylenediaminetetraacetic acid (EDTA), and 100 mM potassium phosphate buffer in ddH2O (20 mM monobasic potassium phosphate, 79 mM dibasic potassium phosphate in ddH2O). The constructs were placed in microcentrifuge tubes containing papain solution and left overnight at 60 °C. After digestion, the scaffolds were centrifuged at 10,000 rpm for 5 minutes to form a pellet of the polymer and other impurities and later stored at −20 °C for biochemical analysis. The supernatant was later used to analyze DNA, and hydroxyproline (HYP) content, and for the chondrogenic and control groups, also the glycosaminoglycan (GAG) content.
The DNA content was analyzed using a PicoGreen kit (Molecular Probes, Eugene, OR) according to the manufacturer’s protocol. GAG content was determined using a dimethylmethylene blue (DMMB) assay kit (Biocolor, Newtownabbey, Northern Ireland) as we have described previously [39]. Hydroxyproline content was determined using a modified HYP assay method [40], as we have also described previously [39]. A conversion factor of 11.5 can be used to convert the hydroxyproline mass to collagen content based on our unpublished data.
To determine alkaline phosphatase (ALP) activity and calcium content for the osteogenic and control groups, constructs (n=4) were homogenized in 1 mL of 0.05% Triton X-100 (Sigma) and stored at −20 °C. The total ALP activity was expressed as a measure of liberated pnitrophenol concentration/µg protein/min, as described elsewhere [41]. The protein content was determined by combining 20 µL of the lysate with 40 µL BioRad reagent and subsequent reading at 600 nm.
Calcium content was determined by using QuantiChrom™ Calcium Assay Kit (DICA-500; QuantiChrom) according to the manufacturer’s instructions.
2.6 Mechanical Testing
Mechanical characterization of the constructs (n=5) was carried out using a uniaxial testing apparatus (Instron Model 5848, Canton, MA, 50 N load cell) under unconfined compression using a custom-built bath-platen apparatus [42]. Cylindrical scaffolds prepared by CO2 sintering were tested under hydrated conditions (phosphate buffered saline (PBS) comprised of 0.138 M sodium chloride, 0.0027 M potassium chloride) at 37 °C. At week 0, cell-seeded scaffolds of PCL and one group of PLGA were tested (7.8 ± 0.2 mm diameter, 1.7 ± 0.7 mm height for PCL constructs; 8.3 ± 0.3 mm diameter, 1.8 ± 0.5 mm height for PLGA constructs). It was assumed that there would be no detectable differences in the mechanical properties of the three PLGA groups at week 0 given that those scaffolds were all identical. At week 6, all four groups were tested. The dimensions of the constructs at week 6 were 13.7 ± 1.4 mm in diameter and 1.1 ± 0.5 mm in height for the Control PLGA constructs. The Chondrogenic PLGA group constructs were 13.6 ± 1.2 mm in diameter and 2.5 ± 0.8 mm in height. The Osteogenic groups had a diameter of 11.8 ± 1.7 mm and a height of 1.9 ± 0.6 mm for the PLGA group and a diameter of 7.5 ± 0.2 mm and a height of 1.9 ± 0.4 mm for the PCL group. Compressive moduli of elasticity were obtained from the linear regions of the stress-strain curves. A continuous deformation rate of 5 mm/min was used during the compression testing. The stress was defined as the ratio of the load to the initial cross-sectional area, and the strain was defined as the ratio of the change in length to the original length.
2.7 Statistical Analyses
Statistical analyses were performed using a single factor analysis of variance with a Tukey’s post hoc test (ANOVA) in Origin 6.0 software. All quantitative results (numerical values and representative diagrams) were expressed as the average ± standard deviation.
3.0 Results
3.1 Microspheres and Scaffold Fabrication
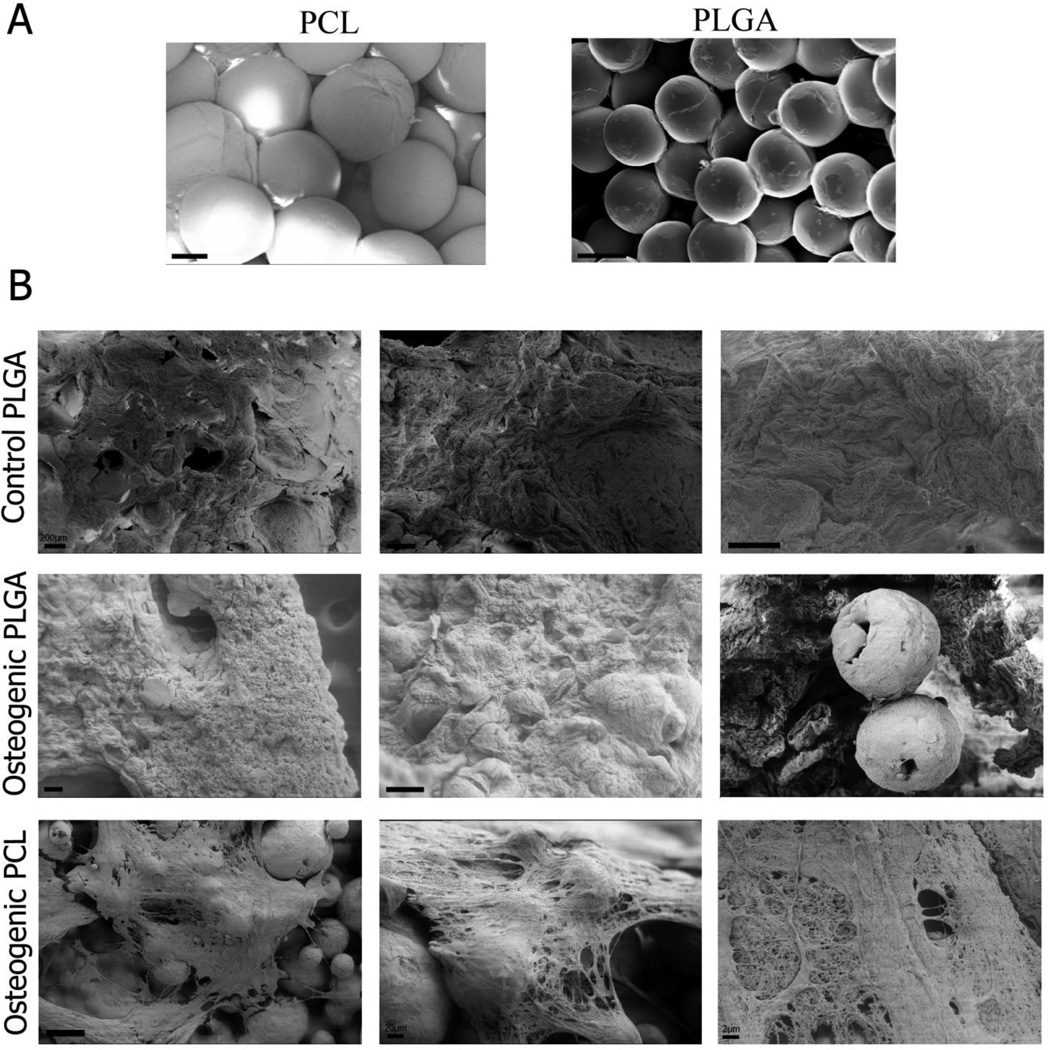
PLGA and PCL microspheres having uniform diameter were prepared using the Precision Particle Fabrication method [34]. The microspheres displayed high monodispersity and had a nominal diameter of ~200 µm. Cylindrical scaffolds containing two types of polymers, PLGA and PCL, were produced using subcritical CO2. Morphological assessment of the scaffolds using scanning electron microscopy (SEM) revealed that there were slight distortions on the surfaces of the microspheres, but the spherical shape was largely retained as seen in Figure 1. The porosity was calculated according to equation (1) and was found to be 44.6 ± 4.1% for PCL scaffolds and 43.2 ± 0.2% for PLGA scaffolds (no statistically significant difference). SEM imaging after 6 weeks showed that PCL microspheres retained their spherical shape while PLGA microspheres had degraded (Figure 1).
