Abstract
Bone marrow (BM) hematopoietic stem and progenitor cells (HSPCs) can be activated by type I IFNs, TLR agonists, viruses, and bacteria to increase hematopoiesis. In this study, we report that endotoxin treatment in vivo induces TLR4, MyD88, and Toll/IL-1 resistance domain-containing adaptor-inducing IFN-β (TRIF)-dependent expansion of BM HSPCs. Bacterial infection by Staphylococcus aureus or cecal ligation and puncture also induces HSPC expansion, but MyD88, TRIF, type I IFN, cytokine, PG, or oxidative stress pathways are not required for their expansion. S. aureus-induced HSPC expansion in MyD88−/−TRIF−/− mice is also normal, but is associated with BM remodeling as granulocyte stores are released peripherally. Importantly, reduction in BM cellularity alone can reproduce HSPC expansion. These data show in vivo HSPC responses to bacterial infection are complex and not absolutely dependent upon key inflammatory signaling pathways.
Long-term reconstituting (LTR) hematopoietic stem cells (LT-HSCs) are the source of all circulating blood cells and are defined by their capacity for self-renewal and multilineage differentiation. Mouse hematopoietic stem and progenitor cells (HSPCs), including LT-HSCs, can be identified within the lineage−/lowSca-1+c-kit+ (LSK) subset of cells (1-3), and LT-HSCs can be differentiated from those with short-lived, rapidly dividing HSPCs short-term reconstituting-HSCs (ST-HSC) by the expression of additional cell surface markers (4). Although LT-HSCs maintain blood cell production during homeostasis, HSPCs expand in vivo following IFN-α treatment (4-6), bone marrow (BM) ablation (7), or bleeding (8), dramatically increasing the production of leukocytes.
TLRs are central to innate immunity by recognizing conserved molecular patterns found on pathogens. All TLRs, except TLR3, signal via the adaptor protein, MyD88, leading to NF-κB activation. TLR4, the receptor for LPS, and TLR3 also signal through the adaptor protein, Toll/IL-1 resistance domain-containing adaptor-inducing IFN-β (TRIF)/Toll-IL1 receptor domain-containing adaptor molecule 1, which leads to IFN-I production. HSPCs express TLRs and are activated by TLR2 and TLR4 agonists in vitro through MyD88 signaling (9). HSPCs are also activated in vivo by the TLR3 agonist polyinosinic-polycytidylic acid through IFN-I signaling (5, 6). Similarly, vaccinia virus infection causes MyD88-dependent HSPC expansion (10). Although Escherichia coli infection induces LSK expansion (11), the mechanism(s) of bacterial-induced HSPC activation have not been fully elucidated. In this study, we used two infectious models, Staphylococcus aureus infection and polymicrobial sepsis induced by cecal ligation and puncture (CLP), to examine the regulation of in vivo HSPC expansion.
Materials and Methods
Mice
C57BL/6, TLR4 mutant C3H/HeJ mice, and control C3H/HeOuJ, TRIF−/−, IL-1R−/− IL-6−/−, and Rag1−/− mice on a B6 background were from The Jackson Laboratory (Bar Harbor, ME). IFN-αβR/A129−/− (IFNAR−/−) and wild-type SvEv.129 mice were purchased from B&K Universal (Grimston, Aldbrough, U.K.).B6.MyD88−/− mice were obtained from S. Akira throughA.Ayala atRhode Island Hospital (Providence, RI). Experiments utilizing MyD88−/−TRIF−/− double knockout (DKO) mice were performed at Osaka University (Osaka, Japan). Mice were maintained in specific pathogen-free conditions, and experiments were performed under institutional guidelines.
Reagents
Anti–Gr-1 (RB6-8C5) and anti-human IL-4 (IgG2b isotype) were kindly provided by P. Heyworth from Schering-Plough Biopharma (Palo Alto, CA). Endotoxin-reduced staphylococcal enterotoxin B (SEB), E. coli endotoxin (LPS; strain O55:B5), N-acetyl cysteine (used at 100 mg/kg), and apocynin (50 mg/kg) were purchased from Sigma-Aldrich (St. Louis, MO). Pegylated soluble TNFR1 was a kind gift of Amgen (Thousand Oaks, CA; used at 100 mg/kg).
