Abstract
Purpose
Standard of care treatment for most stage I rectal cancers is total mesorectal excision (TME). Given the morbidity associated with TME, local excision (LE) for early-stage rectal cancer has been explored. This study examines practice patterns and overall survival (OS) for early-stage rectal cancer.
Methods
All patients in the National Cancer Data Base diagnosed with rectal cancer from 1998 to 2010 were initially included. Use of LE versus proctectomy and use of adjuvant radiation therapy were compared over time. Adjusted Cox proportional hazards models were used to compare OS based on treatment.
Results
LE was used to treat 46.5% of patients with T1 and 16.8% with T2 tumors. Use of LE increased steadily over time (P < .001). LE was most commonly used for women, black patients, very old patients, those without private health insurance, those with well-differentiated tumors, and those with T1 tumors. Proctectomy was associated with higher rates of tumor-free surgical margins compared with LE (95% v 76%; P < .001). Adjuvant radiation therapy use decreased over time independent of surgical procedure or T stage. For T2N0 disease, patients treated with LE alone had significantly poorer adjusted OS than those treated with proctectomy alone or multimodality therapy.
Conclusion
Guideline-concordant adoption of LE for treatment of low-risk stage I rectal cancer is increasing. However, use of LE is also increasing for higher-risk rectal cancers that do not meet guideline criteria for LE. Treatment with LE alone is associated with poorer long-term OS. Additional studies are warranted to understand the factors driving increased use of LE.
INTRODUCTION
The goal of treatment for early-stage rectal cancer is to optimize oncologic control while minimizing the long-term impact of treatment on quality of life. The standard of care treatment for most stage I rectal cancers is surgery alone, specifically total mesorectal excision (TME).1 For early rectal cancers, this procedure is usually curative but can have a substantial impact on quality of life, including the possibility of permanent colostomy and the potential for short- and long-term bowel, bladder, and sexual dysfunction.2 Given the morbidity associated with TME, alternative approaches to management of rectal cancer have been explored, including local excision (LE) via transanal excision (TAE) or transanal endoscopic microsurgery (TEMS).
Studies have shown that LE alone is inferior to TME for oncologic control.3,4 The poorer outcomes associated with LE primarily result from occult nodal disease, which occurs in approximately 10% of patients with T1 rectal cancers.3 Attempts to identify a subgroup of patients for whom LE would be safe have led to recommendations from the National Comprehensive Cancer Network and American Society of Colorectal Surgeons supporting LE as an acceptable alternative for T1 tumors that are < 30% of the bowel circumference, < 3 cm in size, mobile, well to moderately differentiated, and lack lymphovascular invasion.5–7
Multimodality therapy has also been proposed as an alternative to TME for patients with rectal cancer.8,9 Specifically, chemoradiotherapy has been added to LE in an attempt to clear subclinical nodal disease. Although early results are promising, this approach is investigational and has not been adopted as standard of care.
A 2007 National Cancer Data Base (NCDB) study examining patterns of surgical care for rectal cancer documented a steady increase in the use of LE for stage I rectal cancers from 1989 to 2003.10 The primary goal of this study was to determine whether rates of LE continue to rise despite a lack of data to support the widespread adoption of this technique for higher-risk tumors. This study also examined concomitant trends in the use of adjuvant radiation therapy for stage I tumors. Finally, long-term overall survival (OS) after LE versus proctectomy with or without irradiation for early-stage rectal cancer was examined. Our hypotheses were that use of LE continues to increase for both well-differentiated T1N0 tumors and for higher-risk tumors and that patients with stage I rectal cancer treated with LE have poorer long-term OS than patients treated with proctectomy.6,7
METHODS
The NCDB is a nationwide oncology database that includes incident cases of cancer from the > 1,500 hospitals in the United States with Commission on Cancer–accredited cancer programs. The database includes approximately 70% of all newly diagnosed cancers in the United States. Variables captured, including basic demographics, zip code and county-level area characteristics, tumor staging, initial course of treatment, and vital status, are similar to those captured by other tumor registry databases, such as the National Cancer Institute SEER program.
All patients diagnosed with invasive rectal cancer from 1998 to 2010 were initially included. This study period captured a time during which there were many advances in rectal cancer therapy, such as adoption of neoadjuvant chemoradiotherapy and development of minimally invasive transabdominal and transanal surgical techniques. At the time of analysis, 2010 data were the most recent data available. To focus on surgery performed with curative intent, we excluded patient cases for which there was any indication of distant metastatic disease, those for which tumor staging information was unknown, and those involving a history of prior malignancy. Similarly, patient cases were excluded if initial chemotherapy, irradiation, or surgery was indicated to be administered with palliative intent. To determine stage, the NCDB analytic stage, which is primarily based on pathologic tumor stage, was used.11 Patients who underwent neoadjuvant therapy (chemotherapy and/or radiation therapy before surgical therapy) were excluded from the main analysis, because pretreatment pathologic stage could not be determined.
Surgical procedures were defined as proctectomy, LE, or other. Proctectomy included any segmental resection from partial proctectomy to total abdominal proctocolectomy. Although this group was intended to capture patients undergoing TME, adoption of TME was ongoing during this study period, and the completeness of TME could not be verified from registry data. The LE group included patient cases in which the tumor was removed without mesenteric resection. Before 2009, the NCDB did not distinguish between TEMS and traditional TAE. As with proctectomy, the quality of the LE (ie, depth of excision) could not be verified. The other category included patient cases in which type of surgical procedure was unknown and those involving any procedure for which a pathology specimen was not generated (eg, fulgurations, cryoablations, and so on). Patients who underwent these so-called other procedures were excluded from further analysis.