Figure 1.
Representative scanning electron microscopy (SEM) images of week 0 (A) and week 6 (B) samples. Scaffolds were fabricated using PLGA and PCL microspheres at customized processing conditions for sintering (CO2 exposure at 25 bar for 1 h at 25°C followed by depressurization at a rate of 0.101 psi s−1 for PLGA; 45 bar for 4 hrs at 45 °C followed by depressurization at a rate of 0.2 psi s−1 for PCL). Comparable degrees of sintering were observed at week 0, and microsphere shape was better preserved in the PCL constructs by week 6. Chondrogenic PLGA scaffolds are not shown as they did not withstand SEM processing. Scale bars = 100 µm.
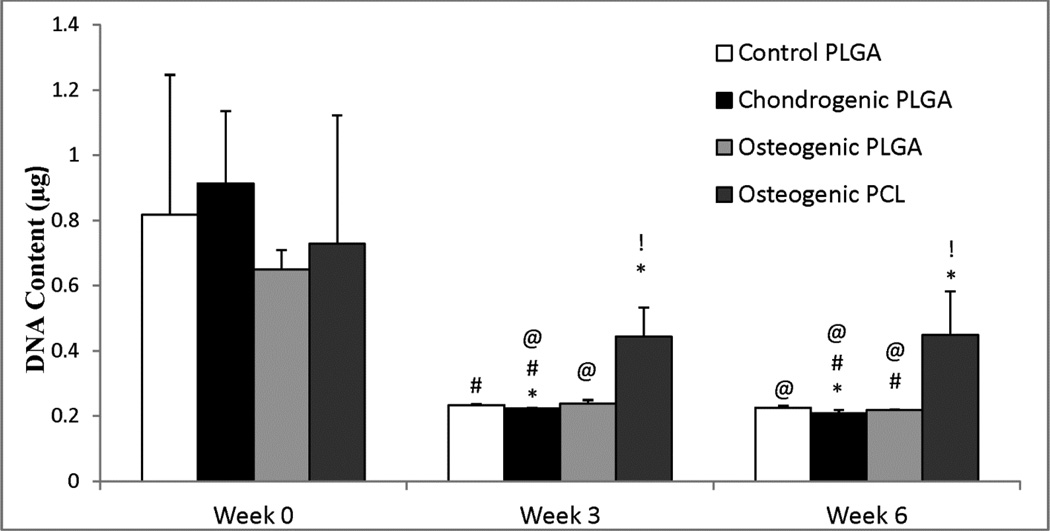
3.2 DNA Content
The overall DNA content for all constructs decreased over the period of 6 weeks (p < 0.05) as seen in Figure 2. There was a statistically significant decrease in DNA content for each group at weeks 3 and 6 compared to their week 0 values. The three PLGA groups decreased by about 70% at week 6 (p < 0.005) compared to their week 0 values, while the Osteogenic PCL group decreased by 38% over the 6 week time period (p ≤ 0.01). However, there were 1.8 times and 2.0 times higher DNA contents in the Osteogenic PCL group compared to the Osteogenic PLGA group at weeks 3 (p < 0.005) and 6 (p ≤ 0.01), respectively. The average DNA content in the Control PLGA and Osteogenic PLGA groups were not found to be statistically significantly different from each other at week 6.
Figure 2.
DNA content for all groups. All three PLGA groups had a statistically significant decrease in cell number by week 6 compared to the week 0 Control group. Only Osteogenic PCL group had similar cell number throughout 6 weeks, although it had significantly higher cell number compared to the control group at weeks 3 and 6. Values are reported as mean ± standard deviation, n = 4. Statistically significant difference @ = from the Control PLGA group at week 0 (p < 0.05), # = from its value at the previous time point (p < 0.05), and * = from the Control PLGA group at that time point (p < 0.05), and ! = between the two osteogenic groups at that time point (p < 0.05).