Mouse infection models
To induce polymicrobial sepsis, mice underwent CLP or sham procedure (12) to obtain an expected mortality of ~10% by 7 d. For S. aureus infection, mice were inoculated with 100 μl of midlogarithmic phase S. aureus, SH1000, in sterile saline, s.c., i.p. (5 × 107 CFUs), or i.v. (5 × 106 CFUMs).
For toxin administration, mice were treated i.p. with 5 μg LPS or 10 μg SEB. Mice were sacrificed 18–36 h later, and BM was harvested for further analysis. To deplete mature neutrophils, mice were treated with 500 μg anti–Gr-1 or control Ab twice separated by 18 h.
Flow cytometry
BM cells were analyzed as previously described (12). Abs were from eBioscience (San Diego, CA) and included anti-CD16/32 Fc block, anti-mouse biotin Lineage mixture (Lin; BD Biosciences, San Jose, CA), anti-CD150, anti–Sca-1, anti–c-kit, anti-CD135/Flk-2, anti–Gr-1, and anti-CD11b. All samples were stained with SYTOX Blue (Invitrogen, Carlsbad, CA) for cell viability analysis and collected on a Becton-Dickinson LSRII flow cytometer using FACSDiva software (BD Biosciences). Total cell numbers from select experiments were calculated and are shown in Supplemental Fig. 7A-D.
Cell purification and culture
Lin− progenitor cells were obtained using biotin-Lin followed by antibiotin magnetic beads (Miltenyi Biotec, Auburn, CA). To obtain LT-HSC–enriched cells, the Lin− cell fraction was incubated with Lin, c-kit, sca-1, CD150, and CD135 Abs, and cells were sorted using an FACSAria sorter (BD Biosciences). Lin− progenitors were cultured in Methocult H4100 base methylcellulose media (Stem Cell Technologies, Vancouver, British Columbia, Canada) supplemented with erythropoietin, GM-CSF, G-CSF, or IL-7 (R&D Systems, Minneapolis, MN) for 14 d.
Histologic analysis
Samples were fixed in 10% neutral formalin (Sigma-Aldrich), paraffin-embedded, decalcified with EDTA, sectioned (5-μm sections), and mounted for H&E staining.
Statistics
Differences among groups were evaluated by ANOVA for multiple groups and the Student t test for two groups. Tukey test was used for post hoc analysis when p < 0.05 by ANOVA.
Results and Discussion
LPS-induced HSPC expansion involves TLR4 inflammatory signaling through MyD88 and TRIF
The LSK population is heterogeneous, and <10% are true repopulating HSCs (2). Many additional markers have been used to obtain higher purities of LT-HSCs. Enriched HSC populations with higher LTR potential can be purified within the CD150+ (13, 14) and the Flk-2/CD135− (15) populations. LSKs expressing CD150 retain their LTR ability even when obtained from cyclophosphamide or G-CSF–treated mice (16), whereas CD135 expression defines ST-HSCs in an activated state (15, 17). Because CD150+CD135− LSKs have been used as a novel identification strategy for LT-HSCs (18, 19), we examined the effects of infection on the CD150+CD135− LT-HSC–enriched population, as well as the activated CD150−CD135+ ST-HSC population (13, 15, 20).
Because LPS is a potent activator of HSPC proliferation in vitro, we initially examined whether LPS affects HSPC responses in vivo. A single dose of LPS (100-fold lower than the LD50 dose) induces a transient LSK expansion (8- to 9-fold; Supplemental Fig. 1A-C), beginning 18 h postinjection, lasting 72 h before declining (data not shown). When examining the subpopulations of expanding LSKs following LPS injection, both LT-HSC–enriched and highly proliferative ST-HSCs subtypes of LSK expand (Supplemental Fig. 1C).
Expectedly, we find LPS-induced HSPC expansion is absent in TLR4 mutant C3H/HeJ mice in vivo, confirming the dependence on TLR4 (Supplemental Fig. 1B, 1C). However, unlike the absolute requirement of MyD88 for LPS-induced in vitro LSK proliferation (9), in vivo LPS treatment induces partial LSK expansion in MyD88−/− or TRIF−/− mice to 61% and 36% of wild-type levels, respectively (Supplemental Fig. 1D), indicating participation of both TLR adaptors in vivo.