Logistic and segmented regressions were used to test for differences in use of LE over time. For stage I tumors, patient and tumor characteristics associated with procedure type and use of radiation therapy were examined in bivariate and multivariate analyses. Interactions between T stage and demographic variables with regard to receipt of LE versus proctectomy were also examined. Pearson's χ2 test, t test, and multivariate logistic regression analyses were used to examine differences between the surgical groups. Because few patients have a comorbidity score (Charlson-Deyo score) > 2, the NCDB reports only three categories (ie, 0, 1, ≥ 2). Comorbidity scores were only available starting in 2003, so a subset analysis was performed for each analysis, limiting the study population to these more recent patient cases.
In accordance with NCDB participant user file data-use agreements, survival analysis was limited to patients diagnosed before 2006 to allow for ≥ 5 years of follow-up for all patients. Survival was calculated in months from date of diagnosis to date of last contact or confirmed death. Adjusted Cox proportional hazards models, controlling for patient and tumor characteristics, were used to compare OS based on treatment received. Given the inherent confounding when comparing treatment effectiveness using observational data, a number of sensitivity analyses were performed to address the effect of design assumptions on survival outcomes. All statistical analyses were performed using STATA software (version 11.2; STATA, College Station, TX). Institutional review board exemption was obtained from the University of North Carolina Institutional Review Board.
RESULTS
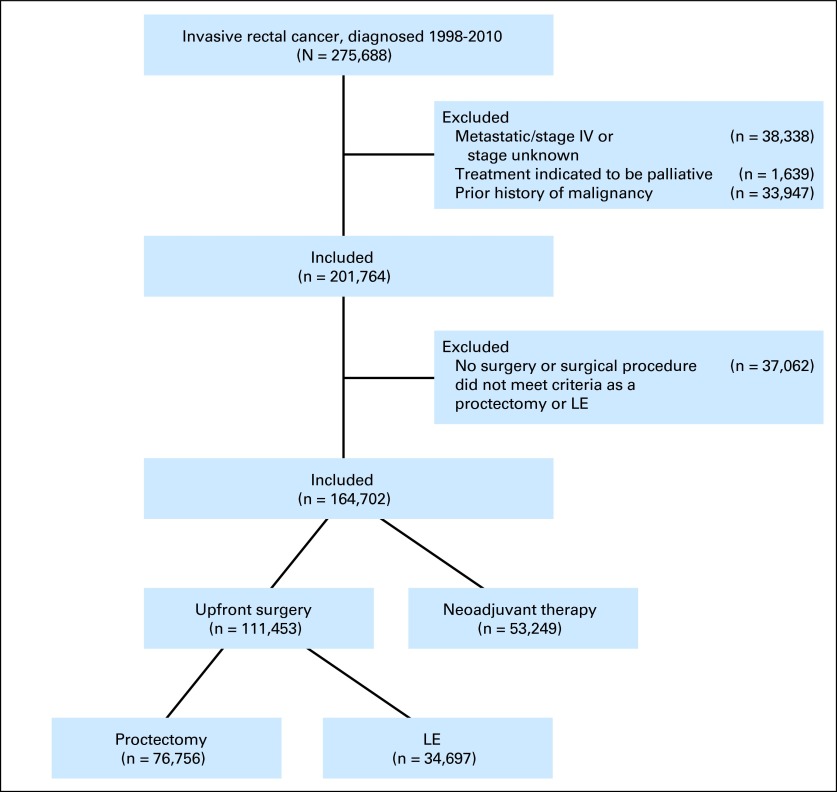
A total of 201,764 patients met initial inclusion criteria (Fig 1); 37,062 patients (18%) were excluded because they did not undergo surgery or because they underwent a surgical procedure that did not meet criteria as proctectomy or LE. The sequencing of multimodality treatment evolved over time, with the proportion of patients for whom radiation was delivered neoadjuvantly increasing from 36.6% in 1998 to 82.8% in 2010 (P < .001). However, because pretreatment pathologic tumor stage was not available for patients treated with neoadjuvant therapy, the 53,249 patients who received neoadjuvant therapy were excluded from further analysis (Data Supplement).
Fig 1.
CONSORT diagram. LE, local excision.