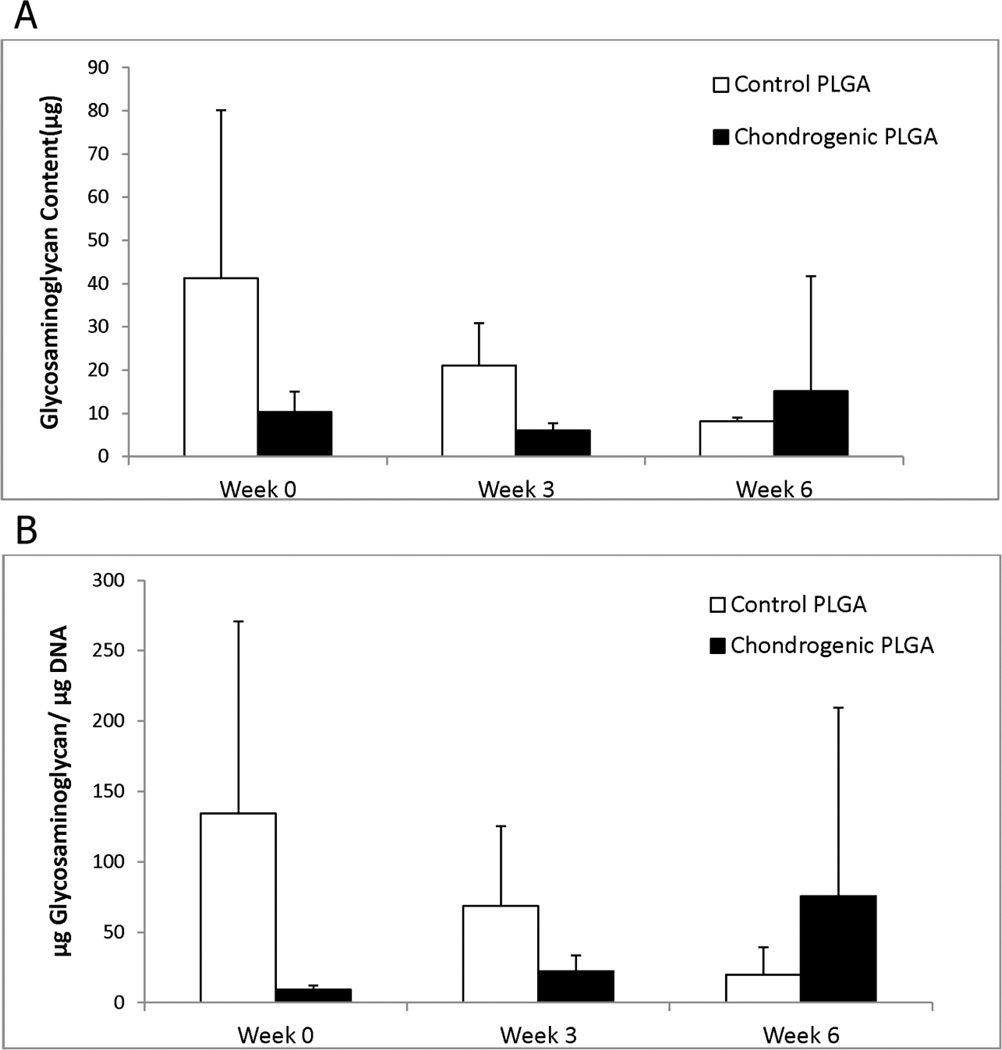
3.3 Glycosaminoglycan Content
GAG analysis was carried out for the Chondrogenic PLGA group and the Control PLGA group. Higher GAG values were observed in the Control PLGA group over the chondrogenic group at weeks 0 and 3, however, statistically significant differences in the GAG content were not observed in either group over time (Figure 3).
Figure 3.
Glycosaminoglycan (GAG) content for the chondrogenic groups, (A) per construct and (B) normalized to DNA content. Significant differences in the GAG content were not observed. Values are reported as mean ± standard deviation, n = 4.
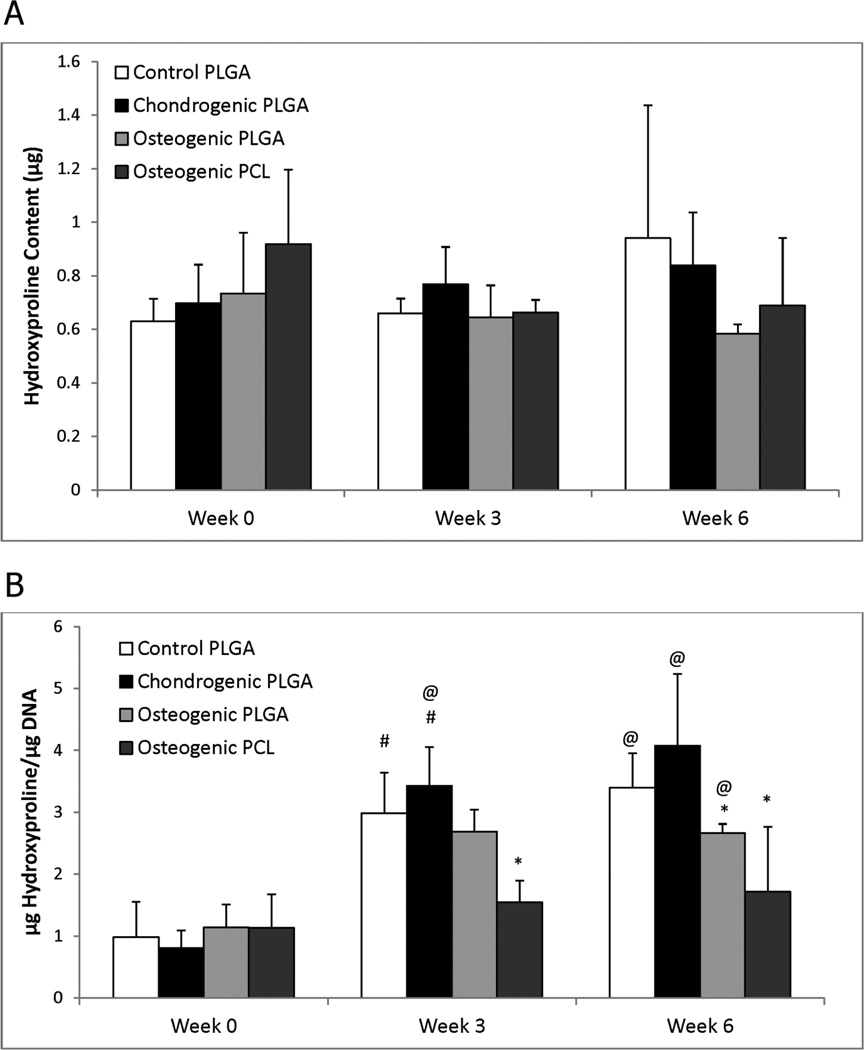
3.4 Hydroxyproline Content
The hydroxyproline (HYP) data for all groups is shown in Figure 4. Statistically significant differences in the absolute HYP content were not observed among the groups during the 6 weeks. However, with the aforementioned decrease in DNA content over time, all three of the PLGA groups showed a significant increase in HYP/DNA content at weeks 3 and 6 compared to the week 0 Control PLGA group (p < 0.01). For example, HYP/DNA contents of the Control PLGA and the Chondrogenic PLGA constructs at week 3 were 3.0 times (p < 0.005) and 4.2 times (p < 0.001) higher than at week 0, respectively. In contrast, no significant changes were observed in the Osteogenic PCL group throughout the 6 weeks. The HYP/DNA content of the Osteogenic PCL group was about 47% lower than the Control PLGA group both at week 3 (p < 0.01) and at week 6 (p < 0.05). Significant differences between the two osteogenic groups were not observed at any of the time points.
Figure 4.
Hydroxyproline (HYP) content for all groups, (A) per construct and (B) normalized to DNA content. (A) Statistically significant differences in HYP content were not observed. (B) All groups had statistically significant increases in HYP/DNA content at week 3 and 6 relative to week 0. There were no statistically significant differences between the two osteogenic groups at any time point. Values are reported as mean ± standard deviation, n = 4. Statistically significant difference @ = from the Control PLGA group at week 0 (p < 0.05), # = from its value at the previous time point (p < 0.05), and * = from the Control PLGA group at that time point (p < 0.05).