Ly6A/E (Sca-1), one of the primary markers of HSCs, is an IFN-I-inducible gene, and IFN-I signaling is necessary for in vivo polyinosinic-polycytidylic acid/TLR3-induced HSPC activation (5, 6). We therefore examined whether IFN-I signaling contributes to TLR4-induced HSPC activation. Using IFNAR−/− mice, we found that HSPC expansion is completely intact in the absence of IFN-I signaling (Supplemental Fig. 1E), indicating that LPS stimulates in vivo HSPC activation through TLR4-mediated inflammatory, but not IFN-I–dependent, signaling.
Bacterial infection induces expansion of LT-HSCs and ST-HSCs
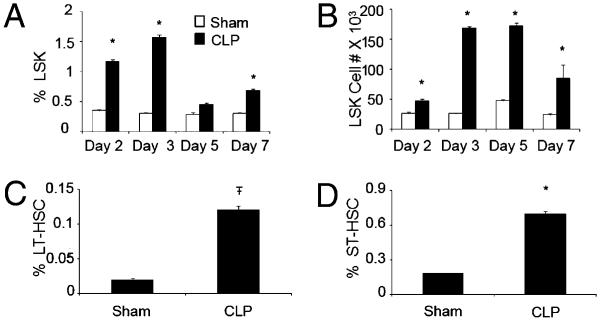
We previously found that bacterial sepsis induced by CLP causes myeloid cell expansion in the BM, spleen, and lymph nodes (12). Because HSPCs are precursors for immature myeloid cells, we examined the effects of bacterial infection on HSPC expansion. Following CLP, we observe a tripling in the percentage and a doubling in the absolute number of the total LSK population within 36 h of CLP (Fig. 1A, 1B). Both the percentage and absolute number of LSK remain elevated for at least 7 d (Fig. 1A, 1B). Similar to HSPC activation by LPS, we find an increase in both the LT-HSC and ST-HSC subpopulations by 36 h post CLP (Fig. 1C, 1D; gating scheme illustrated in Supplemental Fig. 2A).
FIGURE 1.
Expansion of HSPCs in response to CLP. Time course demonstrating percentage (A) and absolute number (B) of BM LSK expansion following CLP. Demonstration of percentage of LT-HSCs (CD150+CD135−LSK) (C) and percentage of ST-HSCs (CD150−CD135+LSK) (D) 36 h following CLP. *p < 0.05; Ŧp < 0.001 control versus treatment.
Because cell surface phenotype alone only suggests that these cells are capable of LTR, we next confirmed whether the CD150+CD135− LSK population from infected mice truly possesses LTR potential. Following 36 h of CLP or sham treatment, highly enriched CD150+CD135− LSKs were obtained (Supplemental Fig. 2B, 2C), and 101 of these cells were injected i.v. into lethally irradiated mice with 5 × 105 Lin+ cells for radioprotection. At 16 wk following transfer, we observed similar survival in mice reconstituted with sham or CLP-treated CD150+CD135− LSKs (Supplemental Fig. 2D), whereas no lethally irradiated mice receiving Lin+ cells alone survived, indicating LT-HSCs following bacterial infection retain LTR potential.
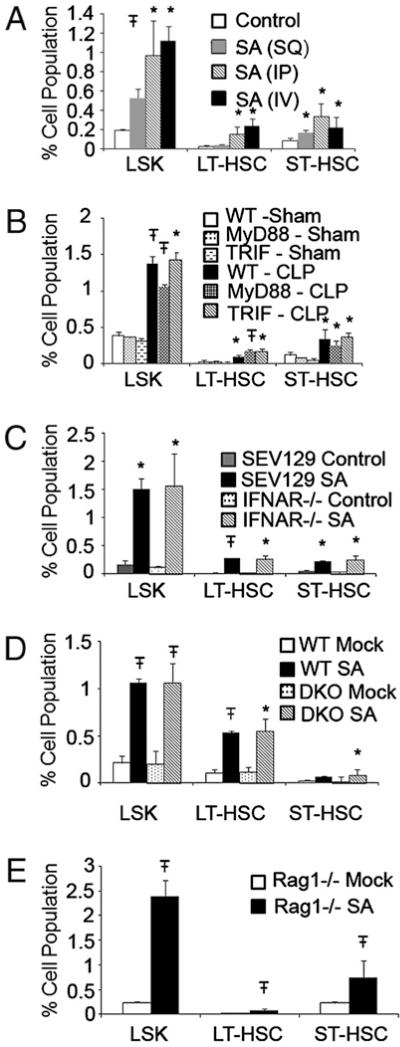
Following CLP, rapid dissemination of multiple bacterial strains occurs to the systemic circulation. To dissect the effect of route of bacterial entry as well as to better quantify the HSPC response to bacteria, we also analyzed the effects of i.p., i.v., or s.c. S. aureus infection. We find that although all three routes of infection were able to induce LSK expansion, the expansion was more robust (required 10-fold less bacteria) in response to i.v. S. aureus infection compared with either s.c. or i.p. infection, indicating that systemic dissemination of bacteria is required for a maximal response (Fig. 2A).