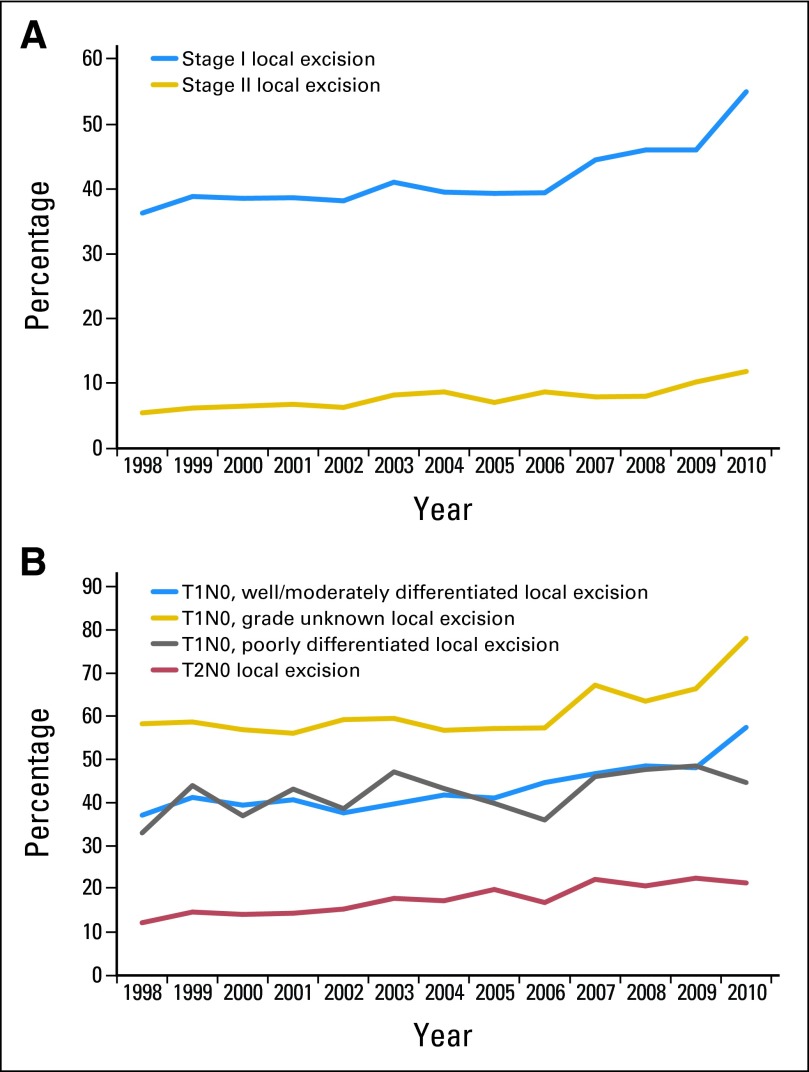
Of the 111,453 patients remaining after exclusions, 31% were treated with LE. Of the 2,452 LEs performed in 2010, 60.5% were performed via TEMS, and 33.7% were performed via traditional TAE; for 5.8%, surgical approach was unknown. LE was used commonly for patients with stage I disease (41.2%) and less commonly for those with stage II disease (7.3%); 46.5% of patients with T1 and 16.8% with T2 tumors were treated with LE. Use of LE increased steadily and statistically significantly over time for every tumor stage group (Fig 2). For T1 tumors, use of LE increased from 39.8% in 1998 to 62.0% in 2010; for T2 tumors, use of LE increased from 12.2% to 21.4%.
Fig 2.
Changes in use of local excision (LE) to treat rectal cancer over time (patients who received neoadjuvant therapy excluded) by (A) disease stage and (B) subtype of stage I tumors. LE is considered standard-of-care therapy only for select patients with well or moderately differentiated T1N0 rectal cancers.
Among patients with stage I disease, LE was most commonly used for women, black patients, very old patients, those without private health insurance, those with well-differentiated tumors, and those with T1 tumors (Table 1). In the multivariate analysis, type of procedure was significantly associated with sex, age, race, insurance, educational attainment, tumor grade, and tumor size. T stage was the factor most strongly associated with surgical procedure; patients with T2 tumors were much less likely (odds ratio [OR], 0.25; 95% CI, 0.24 to 0.26) to undergo LE compared with patients with T1 tumors. Older age was associated with use of LE starting at age 70 years compared with patients age 50 to 59 years. The magnitude of this association was greater for T1 than for T2 tumors. The interaction between T stage and age was the only significant interaction. Black patients were more likely to undergo LE (OR, 1.44; 95% CI, 1.31 to 1.57) than white patients and uninsured patients were more likely (OR, 1.38; 95% CI, 1.15 to 1.64) than privately insured patients to undergo LE. For the subset of patients from 2003 to 2010, LE was less common in those with comorbidity score ≥ 2 compared with patients with comorbidity score of 0 (OR, 0.83; 95% CI, 0.72 to 0.96).
Table 1.