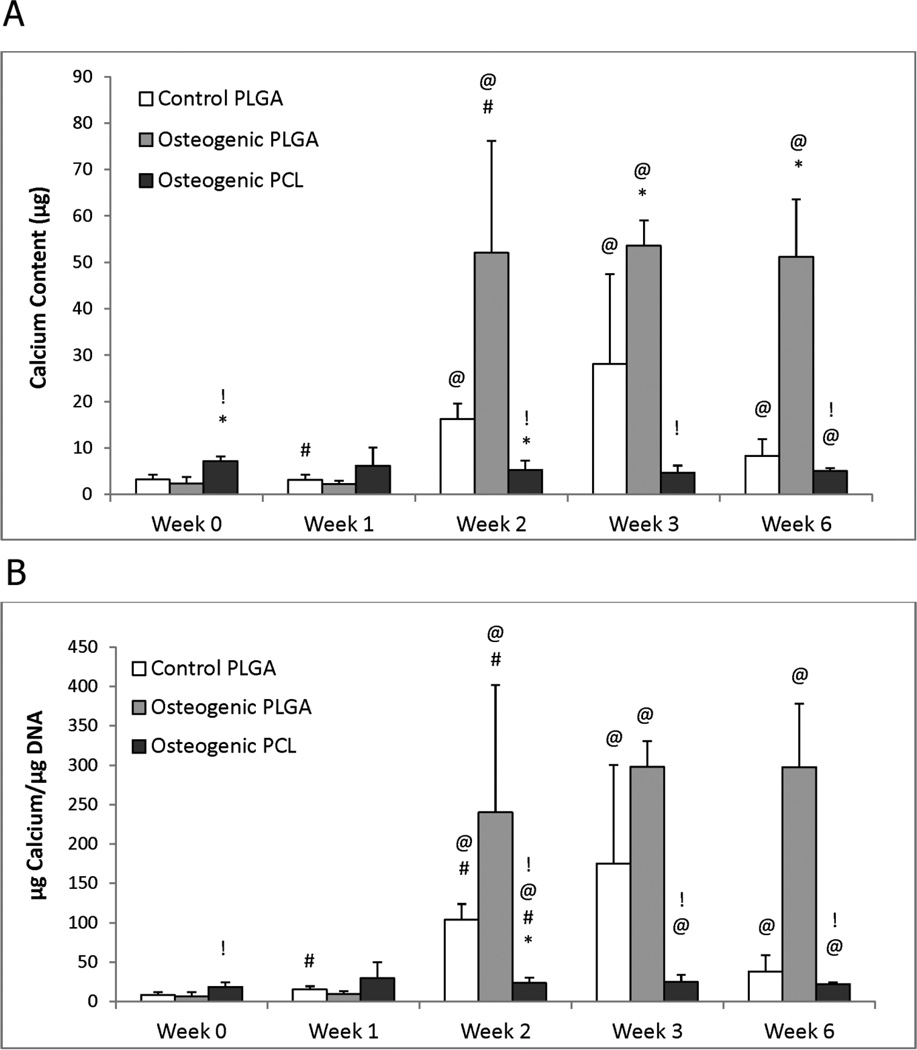
3.5 Calcium Content
Calcium contents for any given group did not increase significantly from week 0 to week 1 (Figure 5). However, at week 2, the Osteogenic PLGA group increased to 22 times its week 0 value (p ≤ 0.02), and the Control PLGA group increased by a factor of 5.1 (p < 0.001), but the Osteogenic PCL group did not change significantly. By week 6, the Osteogenic PLGA group had 6.2 times more calcium than the control group (p < 0.001) and 10.0 times more than the Osteogenic PCL group (p < 0.001).
Figure 5.
Calcium content for the osteogenic groups, (A) per construct and (B) normalized to DNA content. (A) Most groups had statistically significant increases in calcium content from week 0 to week 6, but only the Osteogenic PLGA constructs had significantly higher production relative to the control at 6 weeks. Notably, this value was at least 6 times higher than any other group at the end of the culture. At 6 weeks, the Osteogenic PCL group showed a lower calcium content when compared to the Control group. (B) Osteogenic PLGA constructs produced more Calcium/DNA than Control and PCL groups at week 6. An continuously increasing Calcium/DNA content trend was observed for Control and Osteogenic PCL groups until week 3, although both decreased from week 3 to week 6. Values are reported as mean ± standard deviation, n = 4. Statistically significant difference @ = from the Control PLGA group at week 0 (p < 0.05), # = from its value at the previous time point (p < 0.05), * = from the Control PLGA group at that time point (p < 0.05) and ! = between the two osteogenic groups at that time point (p < 0.05).
Normalized data showed a similar trend with increases in calcium content from week 2 onward. The Osteogenic PLGA and Control PLGA groups had significantly higher calcium/DNA contents at week 2 (35.9 times higher, p ≤ 0.02 for Osteogenic PLGA; 12.6 times higher, p < 0.001 for Control PLGA) and week 3 (44.6 times higher, p < 0.001 for Osteogenic PLGA; 21.3 times higher, p ≤ 0.05 for Control PLGA) compared to the their respective week 0 values. In contrast, the Osteogenic PCL group did not show any significant increase in calcium/DNA content over its week 0 value. In comparing the groups to each other, the calcium/DNA content in the Osteogenic PLGA group was found to be significantly higher than the PCL group at weeks 2 (10.2 times higher, p ≤ 0.03), 3 (12 times higher, p < 0.001) and 6 (13.5 times higher, p < 0.001).