FIGURE 2.
TLR, IFN-I, and superantigen signaling do not participate in bacterial infection induced HSPC expansion. A, Expansion of LSK, LT-HSCs, and ST-HSCs 36 h following the s.c. (SQ) or i.p. (IP) injection of 5 × 107 S. aureus (SA) or the i.v. (IV) injection of 5 × 106 SA. B, Expansion of LSK, LT-HSCs, and ST-HSCs 36 h following CLP does not involve MyD88 or TRIF pathways. Expansion of LSK, LT-HSCs, and ST-HSCs 36 h following i.v. SA infection in SEV129 and IFNAR−/− mice (C), B6 and MyD88−/−TRIF−/− DKO mice (D), or Rag−/− mice (E). *p < 0.05; Ŧp < 0.001 control versus treatment at the time point indicated.
HSPC expansion during bacterial infection does not require TLR inflammatory or IFN-I signaling
Although bacterial infection is more complex than individual TLR stimulation, we hypothesized that HSPC responses to infection would also be regulated through TLR pathways. However, MyD88 or TRIF deficiency individually did not affect BM HSPC expansion in response to CLP or S. aureus infection (Fig. 2B and data not shown).
Infection and inflammation elicit changes in BM leukocyte development. Adjuvant-induced inflammation favors myeloid cell development at the expense of lymphoid cells (21, 22). Previously, we observed that CLP causes increased BM and splenic myeloid cell development that was partially MyD88 dependent (12). However, lymphocyte development following CLP was not examined. In this study, we find that similar to adjuvant treatment, CLP causes a failure in BM B cell development within 3 d (Supplemental Fig. 3A) and depletion of AA4.1+ BM pre-B cells by 36 h that lasts up to 7 d (Supplemental Fig. 3B). MyD88−/− mice displayed a delay in pre-B cell depletion following CLP (Supplemental Fig. 3C), whereas other genetic knockout mice including IFNAR−/−, TRIF−/−, IL-1R−/−, and IL-6−/− mice did not (data not shown). This indicates that the presence of MyD88-dependent inflammation contributes to the early depletion of lymphoid BM elements but is not required for the BM phenotype caused by infection.
Interestingly, following CLP, myeloid progenitors (Lin−c-kit+sca-1− cells) and common lymphoid progenitors (Lin−c-kitlowsca-1lowCD127+ cells) were decreased by ~20–30% by 36 h (data not shown). Not surprisingly, total Lin− cells (containing both stem and progenitor cells) from septic mice formed more myeloid colonies (GM-CSF or G-CSF) than progenitors from sham mice (Supplemental Fig. 3D). Surprisingly, these Lin− cells from septic mice possessed a similar ability to form erythroid (erythropoietin) or lymphoid (IL-7) colonies when compared with sham progenitors (Supplemental Fig. 3D). This suggests that the inflammatory BM microenvironment causes an in vivo block of erythroid/lymphoid cell development without depleting these cells from the BM.
Blockade of other inflammatory factors that regulate hematopoiesis, including TNF-α, IL-1, IL-6, PG, or free radical/oxidative stress, also had no effect on CLP-induced HSPC expansion (Supplemental Fig. 4). Not surprisingly, given a lack of effect of IFN-I on LPS-induced HSPC expansion, loss of IFN-I signaling also did not affect HSPC expansion to S. aureus infection or CLP (Fig. 2C and data not shown).
S. aureus induces HSPC expansion in the absence of all TLR signaling
Because the lack of individual MyD88 or TRIF signaling did not affect HSPC expansion, an interpretation of the data may be that MyD88 and TRIF signaling are redundant, and activation of either pathway alone is sufficient for complete HSPC expansion. To eliminate this possibility, we used MyD88−/−TRIF−/− (DKO) mice, which lack all TLR signaling. Surprisingly, HSPC expansion was completely intact in DKO mice (Fig. 2D) in response to S. aureus infection, indicating that TLR signaling is not required for HSPC activation to S. aureus infection.