Surgery Type and Adjusted Odds of Receiving LE and Irradiation for Stage I Rectal Cancer (1998-2010 incident cases)*
| Characteristic | Procedure |
LE |
RT |
||||||
|---|---|---|---|---|---|---|---|---|---|
| LE (n = 18,961) |
Proctectomy (n = 27,043) |
P | |||||||
| No. | % | No. | % | OR | 95% CI | OR | 95% CI | ||
| Sex | < .001 | ||||||||
| Male | 9,889 | 39.6 | 15,095 | 60.4 | 1.00 | 1.00 | |||
| Female | 9,072 | 43.2 | 11,948 | 56.8 | 1.11 | 1.05 to 1.16 | 0.87 | 0.81 to 0.94 | |
| Age, years | < .001 | ||||||||
| < 30 | 98 | 52.7 | 88 | 47.3 | 1.03 | 0.70 to 1.51 | 0.79 | 0.41 to 1.53 | |
| 30 to 39 | 432 | 43.6 | 560 | 56.5 | 1.01 | 0.85 to 1.20 | 1.19 | 0.94 to 1.52 | |
| 40 to 49 | 1,596 | 39.4 | 2,459 | 60.6 | 1.00 | 0.91 to 1.10 | 1.18 | 1.04 to 1.35 | |
| 50 to 59 | 4,155 | 41.1 | 5,946 | 58.9 | 1.00 | 1.00 | |||
| 60 to 69 | 4,510 | 38.5 | 7,195 | 61.5 | 1.02 | 0.95 to 1.10 | 0.96 | 0.86 to 1.07 | |
| 70 to 79 | 4,771 | 40.1 | 7,117 | 59.9 | 1.22 | 1.12 to 1.33 | 0.79 | 0.70 to 0.90 | |
| 80 to 89 | 2,983 | 46.9 | 3,381 | 53.1 | 1.76 | 1.59 to 1.94 | 0.45 | 0.39 to 0.53 | |
| ≥ 90 | 416 | 58.4 | 297 | 41.7 | 2.78 | 2.27 to 3.41 | 0.18 | 0.11 to 0.29 | |
| Race | < .001 | ||||||||
| White | 16,146 | 40.1 | 24,153 | 59.9 | 1.00 | 1.00 | |||
| Black | 1,793 | 52.0 | 1,654 | 48.0 | 1.39 | 1.27 to 1.52 | 0.98 | 0.86 to 1.13 | |
| Native American | 35 | 34.7 | 66 | 65.4 | 0.76 | 0.45 to 1.27 | 0.99 | 0.47 to 2.05 | |
| Asian | 568 | 45.0 | 695 | 55.0 | 0.93 | 0.80 to 1.08 | 0.85 | 0.67 to 1.08 | |
| Other/unknown | 419 | 46.9 | 475 | 53.1 | 1.20 | 1.01 to 1.42 | 0.73 | 0.55 to 0.97 | |
| Insurance | < .001 | ||||||||
| Uninsured | 354 | 42.9 | 472 | 57.1 | 1.35 | 1.13 to 1.61 | 0.83 | 0.64 to 1.06 | |
| Private | 2,460 | 38.5 | 3,938 | 61.6 | 1.00 | 1.00 | |||
| Medicaid | 502 | 43.8 | 643 | 56.2 | 1.02 | 0.87 to 1.20 | 0.88 | 0.70 to 1.11 | |
| Medicare | 9,184 | 41.2 | 12,786 | 58.8 | 1.01 | 0.95 to 1.08 | 0.93 | 0.84 to 1.02 | |
| No high school diploma, % | .031 | ||||||||
| < 14 | 2,877 | 42.2 | 3,949 | 57.9 | 1.00 | 1.00 | |||
| 14 to 19.9 | 4,129 | 40.4 | 6,084 | 59.6 | 0.96 | 0.90 to 1.02 | 1.03 | 0.94 to 1.13 | |
| 20 to 28.9 | 4,318 | 40.0 | 6,354 | 60.0 | 0.92 | 0.86 to 0.98 | 1.18 | 1.07 to 1.29 | |
| ≥ 29 | 6,618 | 42.0 | 9,264 | 58.0 | 1.00 | 0.93 to 1.07 | 1.17 | 1.05 to 1.30 | |
| Rural location | .05 | ||||||||
| Yes | 363 | 38.0 | 589 | 62.0 | 0.99 | 0.84 to 1.16 | 0.91 | 0.71 to 1.16 | |
| No | 17,526 | 41.0 | 24,913 | 59.0 | 1.00 | 1.00 | |||
| Tumor grade | < .001 | ||||||||
| Well differentiated | 3,496 | 50.0 | 3,492 | 50.0 | 1.00 | 1.00 | |||
| Moderately differentiated | 9,968 | 34.0 | 19,182 | 66.0 | 0.69 | 0.65 to 0.74 | 1.38 | 1.23 to 1.54 | |
| Poorly differentiated | 1,236 | 35.6 | 2,234 | 61.4 | 0.80 | 0.72 to 0.88 | 2.10 | 1.81 to 2.44 | |
| Unknown | 4,261 | 66.6 | 2,135 | 33.4 | 1.62 | 1.48 to 1.77 | 0.72 | 0.61 to 0.85 | |
| T classification | < .001 | ||||||||
| pT1 | 9,431 | 46.5 | 10,854 | 53.5 | 1.00 | 1.00 | |||
| pT2 | 2,920 | 16.8 | 14,461 | 83.2 | 0.26 | 0.25 to 0.27 | 3.96 | 3.64 to 4.30 | |
| Margins | < .001 | ||||||||
| Positive or unknown | 4,494 | 23.7 | 1,430 | 5.3 | 3.14 | 2.76 to 3.58 | |||
| Negative | 14,467 | 76.3 | 25,613 | 94.7 | 1.00 | ||||
| Surgery | |||||||||
| Local excision | 5.19 | 4.78 to 5.63 | |||||||
| Proctectomy | 1.00 | ||||||||
| Comorbidity score* | < .001 | ||||||||
| 0 | 8,925 | 45.5 | 10,708 | 54.5 | 1.00 | 1.00 | |||
| 1 | 1,610 | 37.6 | 2,676 | 62.4 | 0.76 | 0.70 to 0.83 | 0.83 | 0.73 to 0.96 | |
| 2 | 492 | 39.2 | 764 | 60.8 | 0.81 | 0.70 to 0.93 | 0.67 | 0.52 to 0.86 | |
Abbreviations: LE, local excision; OR, odds ratio; RT, radiation therapy.
ORs for comorbidity score based on subset analysis using 2003 to 2010 incident patient cases only (n = 25,175).
Patients who underwent proctectomy were much more likely than those who underwent LE to have documented tumor-free final surgical margins (95% v 76%; P < .001). This was true for both T1 and T2 tumors.