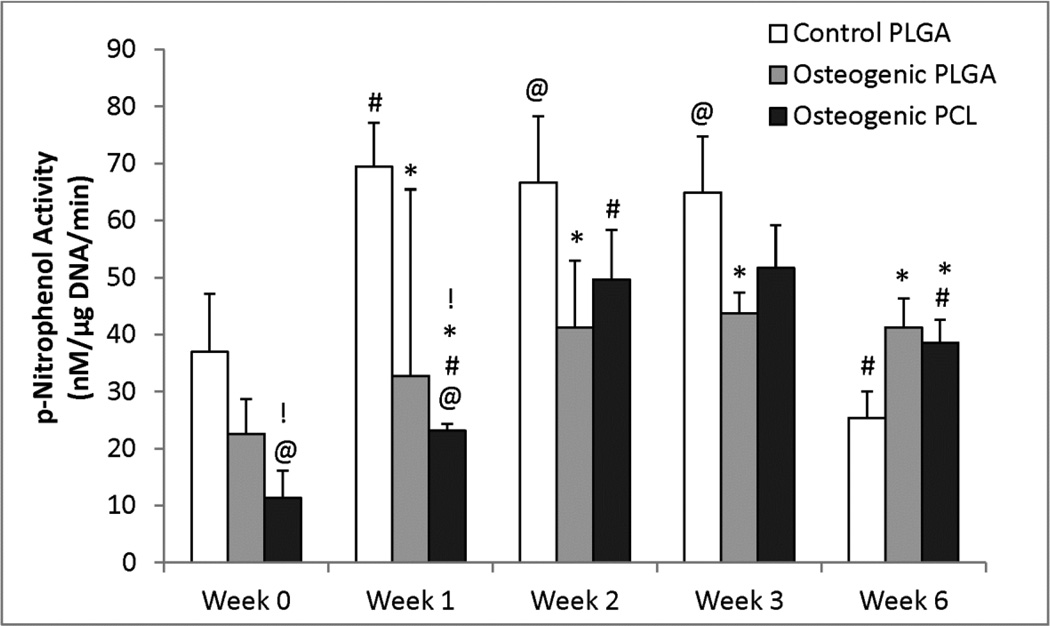
3.6 Alkaline Phosphatase Activity
The Osteogenic PCL and PLGA groups both had significantly lower ALP activity than the Control PLGA group at weeks 1 and 6 (Figure 6). The Control PLGA group showed a significantly higher ALP activity (p < 0.005) at the end of weeks 1, 2 and 3 when compared to its week 0 value, and the Osteogenic PLGA group also experienced an increase in ALP activity from week 0 to week 6, being 1.82 times higher (p < 0.005). The ALP activity of the Osteogenic PCL group also increased by 3.4 times at the end of 6 weeks relative to its week 0 value (p < 0.005). The ALP activity of the Osteogenic PLGA group was 2.0 times higher than the Osteogenic PCL group at week 0 (p < 0.001) and 1.4 times higher at week 1 (p ≤ 0.01).
Figure 6.
Alkaline Phosphatase activity of the osteogenic groups. Alkaline phosphatase activity for all constructs showed a varying trend throughout the 6 weeks. The Control PLGA group and the Osteogenic PCL had an increasing ALP content at the end of week 3. Values are reported as mean ± standard deviation, n = 4. Statistically significant difference @ = from the Control PLGA group at week 0 (p < 0.05), # = from its value at the previous time point (p < 0.05), * = from the Control PLGA group at that time point (p < 0.05) and ! = between the two osteogenic groups at that time point (p < 0.05).
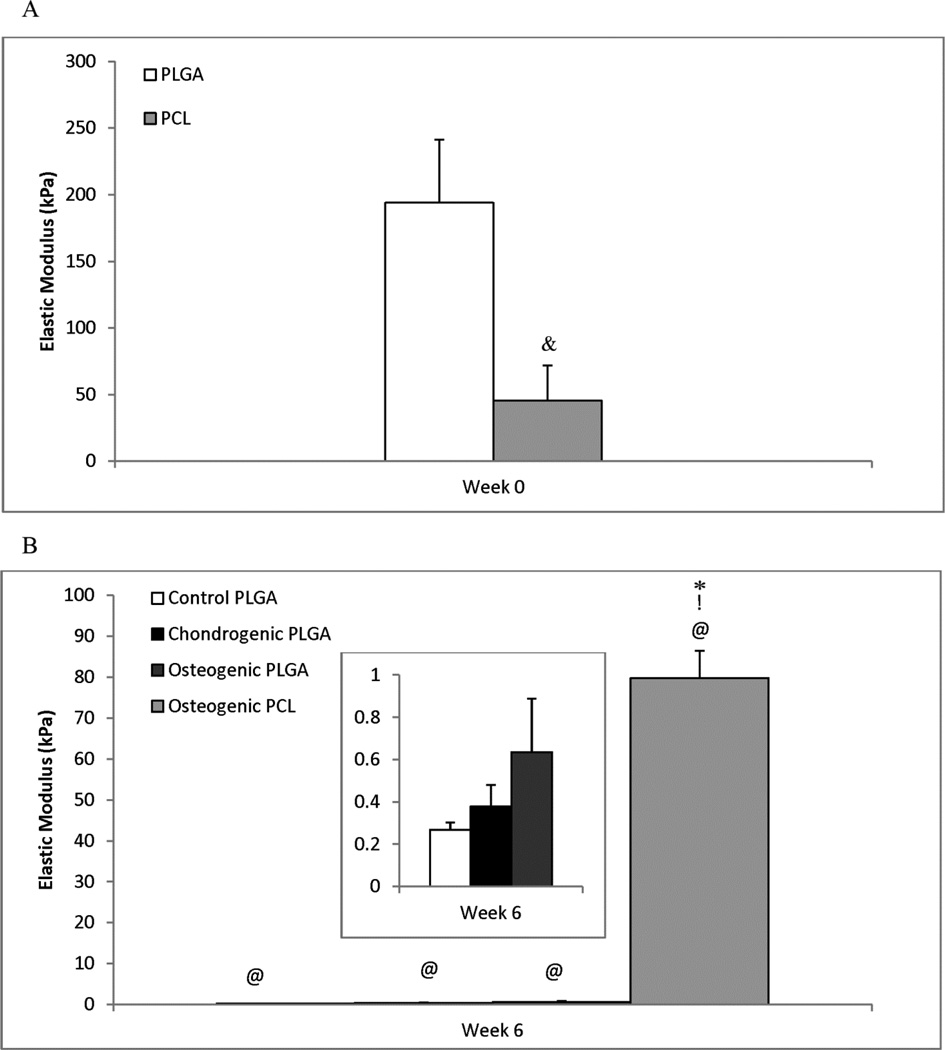
3.7 Mechanical Testing
From unconfined compression testing, the modulus of elasticity was determined for all groups at week 0 and week 6 (Figure 7). All of the groups except week 6 Osteogenic PCL group showed an initial linear region followed by a nonlinear region in the stress-strain curve. The compressive moduli were determined from the stress-strain plots using the initial linear regions which were chosen before the onset of the nonlinear region which generally extended up to 40% strain for all the PLGA groups, while the week 0 PCL and PLGA groups were linear up to 20% strain. For the week 6 Osteogenic PCL group, the modulus of elasticity was calculated from the initial linear region that extended up to only 10% strain.
Figure 7.
Elastic Modulus (kPa) at (A) Week 0 (following cell-seeding) and (B) Week 6. At week 0, the PLGA scaffolds were 4.2 times stiffer than the PCL scaffolds (p < 0.05). However, the opposite was true at 6 weeks, with the PCL scaffolds being over two orders of magnitude stiffer than any of the PLGA groups. The inset graph in panel B represents the three PLGA groups (y-axis units the same). While PCL scaffolds saw a statistically insignificant increase of 75% from week 0 to week 6, a statistically significant decrease in elastic modulus was observed for all three PLGA groups. The Osteogenic PLGA group was statistically higher over the control at week 6. Values are expressed as mean ± standard deviation, n = 5. Statistically significant difference & = between the two groups at week 0, @ = from the Control PLGA group at week 0 (p < 0.001), * = from the Control PLGA group at that time point and ! = between the two osteogenic groups at week 6 (p < 0.001).