Because S. aureus can produce superantigens, and superantigenic stimulation of TCR–MHC complexes in DKO mice may induce HSPC expansion, we tested whether this may be participating in our model. We found that i.p. treatment of mice with SEB is capable of HSPC expansion (Supplemental Fig. 5A). However, when we treated RAG−/− mice (which cannot mount a T cell response to the superantigen) with S. aureus, we find that HSPC responses are similar to WT mice (Fig 2E), indicating that by itself, superantigen production by S. aureus is not the dominant pathway resulting in HSPC expansion.
Infection induces BM remodeling, and Ab-mediated neutrophil depletion is capable of BM remodeling and is sufficient for HSPC expansion
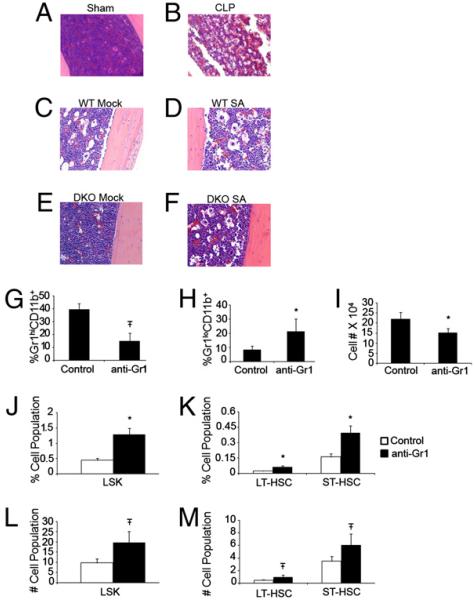
The BM is a fixed space with limited space for expansion of cell populations. Following an infectious or inflammatory insult, BM leukocyte stores are released into the blood. Despite this, many bacterial infections of mice cause an initial leukopenia followed by a delayed leukocytosis when the hematopoietic system is activated sufficiently (12; Supplemental Fig. 5B). We examined serial H&E sections of BM from sham and CLP-treated mice to determine the effects of infection on BM homeostasis. By 12 h, the BM of septic mice shows dilated sinusoids and release of mature neutrophils (Supplemental Fig. 6A). Within 36 h, the BM shows extreme sinusoidal dilitation with loss of mature neutrophils and increased space (Fig. 3A, 3B, Supplemental Fig. 6B). Immature islands of cells are located close to bone-lining osteoblasts and BM vascular cells. By 96 h, the BM regains cellularity, predominated by developing myeloid cells, but a persistent paucity in cellularity remains (Supplemental Fig. 6C). By 7 d, however, the BM is completely repopulated with mostly myeloid cells (Supplemental Fig. 6D). This increased space correlates well with total BM cellularity (Supplemental Fig. 6E) and was also observed in mice following S. aureus infection, occurring to the same extent in wild-type and MyD88−/−TRIF−/− DKO mice (Fig. 3C-F). These results led us to speculate whether emigration of myeloid cells to the periphery causes phenotypic changes in the BM, allowing the BM to be more conducive to HSPC expansion. To test this theory, mice were treated with anti-Ly6G/C (Gr-1) Ab to deplete mature neutrophils. Following 2 d of treatment, there is loss of Gr-1high neutrophils from the BM (Fig. 3G) and an increase in the developing Gr-1low myeloid cells (Fig. 3H). Total BM cellularity is decreased by ~30% (Fig. 3I). With decreased cellularity, there is an increased percentage and an approximate doubling of total LSK number (Fig. 3J, 3L). When examining LSK subpopulations, we find expansion of LT-HSCs and ST-HSCs by both percentage and total number (Fig. 3K, 3M). Although inflammation induced by clearance of dying neutrophils within the BM post Ab administration likely participates in HSPC expansion following anti–Gr-1 treatment, these data suggest a possible BM intrinsic mechanism whereby HSPCs interacting with the BM microenvironment respond to decreased cellularity in their microenvironment caused by inflammatory, infectious, or cytotoxic agents, causing them to expand to repopulate the area left by the cellular void.
FIGURE 3.