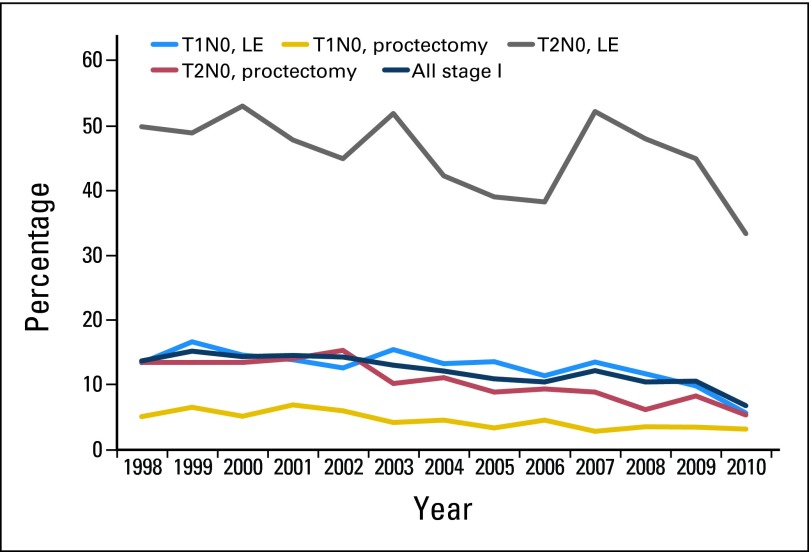
Adjuvant radiation therapy rates for stage I tumors decreased over time (Fig 3). In the multivariate analyses, patients who underwent LE (OR, 4.92; 95% CI, 4.53 to 5.33), those with higher-grade tumors, those with T2 tumors (OR, 4.10; 95% CI, 3.78 to 4.46), and those with positive margins (OR, 3.06; 95% CI, 2.69 to 3.49) were most likely to receive adjuvant radiation (Table 1). Patients age ≥ 80 years were less likely to receive adjuvant radiation. Those with higher comorbidity scores were also less likely to receive adjuvant radiation.
Fig 3.
Adjuvant radiation therapy use for stage I rectal cancers by T stage and type of surgical procedure. LE, local excision.
For patients treated with LE, unadjusted 30-, 60-, and 90-day mortality was 0.9%, 1.4%, and 1.9%, compared with 1.5%, 2.1%, and 2.5% for patients treated with proctectomy, respectively. In the multivariable analysis, increasing age, increasing comorbidity score, and male sex were the only factors associated with increased risk of short-term death. Notably, there was no difference associated with type of surgery.
Increasing age, male sex, higher comorbidity score, and positive/unknown final surgical margins were associated with poorer long-term adjusted OS (Table 2). Patients with Medicaid and those from areas with lower high school graduation rates also had poorer OS. For T1N0 patient cases, there were small but statistically significant differences in adjusted OS based on treatment approach, with patients undergoing LE alone or proctectomy plus irradiation having poorer OS than those undergoing proctectomy alone or LE plus irradiation (Fig 4). In the subset analysis of years 2003 to 2005, controlling for comorbidity score, adjusted OS for all four treatment groups was equivalent. For T2N0 patient cases, adjusted OS among patients treated with proctectomy alone, proctectomy plus irradiation, and LE plus irradiation were equivalent. Meanwhile, patients treated with LE alone had significantly poorer adjusted OS, even after controlling for comorbidity score.
Table 2.
Adjusted OS for Patients With Stage I Rectal Cancer Diagnosed From 1998 to 2005
| Characteristic | T1 |
T2 |
||
|---|---|---|---|---|
| HR | 95% CI | T2 | 95% CI | |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.76 | 0.71 to 0.81 | 0.78 | 0.73 to 0.82 |
| Age, years | ||||
| < 30 | 1.38 | 0.61 to 3.09 | 0.64 | 0.21 to 2.00 |
| 30 to 39 | 0.87 | 0.59 to 1.28 | 0.56 | 0.37 to 0.86 |
| 40 to 49 | 0.77 | 0.61 to 0.97 | 0.75 | 0.62 to 0.91 |
| 50 to 59 | 1.00 | 1.00 | ||
| 60 to 69 | 1.73 | 1.50 to 1.99 | 1.35 | 1.19 to 1.52 |
| 70 to 79 | 3.13 | 2.71 to 3.62 | 2.74 | 2.42 to 3.10 |
| 80 to 89 | 6.84 | 5.88 to 7.96 | 5.23 | 4.60 to 5.96 |
| ≥ 90 | 14.33 | 11.40 to 18.05 | 9.02 | 7.38 to 11.02 |
| Race | ||||
| White | 1.00 | 1.00 | ||
| Black | 1.30 | 1.43 to 1.50 | 1.29 | 1.13 to 1.47 |
| Native American | 1.29 | 0.64 to 2.59 | 2.11 | 1.09 to 4.08 |
| Asian | 0.68 | 0.51 to 0.91 | 0.84 | 0.67 to 1.06 |
| Other/unknown | 0.74 | 0.55 to 0.99 | 0.87 | 0.66 to 1.14 |
| Insurance | ||||
| Uninsured | 1.30 | 0.92 to 1.84 | 1.34 | 1.02 to 1.77 |
| Private | 1.00 | 1.00 | ||
| Medicaid | 1.60 | 1.22 to 2.11 | 1.67 | 1.34 to 2.07 |
| Medicare | 1.46 | 1.33 to 1.60 | 1.17 | 1.08 to 1.27 |
| No high school diploma, % | ||||
| < 14 | 1.00 | 1.00 | ||
| 14 to 19.9 | 1.10 | 1.01 to 1.20 | 1.12 | 1.04 to 1.21 |
| 20 to 28.9 | 1.16 | 1.06 to 1.27 | 1.18 | 1.09 to 1.27 |
| ≥ 29 | 1.24 | 1.12 to 1.38 | 1.19 | 1.08 to 1.30 |
| Rural location | ||||
| Yes | 0.96 | 0.76 to 1.21 | 0.88 | 0.73 to 1.08 |
| No | 1.00 | 1.00 | ||
| Tumor grade | ||||
| Well differentiated | 1.00 | 1.00 | ||
| Moderately differentiated | 1.02 | 0.93 to 1.11 | 1.06 | 0.96 to 1.17 |
| Poorly differentiated | 1.07 | 0.92 to 1.25 | 1.12 | 0.98 to 1.28 |
| Unknown | 0.92 | 0.81 to 1.03 | 0.95 | 0.80 to 1.13 |
| Margins | ||||
| Positive or unknown | 1.25 | 1.13 to 1.38 | 1.22 | 1.11 to 1.35 |
| Negative | 1.00 | 1.00 | ||
| Treatment | ||||
| LE alone | 1.19 | 1.10 to 1.28 | 1.39 | 1.26 to 1.53 |
| Proctectomy alone | 1.00 | 1.00 | ||
| LE plus RT | 1.17 | 0.71 to 1.92 | 0.96 | 0.66 to 1.38 |