At week 0, the modulus of elasticity was 4.25 times higher for the PLGA constructs than for the PCL constructs (p < 0.001). However, after 6 weeks the PCL constructs were at least 125 times stiffer than the PLGA groups (p < 0.001). The moduli of the PLGA constructs dropped drastically after 6 weeks in culture. They decreased by over 99% of their week 0 values at the end of week 6 (p < 0.001). On the other hand, the elastic modulus for the PCL constructs actually increased by 75% from week 0 to week 6 (not statistically significant).
4.0 Discussion
We have demonstrated the feasibility of using a novel microsphere based scaffold sintered using subcritical CO2 for osteogenic and chondrogenic tissue engineering. This work builds on our previous efforts with CO2-sintered microsphere-based scaffolds [32], where only cell viability was demonstrated, by completing a full 6-week in vitro study with both osteogenesis and chondrogenesis, as well as establishing sintering parameters for PCL that include modification of temperature in addition to pressure.
The overall objective of this study was to investigate the use of a novel microsphere based scaffold formed by subcritical CO2 sintering for osteogenic and chondrogenic tissue engineering. Two commonly used polymeric scaffold materials, PLGA and PCL, were used in this study. Subcritical CO2 sintering (~25 bar for PLGA at 25°C, and ~47 bar for PCL at 45°C) was used to prepare scaffolds due to the high solubility of CO2 in polymers and its ability to plasticize polymers by lowering the glass transition temperature or melting point [43]. From the SEM images at week 0 and the porosity data, comparable degrees of sintering were observed in both PCL and PLGA constructs. Since the molecular structure and morphology of the polymers greatly influence CO2 solubility and diffusivity, PLGA having more accessible free volume (due to the methyl group from the lactic acid protruding from the polymer chain) may allow a greater solubility of CO2 than PCL [44]. Therefore, a shorter equilibration time with CO2 was needed for PLGA (1 hour), while PCL needed a relatively longer time (4 hours) at a higher pressure and slightly elevated temperature of 45°C. Due to the relatively short exposure time, plasticization of the entire polymer was avoided and the microspheres were only fused with each other, thereby retaining their spherical shape. A moderate depressurization rate was found to be desirable (0.101 psi s−1 for PLGA scaffolds and ~0.2 psi s−1 for PCL scaffolds) for the production of sintered matrices. Instantaneous depressurization was avoided because it results in foaming of the prepared scaffolds. During depressurization, thermodynamic instability occurs within the polymer, resulting in nucleation of gas cells. These gas cells give rise to nanopores within the scaffold. Faster depressurization rates could be applied in the future to increase porosity, or alternatively porogens like NaCl can be added to increase the porosity.
The slow degradation rate of PCL enabled the microspheres to better retain their shape throughout the 6 weeks in culture. On the other hand, the selected PLGA formulation has a faster degradation rate than PCL. Although the swelling rates, water uptake and degradation rates were not calculated here, it was observed that swelling and change in size occurred as early as ten days in culture for the PLGA constructs. Changes in pH were also noticeable at this stage for the PLGA constructs, evidenced by medium color change. Hence, to prevent further damage to cells, HEPES buffer was added to the culture media around week 3 to maintain the pH around 7. In future studies, steps may be taken to maintain the culture medium near a pH of 7 at all times, either by buffering of the medium or by encapsulation of buffers such as sodium bicarbonate or calcium carbonate [45].
During the culture period, a loss of cells was observed. We have previously observed a similar phenomenon in a different scaffolding system, whereby a rapidly degrading PGA mesh was accompanied by a decrease in DNA content over time [46]. It was noted in our previous study that the degradation rate was not equal to the rate of extracellular matrix synthesis, resulting in a contraction of scaffolds. Since the cell number (DNA content) in the PLGA scaffolds in the current study did not further decrease from week 3 onward, it indicates that a more stable construct may have been achieved. The cell loss was accompanied by a GAG loss in the Control PLGA constructs. However, the Chondrogenic PLGA constructs showed an increased GAG production after week 3.
In a comparison of the two osteogenic groups, i.e., comparing the PLGA and PCL scaffolds, instead of finding one to be clearly better than the other, it appeared that each had associated advantages and disadvantages. For example, the PLGA constructs were superior in calcium production, with over an order of magnitude more calcium than the PCL group. In contrast, the PCL constructs had far superior mechanical integrity (125 times stiffer than PLGA constructs at week 6) and structural integrity (change in geometry was far less drastic), higher ALP expression activity toward the end of the study, and retained twice as many cells as the PLGA constructs. The bottom line was that the PLGA scaffolds appeared to be better for osteogenic differentiation than the PCL scaffolds, but at the expense of mechanical and structural integrity. As our group has moved toward more in vivo studies with microsphere-based scaffolds, the results of the current study and others have led us to more slowly degrading PLGA formulations, which may provide more of a middle ground to better boost cell performance as well as retain mechanical and structural integrity.
Certain strategies may be implemented in future studies to improve the performance of constructs. First, selection of an adequate PLGA polymer having higher lactic acid content, higher molecular weight and Tg can be utilized in microsphere preparation to decrease the degradation rate of the polymer. Second, a bimodal distribution of microspheres can be used during scaffold fabrication [32] to improve the mechanical properties of the scaffold, although this would result in lower porosity. Addition of nanocomposite materials such as calcium carbonate, hydroxyapatite, or tricalcium phosphate within the microspheres can also help in maintaining the pH changes that occur during PLGA degradation, as well as providing “raw materials” [47] to the cells and regenerating tissues in vivo. Third, faster depressurization rates can be explored and porogens can be added during CO2 sintering to improve porosity. Finally, cell attachment and viability can be improved by functionalizing the polymer with haptotactic cues such as Arg-Gly-Asp (RGD) or Tyr-Ile-Gly-Ser-Arg, (YIGSR) [48] or by encapsulating natural “raw materials” such as those noted earlier. Additional studies on the mechanisms of cell adhesion and migration could further lend insight into targeted functionalization.
The PLGA degradation rate for CO2 sintered microspheres in this study appeared to be too high, at the expense of chondrogenesis, which may be addressed in the future with encapsulated buffers and/or a slower degrading PLGA, although we must emphasize that sintering conditions will necessarily need to be adjusted to suit any given PLGA formulation. In selecting sintering conditions for microsphere-based scaffolds [49], whether by CO2heat [50–54], ethanol [25, 55, 56], ethanol/acetone [35, 57], or any solvent/non-solvent combination [22], we recommend SEM and porosity as a general guideline for selecting conditions to achieve a desired degree of sintering, with mechanical testing to confirm integrity of the structure.