BM remodeling is associated with HSPC expansion. Representative low-power (original magnification ×200) example of H&E sections of tibias 36 h following sham (A) and CLP procedure (B) in B6 mice or mock (control) infection (C and E) in B6 mice or MyD88−/−TRIF−/− DKO mice. D and F, Intravenous S. aureus injection in B6 mice or MyD88−/−TRIF−/− DKO mice. Percentage of Gr1hiCD11b+ (G), percentage of Gr1loCD11b+ (H), or total number of BM cells (I) 36 h postinjection of 500 μg control or anti-Ly6G/C(Gr-1) Ab. Percentage (J) or total number ×103 (L) of LSK, percentage (K) or total number ×103 (M) LT-HSCs and ST-HSCs 36 h postinjection of 500 μg control or anti-Ly6G/C (Gr-1) Ab. Experiments in A and B are representative of three experiments with n ≥ 4 mice per group. C–F are representative of one experiment with n = 4 in each group, and G–M are representative of two experiments with n ≥ 4 mice per group. *p < 0.05; Ŧp < 0.001 control versus treatment.
In summary, we demonstrate that although they likely contribute, the absence of key innate immune pathways do not solely govern the highly conserved processes of HSPC activation and expansion in vivo following bacterial infection. Perhaps the most surprising finding of this study is the lack of in vivo requirement for TLR signaling in HSPC activation using two different infectious models, despite the fact that LPS required TLR signals to induce HSPC expansion. These results highlight the likely presence of other innate immune bacterial recognition pathways during infection that can completely compensate for the loss of TLR signaling to induce hematopoietic activation. This pathway likely affects global signaling and does not use single inflammatory mediators to induce their response.
We found that the BM microenvironment is considerably altered with dilated BM sinusoids, disruption of the BM matrix, and loss of mature cells from the BM, resulting in increased space. Although not specifically addressed in this paper, we hypothesize that complex cross talk between HSPCs interacting with supporting stromal cells within their microenvironment may sense the loss of cells from the BM and provide the signals to expand within the BM space left void following infection or chemotherapeutic BM ablation, although further investigation is necessary.
Supplementary Material
Acknowledgments
We thank N. Benson for technical assistance with the cell sorting and L. Miller for kindly providing the S. aureus.
This work was supported in part by Grants R01 GM-40586-21 and R01 GM-81923-02 awarded by the National Institute of General Medical Sciences.
Abbreviations
- BM
bone marrow
- CLP
cecal ligation and puncture
- DKO
double knockout
- HSPC
hematopoietic stem and progenitor cell
- IFNAR−/−
IFN-αβR/A129−/−
- Lin
lineage mixture
- LSK
lineage−/lowSca-1+c-kit+
- LT-HSC
long-term reconstituting hematopoietic stem cell
- LTR
long-term reconstituting
- SEB
staphylococcal enterotoxin B
- ST-HSC
short-term reconstituting hematopoietic stem cell
- TRIF
Toll/IL-1 resistance domain-containing adaptor-inducing IFN-β.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 2.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin-Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J. Exp. Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida N, Aguila HL, Fleming WH, Jerabek L, Weissman IL. Rapid and sustained hematopoietic recovery in lethally irradiated mice transplanted with purified Thy-1.1lo Lin-Sca-1+ hematopoietic stem cells. Blood. 1994;83:3758–3779. [PubMed] [Google Scholar]
- 4.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNα activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat. Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- 7.Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc. Natl. Acad. Sci. USA. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheshier SH, Prohaska SS, Weissman IL. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16:707–717. doi: 10.1089/scd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 9.Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh P, Yao Y, Weliver A, Broxmeyer HE, Hong SC, Chang CH. Vaccinia virus infection modulates the hematopoietic cell compartments in the bone marrow. Stem Cells. 2008;26:1009–1016. doi: 10.1634/stemcells.2007-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Nelson S, Bagby GJ, Siggins R, 2nd, Shellito JE, Welsh DA. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26:1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Kiel MJ, Yilmaz OH, Morrison SJ. CD150-cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood. 2008;111:4413–4414. doi: 10.1182/blood-2007-12-129601. author reply 4414-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasumura M, Imada C, Nawa K. Expression change of Flk-2/Flt-3 on murine hematopoietic stem cells in an activating state. Exp. Hematol. 2003;31:1331–1337. doi: 10.1016/j.exphem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papathanasiou P, Attema JL, Karsunky H, Xu J, Smale ST, Weissman IL. Evaluation of the long-term reconstituting subset of hematopoietic stem cells with CD150. Stem Cells. 2009;27:2498–2508. doi: 10.1002/stem.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.