| Proctectomy plus RT | 1.28 | 1.06 to 1.54 | 1.00 | 0.91 to 1.11 |
| Comorbidity score* | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 1.52 | 1.30 to 1.79 | 1.56 | 1.36 to 1.80 |
| 2 | 2.82 | 2.24 to 3.54 | 2.49 | 2.03 to 3.06 |
Abbreviations: HR, hazard ratio; LE, local excision; RT, radiation therapy.
HRs for comorbidity score based on subset analysis using 2003 to 2005 incident patient cases only.
Fig 4.
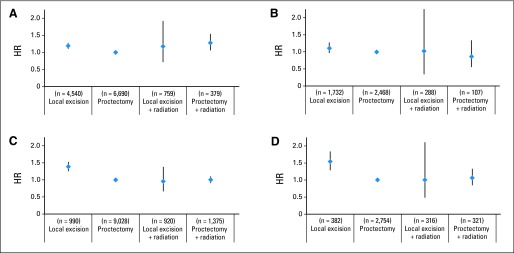
Adjusted overall survival by treatment group for T1N0 tumors from (A) 1998 to 2005 (n = 11,528) and (B) 2003 to 2005 (includes comorbidity adjustment; n = 4,295) and for T2N0 tumors from (C) 1998 to 2005 (n = 11,564) and (D) 2003 to 2005 (includes comorbidity adjustment; n = 3,545). HR, hazard ratio.
Sensitivity Analyses
Because of the lack of mesenteric resection, there may have been understaging in the LE group; however, there was essentially no change in the hazard ratio of death when the proctectomy comparison group was changed from T1N0/T2N0 to T1Nany/T2Nany. Patients with T2 tumors undergoing LE still had statistically inferior survival (Data Supplement). Additionally, because low rectal cancers have higher rates of recurrence but often require abdominoperineal resection, we examined whether preferential use of LE for low-risk cancers might explain inferior survival. However, limiting the proctectomy group to those treated with abdominoperineal resection yielded similar results, with the only statistically significant difference in survival being poorer OS for patients with T2N0 disease treated with LE (Data Supplement).
DISCUSSION
Tumor resection along with TME is the standard of care surgical treatment for the vast majority of rectal cancers.6,7 The incidence of radiographically occult nodal metastasis ranges from 6% for low-risk T1 tumors to as high as 65% for poorly differentiated T2 tumors with lymphovascular invasion.5 As a result, by including complete nodal clearance, TME optimizes locoregional control and tumor staging. TME has been demonstrated to lower the rate of local recurrence, limiting the long-term morbidity and mortality of rectal cancer. Still, TME is associated with substantial morbidity, so alternative approaches to management of rectal cancer continue to be explored.
LE, via traditional TAE or TEMS, removes the full thickness of bowel wall with grossly negative margins but yields few if any lymph nodes for pathologic evaluation. The potential advantages of LE are lower morbidity rates and better long-term functional outcomes.12 In particular, for distal rectal tumors, LE offers the promise of sphincter preservation, whereas TME often results in permanent ostomy creation. Although the long-term functional outcomes after LE have not been systematically examined, it is likely that bowel, urinary, and sexual function are superior compared with outcomes after TME. However, locoregional recurrence rates after LE are high (10% to 22%), primarily because of occult nodal disease.3,10,13 As a result, LE is currently considered an acceptable definitive surgical procedure only for T1N0 rectal cancers that are < 3 cm in size, are well to moderately differentiated, and do not involve lymphovascular or perineural invasion.6,7
Because local recurrences may be amenable to salvage surgical therapy, differences in local recurrence rates may not translate into differences in OS. However, salvage surgical resection is not possible for all recurrences, and salvage procedures generally result in morbidity beyond that associated with TME alone.14–16 Even with multimodality therapy, in one study, treatment of local recurrences required multivisceral pelvic resection in 33% of patients and total pelvic exenteration in 5%.14
Still, the importance of quality of life versus potential risk of recurrence will be valued differently by different patients. As a result, it is reasonable to continue to seek to identify additional groups of patients who might be adequately treated with an LE approach.5,17 This is especially true for patients with T1 tumors, because survival differences between patients treated with LE and those treated with proctectomy seem to be negligible.