5.0 Conclusions
A key point to report in this investigation is that temperature, pressure and exposure time were necessary to control for CO2 sintering of PCL (higher temperature and pressure conditions with longer exposure time), as opposed to PLGA, which was sintered at ambient temperature and pressure conditions (for 1 hour exposure). With osteogenesis, there were marked differences between the PLGA and PCL constructs. Specifically, the PLGA group produced an order of magnitude more calcium, while PCL retained its shape, size and mechanical integrity and had twice as many cells per construct at 6 weeks. With feasibility demonstrated after this first study of cell-seeded, CO2-sintered, microsphere-based scaffolds, an expanded study that includes histology and gene expression is certainly warranted with additional upgrades to the scaffolding system. The overall conclusion here is that PLGA may be the material of choice in a CO2 sintering application, based on the drastically superior calcium content observed in osteogenesis and the more straightforward sintering conditions (controlling the pressure and exposure time at ambient temperature), but that both chondrogenesis and osteogenesis may benefit from a slower degradation rate (different PLGA formulation) and the encapsulation of growth factors, calcium-based nanoparticles, and/or buffers in the microspheres to achieve superior cell performance.
Highlights.
-
➢
The first long-term culture of CO2-sintered microsphere-based scaffolds.
-
➢
Established important thermodynamic differences between sintering PLGA and PCL.
-
➢
PCL sintering with CO2 required manipulation of both temperature and pressure.
-
➢
PLGA may be preferred over PCL in a CO2 sintering application.
-
➢
Microsphere encapsulation may have benefited cell performance.
Acknowledgments
The present work was supported by NIH Grant R21 EB007313. We are grateful to Alan Walker for helping with the design and construction of the stainless-steel CO2 chamber.
Biographies

Aaron Scurto, Ph.D.
Aaron Scurto is an Associate Professor of Chemical & Petroleum Engineering at the University of Kansas. He earned his B.S. from the University of Delaware and his Ph.D. from the University of Notre Dame, and did his post-doctoral training at RWTH-Aachen in Germany and at M.I.T. He was the recipient of a Du Pont Young Professor Award from 2007 – 2010. Professor Scurto’s research interests revolve around homogeneous catalysis and the relationships between solvents and catalysts. In particular, he has worked extensively with supercritical and dense-phase CO2 and ionic liquids as solvents in green/sustainable chemistry applications.

BanuPriya Sridharan, B.S.
BanuPriya Sridharan is a doctoral candidate mentored by Dr. Michael Detamore in the Bioengineering Program at the University of Kansas. She earned her B.S. in bioengineering from SASTRA University, India and graduated with distinction. As a part of her final semester research project she worked in Harvard MIT- HST’s BAMM labs under Dr. Utkan Demirci. Her research interests include using novel materials for osteochondral tissue engineering and cancer diagnostics (ovarian cancer).

Manjari Bhamidipati, M.S.
Manjari Bhamidipati is working as a Research Assistant 1 in the Department of Molecular Carcinogenesis at the University of Texas MD Anderson Cancer Center. She earned her M.S. in Bioengineering under the guidance of Dr. Michael Detamore at the University of Kansas. She earned her B.S. in Biotechnology from Andhra University, India and graduated with distinction. Her research interests include biomaterials, tissue engineering and cancer biology.

Michael Detamore, Ph.D.
Michael Detamore is a Professor of Chemical & Petroleum Engineering and Director of the Biomaterials and Tissue Engineering Laboratory at the University of Kansas. He earned his B.S. in chemical engineering from the University of Colorado and his Ph.D. in bioengineering from Rice University. He is the recipient of the NSF CAREER Award and the Coulter Foundation Translational Research Award, and was a Fulbright Scholar and Visiting Professor at NUI Galway in Ireland in 2011. His research interests are biomaterials, biomechanics, stem cells and tissue engineering. Central research themes include umbilical cord stem cells and gradients in tissue engineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaklenec A, Hinckfuss A, Bilgen B, Ciombor DM, Aaron R, Mathiowitz E. Biomaterials. 2008;29:1518–1525. doi: 10.1016/j.biomaterials.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Kucharska M, Walenko K, Butruk B, Brynk T, Heljak M, Ciach T. Materials Letters. 2010;64:1059–1062. [Google Scholar]
- 3.Jiang T, Nukavarapu SP, Deng M, Jabbarzadeh E, Kofron MD, Doty SB, Abdel-Fattah WI, Laurencin CT. Acta Biomaterialia. 2010;6:3457–3470. doi: 10.1016/j.actbio.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Borden M, Attawia M, Khan Y, Laurencin CT. Biomaterials. 2002;23:551–559. doi: 10.1016/s0142-9612(01)00137-5. [DOI] [PubMed] [Google Scholar]
- 5.Berkland C, Kim K, Pack DW. Pharmaceutical Research. 2003;20:1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- 6.Lu JM, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C. Expert Rev Mol Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui W, Bei J, Wang S, Zhi G, Zhao Y, Zhou X, Zhang H, Xu Y, Biomed J. Mater Res B Appl Biomater. 2005;73:171–178. doi: 10.1002/jbm.b.30189. [DOI] [PubMed] [Google Scholar]
- 8.Wheatley MA, Forsberg F, Oum K, Ro R, El-Sherif D. Ultrasonics. 2006;44:360–367. doi: 10.1016/j.ultras.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ganaha F, Kao EY, Wong H, Elkins CJ, Lee J, Modanlou S, Rhee C, Kuo MD, Yuksel E, Cifra PN, Waugh JM, Dake MD, Vasc J. Interv Radiol. 2004;15:601–608. doi: 10.1097/01.rvi.0000127888.70058.93. [DOI] [PubMed] [Google Scholar]
- 10.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, Lu Z, DeFelice S, Klugherz B, Wilensky R, Levy RJ. Gene Ther. 2003;10:1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 11.Mei L, Sun H, Jin X, Zhu D, Sun R, Zhang M, Song C. Pharm Res. 2007;24:955–962. doi: 10.1007/s11095-006-9214-z. [DOI] [PubMed] [Google Scholar]
- 12.Westedt U, Kalinowski M, Wittmar M, Merdan T, Unger F, Fuchs J, Schaller S, Bakowsky U, Kissel T. J Control Release. 2007;119:41–51. doi: 10.1016/j.jconrel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Mo Y, Lim LY. J Control Release. 2005;108:244–262. doi: 10.1016/j.jconrel.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Peyre M, Sesardic D, Merkle HP, Gander B, Johansen P. J Pharm Sci. 2003;92:957–966. doi: 10.1002/jps.10361. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar S, Lee GY, Wong JY, Desai TA. Biomaterials. 2006;27:4775–4782. doi: 10.1016/j.biomaterials.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Hwang CM, Khademhosseini A, Park Y, Sun K, Lee SH. Langmuir. 2008;24:6845–6851. doi: 10.1021/la800253b. [DOI] [PubMed] [Google Scholar]