The addition of chemoradiotherapy has been proposed to improve oncologic control with LE.8,9 Although early studies are promising with regard to oncologic control, it is unknown whether urinary, sexual, and GI morbidity associated with irradiation may mitigate some of the functional advantages of LE.8,9 As a result, the role of adjuvant chemoradiotherapy for stage I tumors remains unclear. A study of multimodality therapy from Italy, which used a more extensive transanal resection that included resection of adjacent mesenteric tissue, showed promising local control rates (12% local recurrence at 10 years).9 In the United States, a recent American College of Surgeons Oncology Group trial (ACOSOG Z6041) demonstrated an excellent pathologic complete response rate (44%) for the primary tumor in clinically staged T2 rectal cancers after neoadjuvant capecitabine/ oxaliplatin and irradiation, but there were high rates of toxicity during chemoradiotherapy.8 Our study showed OS for both T1 and T2 tumors treated with LE followed by irradiation was similar to survival for similar patients treated with proctectomy alone. However, these results should be interpreted with caution, because the numbers of patients were small, and registry data permit adjustment for only a limited number of covariates. Still, the evidence suggests that patients with stage I rectal cancer treated with LE in combination with chemoradiotherapy may have oncologic outcomes similar to those of patients treated with TME alone.
The prior NCDB study examining patterns of surgical care for rectal cancer brought attention to the steady climb in rates of LE for stage I tumors.10 Use of LE continues to increase for all stage I tumors, with 62% of all T1 and 21% of all T2 cancers treated with LE in 2010. Given the promising early results of multimodality therapy, one might have speculated that the increase in LE over the past decade would be accompanied by a parallel increase in use of radiation therapy.8,9 However, use of adjuvant radiation for stage I rectal cancer declined over the study period. Although some of this decrease may be related to a shift in paradigm to neoadjuvant therapy, it is clear that use of LE as a single-modality therapy is increasing.
The reasons that rates of LE are increasing for higher-risk tumors that do not meet guideline criteria for LE are unclear.6,7 The trend may be patient driven, with more patients demanding therapies with minimal impact on quality of life.18,19 Alternatively, providers may not be aware of the guideline criteria for LE (mobile, < 3 cm, well or moderately differentiated T1 tumors; < 30% of bowel circumference without lymphovascular invasion),6,7 or the growing number of case series describing TEMS may lead some surgeons to push the limits of indications for this approach.19–24 This type of technology-driven, rather than data-driven, evolution in practice patterns has been observed for other cancers.25 Regardless of the reasons for these changes, the data from this study should be cautionary to those considering an LE approach to management of T2 rectal cancer, because patients with T2 tumors who undergo LE alone have statistically significantly poorer oncologic outcomes than patients treated with proctectomy.10
Our study has limitations: registry data provide little detail regarding therapeutic interventions and oncologic outcomes, the data offer limited detail about surgical procedure performed, and some data on first course of therapy may have been missing, such that a patient could have undergone a subsequent surgical procedure or adjuvant therapy after LE that was not captured. The result of this error would be a bias toward the mean, pushing survival curves between the surgical groups closer together than they may actually be. In other words, the differences in outcome between proctectomy and LE groups may be even more substantial than found in this study. Information on rates of local recurrence, salvage surgical procedures, and disease-specific survival is important but cannot be obtained without augmentation of the NCDB through other sources, such as the medical record abstraction completed for the prior study.10 Finally, the reasons why interventions were or were not performed are also unknown. This is demonstrated by the subset of patients who received adjuvant radiation therapy after proctectomy for T1N0 tumors. Because irradiation would not be routinely recommended for these patients, some unmeasured factor may have influenced treatment choice and outcome for these patients. Similarly, the surprising finding that LE was less common for patients with higher comorbidity scores could not be further explored. Ultimately, the inherent selection bias when comparing LE with proctectomy could only be minimally controlled for using the NCDB.
In conclusion, despite the limitations and inherent selection bias of registry data, this study clearly demonstrates rising rates of LE for treatment of stage I rectal cancer. Additional investigation is warranted to understand why a growing proportion of patients with stage I disease are treated in this manner and to ensure that novel treatment paradigms are properly applied in practice.6,7
Supplementary Material
Footnotes
See accompanying editorial on page 4273
Supported by Grant No. UL1TR000083 from the National Center for Research Resources and National Center for Advancing Translational Sciences, National Institutes of Health (NIH); by the Integrated Cancer Information and Surveillance System, University of North Carolina (UNC) Lineberger Comprehensive Cancer Center; by the University Cancer Research Fund via the state of North Carolina; and by NIH Grant No. T32 5T32CA128590-04 for Academic Training in Oncology at UNC Chapel Hill (D.C.P.).
Presented in part in poster format at the 2013 Gastrointestinal Cancers Symposium, San Francisco, CA, January 24-26, 2013, and 66th Annual Cancer Symposium of the Society of Surgical Oncology, National Harbor, MD, March 6-9, 2013.
The data used in this study are derived from a deidentified National Cancer Data Base (NCDB) file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and American Cancer Society. The American Cancer Society, Commission on Cancer, and National Institutes of Health have not verified and are not responsible for the analytic or statistical methodology employed or the conclusions drawn from these data by the investigators.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karyn B. Stitzenberg, Michael O. Meyers
Collection and assembly of data: Karyn B. Stitzenberg
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 2.Bleier JI, Maykel JA. Outcomes following proctectomy. Surg Clin North Am. 2013;93:89–106. doi: 10.1016/j.suc.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 3.You YN. Local excision: Is it an adequate substitute for radical resection in T1/T2 patients? Semin Radiat Oncol. 2011;21:178–184. doi: 10.1016/j.semradonc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum. 2009;52:577–582. doi: 10.1007/DCR.0b013e3181a0adbd. [DOI] [PubMed] [Google Scholar]
- 5.Saraste D, Gunnarsson U, Janson M. Predicting lymph node metastases in early rectal cancer. Eur J Cancer. 2013;49:1104–1108. doi: 10.1016/j.ejca.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Tjandra JJ, Kilkenny JW, Buie WD, et al. Practice parameters for the management of rectal cancer (revised) Dis Colon Rectum. 2005;48:411–423. doi: 10.1007/s10350-004-0937-9. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network; 2012. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer—Version 4.2013. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Aguilar J, Shi Q, Thomas CR, Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: Preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384–391. doi: 10.1245/s10434-011-1933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lezoche E, Baldarelli M, Lezoche G, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211–1218. doi: 10.1002/bjs.8821. [DOI] [PubMed] [Google Scholar]
- 10.You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified? A nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245:726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American College of Surgeons. NCDB Participant User File: AJCC Pathologic T. http://ncdbpufbeta.facs.org/?q=content/ajcc-pathologic-t.
- 12.Doornebosch PG, Tollenaar RA, Gosselink MP, et al. Quality of life after transanal endoscopic microsurgery and total mesorectal excision in early rectal cancer. Colorectal Dis. 2007;9:553–558. doi: 10.1111/j.1463-1318.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- 13.Allaix ME, Arezzo A, Giraudo G, et al. Transanal endoscopic microsurgery vs. laparoscopic total mesorectal excision for T2N0 rectal cancer. J Gastrointest Surg. 2012;16:2280–2287. doi: 10.1007/s11605-012-2046-8. [DOI] [PubMed] [Google Scholar]
- 14.You YN, Roses RE, Chang GJ, et al. Multimodality salvage of recurrent disease after local excision for rectal cancer. Dis Colon Rectum. 2012;55:1213–1219. doi: 10.1097/DCR.0b013e318270837f. [DOI] [PubMed] [Google Scholar]
- 15.Stipa F, Giaccaglia V, Burza A. Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum. 2012;55:262–269. doi: 10.1097/DCR.0b013e318241ef22. [DOI] [PubMed] [Google Scholar]
- 16.Doornebosch PG, Ferenschild FT, de Wilt JH, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum. 2010;53:1234–1239. doi: 10.1007/DCR.0b013e3181e73f33. [DOI] [PubMed] [Google Scholar]
- 17.Chang HC, Huang SC, Chen JS, et al. Risk factors for lymph node metastasis in pT1 and pT2 rectal cancer: A single-institute experience in 943 patients and literature review. Ann Surg Oncol. 2012;19:2477–2484. doi: 10.1245/s10434-012-2303-9. [DOI] [PubMed] [Google Scholar]
- 18.Temple LK, Naimark D, McLeod RS. Decision analysis as an aid to determining the management of early low rectal cancer for the individual patient. J Clin Oncol. 1999;17:312–318. doi: 10.1200/JCO.1999.17.1.312. [DOI] [PubMed] [Google Scholar]
- 19.De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol. 2009;35:1280–1285. doi: 10.1016/j.ejso.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Wu YY, Li S, et al. TEM and conventional rectal surgery for T1 rectal cancer: A meta-analysis. Hepatogastroenterology. 2011;58:364–368. [PubMed] [Google Scholar]
- 21.Ramirez JM, Aguilella V, Valencia J, et al. Transanal endoscopic microsurgery for rectal cancer: Long-term oncologic results. Int J Colorectal Dis. 2011;26:437–443. doi: 10.1007/s00384-011-1132-9. [DOI] [PubMed] [Google Scholar]
- 22.Baatrup G, Breum B, Qvist N, et al. Transanal endoscopic microsurgery in 143 consecutive patients with rectal adenocarcinoma: Results from a Danish multicenter study. Colorectal Dis. 2009;11:270–275. doi: 10.1111/j.1463-1318.2008.01600.x. [DOI] [PubMed] [Google Scholar]
- 23.Guerrieri M, Baldarelli M, Organetti L, et al. Transanal endoscopic microsurgery for the treatment of selected patients with distal rectal cancer: 15 years experience. Surg Endosc. 2008;22:2030–2035. doi: 10.1007/s00464-008-9976-y. [DOI] [PubMed] [Google Scholar]
- 24.Zieren J, Paul M, Menenakos C. Transanal endoscopic microsurgery (TEM) vs. radical surgery (RS) in the treatment of rectal cancer: Indications, limitations, prospectives: A review. Acta Gastroenterol Belg. 2007;70:374–380. [PubMed] [Google Scholar]
- 25.Stitzenberg KB, Wong YN, Nielsen ME, et al. Trends in radical prostatectomy: Centralization, robotics, and access to urologic cancer care. Cancer. 2012;118:54–62. doi: 10.1002/cncr.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.