- 17.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 18.Dai NT, Williamson MR, Khammo N, Adams EF, Coombes AGA. Biomaterials. 2004;25:4263–4271. doi: 10.1016/j.biomaterials.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Tracy MA, Ward KL, Firouzabadian L, Wang Y, Dong N, Qian R, Zhang Y. Biomaterials. 1999;20:1057–1062. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Ding J. Biomaterials. 2004;25:5821–5830. doi: 10.1016/j.biomaterials.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Deville S, Saiz E, Tomsia AP. Biomaterials. 2006;27:5480–5489. doi: 10.1016/j.biomaterials.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 22.Brown JL, Nair LS, Laurencin CT. Journal of biomedical materials research. Part B, Applied biomaterials. 2008;86:396–406. doi: 10.1002/jbm.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. J Bone Joint Surg Br. 2004;86:1200–1208. doi: 10.1302/0301-620x.86b8.14267. [DOI] [PubMed] [Google Scholar]
- 24.Yao J, Radin S, P SL, Ducheyne P. Biomaterials. 2005;26:1935–1943. doi: 10.1016/j.biomaterials.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Tissue Engineering Part C, Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhamidipati M, Scurto AM, Detamore MS. Tissue Engineering Part B, Reviews. 2013;19:221–232. doi: 10.1089/ten.teb.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reverchon E, Cardea S. The Journal of Supercritical Fluids. 2012;69:97–107. [Google Scholar]
- 28.Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Adv Drug Deliv Rev. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Tai H, Popov VK, Shakesheff KM, Howdle SM. Biochem Soc Trans. 2007;35:516–521. doi: 10.1042/BST0350516. [DOI] [PubMed] [Google Scholar]
- 30.Barry JJ, Silva MM, Popov VK, Shakesheff KM, Howdle SM. Philosophical transactions. Series A, Mathematical, physical, and engineering sciences. 2006;364:249–261. doi: 10.1098/rsta.2005.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte MJF, Reis RL ARC. International Materials Reviews. 2009;54:214–222. [Google Scholar]
- 32.Singh M, Sandhu B, Scurto A, Berkland C, Detamore MS. Acta biomaterialia. 2010;6:137–143. doi: 10.1016/j.actbio.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon JH, Bhamidipati M, Sridharan B, Scurto AM, Berkland CJ, Detamore MS. Journal of biomedical materials research. Part B, Applied biomaterials. 2013;101:330–337. doi: 10.1002/jbm.b.32843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berkland C, Kim K, Pack DW. Journal of controlled release : official journal of the Controlled Release Society. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 35.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Tissue Engineering Part A. 2011;17:2845–2855. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 36.Vasita R, Mani G, Agrawal CM, Katti DS. Polymer. 2010;51:3706–3714. [Google Scholar]
- 37.Karakecili AG, Gumusderelioglu M. Journal of biomaterials science. Polymer edition. 2002;13:185–196. doi: 10.1163/156856202317414366. [DOI] [PubMed] [Google Scholar]
- 38.Bassi AK, Gough JE, Zakikhani M, Downes S. J Tissue Eng. 2011:615328. doi: 10.4061/2011/615328. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Annals of biomedical engineering. 2010;38:2167–2182. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards CA, O'Brien WD., Jr Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 41.Boyan BD, Schwartz Z, Bonewald LF, Swain LD. J Biol Chem. 1989;264:11879–11886. [PubMed] [Google Scholar]
- 42.Singh M, Detamore MS. Journal of biomechanical engineering. 2009;131:061008. doi: 10.1115/1.3118776. [DOI] [PubMed] [Google Scholar]
- 43.Areerat S, Funami E, Hayata Y, Nakagawa D, Ohshima M. Polymer Engineering & Science. 2004;44:1915–1924. [Google Scholar]
- 44.Davies OR, Lewis AL, Whitaker MJ, Tai H, Shakesheff KM, Howdle SM. Advanced Drug Delivery Reviews. 2008;60:373–387. doi: 10.1016/j.addr.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal CM, Athanasiou KA. J Biomed Mater Res. 1997;38:105–114. doi: 10.1002/(sici)1097-4636(199722)38:2<105::aid-jbm4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Seshareddy K, Weiss ML, Detamore MS. Tissue Engineering Part A. 2009;15:1009–1017. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renth AN, Detamore MS. Tissue Engineering Part B, Reviews. 2012;18:341–362. doi: 10.1089/ten.teb.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nuttelman CR, Tripodi MC, Anseth KS. Matrix Biol. 2005;24:208–218. doi: 10.1016/j.matbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Dormer NH, Gupta V, Scurto AM, Berkland CJ, Detamore MS. Mater Sci Eng C. 2013 doi: 10.1016/j.msec.2013.06.026. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrie Aronin CE, Sadik KW, Lay AL, Rion DB, Tholpady SS, Ogle RC, Botchwey EA. Journal of biomedical materials research. Part A. 2009;89:632–641. doi: 10.1002/jbm.a.32015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sefcik LS, Petrie Aronin CE, Wieghaus KA, Botchwey EA. Biomaterials. 2008;29:2869–2877. doi: 10.1016/j.biomaterials.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrie Aronin CE, Sefcik LS, Tholpady SS, Tholpady A, Sadik KW, Macdonald TL, Peirce SM, Wamhoff BR, Lynch KR, Ogle RC, Botchwey EA. Tissue Engineering Part A. 2010;16:1801–1809. doi: 10.1089/ten.tea.2009.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lv Q, Nair L, Laurencin CT. Journal of biomedical materials research. Part A. 2009;91:679–691. doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- 54.Amini AR, Adams DJ, Laurencin CT, Nukavarapu SP. Tissue Engineering Part A. 2012 doi: 10.1089/ten.tea.2011.0076. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dormer NH, Singh M, Zhao L, Mohan N, Berkland CJ, Detamore MS. Journal of biomedical materials research. Part A. 2012;100:162–170. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dormer NH, Busaidy K, Berkland CJ, Detamore MS. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2011;69:e50–e57. doi: 10.1016/j.joms.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dormer NH, Qiu Y, Lydick AM, Allen ND, Mohan N, Berkland CJ, Detamore MS. Tissue Engineering Part A. 2012;18:757–767. doi: 10.1089/ten.tea.2011.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]