Abstract
Purpose
The primary purpose of surveillance of patients with esophageal adenocarcinoma (EAC) and/or esophagogastric junction adenocarcinoma after local therapy (eg, chemoradiotherapy followed by surgery or trimodality therapy [TMT]) is to implement a potentially beneficial salvage therapy to overcome possible morbidity/mortality caused by locoregional failure (LRF). However, the benefits of surveillance are not well understood. We report on LRFs and salvage strategies in a large cohort.
Patients and Methods
Between 2000 and 2010, 518 patients with EAC who completed TMT were analyzed for the frequency of LRF over time and salvage therapy outcomes. Standard statistical techniques were used.
Results
For 518 patients, the median follow-up time was 29.3 months (range, 1 to 149 months). Distant metastases (with or without LRF) occurred in 188 patients (36%), and LRF only occurred in 27 patients (5%). Eleven of 27 patients had lumen-only LRF. Most LRFs (89%) occurred within 36 months of surgery. Twelve patients had salvage chemoradiotherapy, but only five survived more than 2 years. Four patients needed salvage surgery, and three who survived more than 2 years developed distant metastases. The median overall survival of 27 patients with LRF was 17 months, and 10 patients (37%) survived more than 2 years. Thus, only 2% of all 518 patients benefited from surveillance/salvage strategies.
Conclusion
Our surveillance strategy, which is representative of many others currently being used, raises doubts about its effectiveness and benefits (along with concerns regarding types and times of studies and costs implications) to patients with EAC who have LRF only after TMT. Fortunately, LRFs are rare after TMT, but the salvage strategies are not highly beneficial. Our data can help develop an evidence-based surveillance strategy.
INTRODUCTION
Esophageal cancer (EC) is a dreadful illness with poor prognosis and high health burden in many societies. Worldwide, 482,300 new cases and 406,800 deaths from EC were estimated in 2013, including 17,990 new cases and 15,210 deaths in the United States.1,2 Although squamous cell carcinoma is common in the endemic area, esophageal adenocarcinoma (EAC) and/or gastroesophageal junction adenocarcinoma are common in the United States.3,4 The incidence of EAC has been rising alarmingly over the past 30 years in the West.4,5 In patients who can withstand surgery, localized EAC is frequently treated by chemoradiotherapy followed by surgery (trimodality therapy [TMT]).6,7 TMT is considered one of the standards of care for patients with localized EAC.6,7 After TMT, patients are surveyed by various schedules and tests. The variations exist because the evidence for patient benefit or lack of benefit has never been well documented (for example, Gastroesophageal Cancer Guidelines from the National Comprehensive Cancer Network provide considerable latitude because they represent the preferences of numerous participating institutions). The cost of surveillance has not been reported, but costs can vary considerably by region and country and also on the basis of health care systems. However, the primary reason to survey patients after successful local therapy (eg, TMT) is to implement a potentially beneficial salvage therapy to overcome locoregional failure (LRF).8 Periodic endoscopy and imaging techniques are often incorporated on a schedule that is preferred by an institution or practice.9,10 Surveillance strategy is also important because it is the cause for considerable anxiety and stress to the patient and relatives at each visit in which outcomes of the tests are reviewed.
The purpose of this study was to analyze a large cohort of patients who had TMT and to assess the outcome of patients for whom salvage therapy was implemented following LRF. We were also interested in the type of LRF and its timing, which can have implications for the appropriateness and timing of certain investigations.
PATIENTS AND METHODS
Patient Selection
We analyzed patients from our prospectively maintained database on EAC in the Department of Thoracic and Cardiovascular Surgery at The University of Texas MD Anderson Cancer Center to find consecutive patients who had histologically confirmed localized EAC and received TMT between 2000 and 2010. All patients had baseline and presurgical (postchemoradiation) staging that included imaging studies and endoscopic biopsies (ultrasonography was performed for each patient at baseline). Before proceeding with therapy, each patient was evaluated by physicians from appropriate disciplines and then discussed by the multidisciplinary team (consisting of radiologists, gastroenterologists, thoracic surgeons, radiation oncologists, pathologists, nutritionists, geneticists [when appropriate], and medical oncologists). Clinical staging was based on the American Joint Committee on Cancer (AJCC) Classification, 6th edition, and pathologic staging was based on AJCC, 7th edition.11,12 The institutional review board approved this analysis.
Therapy
All patients had chemoradiotherapy consisting of radiation and concurrent chemotherapy with a fluoropyrimidine (intravenous or oral) and either a platinum compound or a taxane as described previously.13,14 Approximately 5 to 6 weeks after the completion of chemoradiotherapy, the patients underwent preoperative restaging. All patients completed esophagectomy. The treating thoracic surgeon selected the surgical technique (transthoracic [Ivor-Lewis], transhiatal, total [three-field technique], or minimally invasive esophagectomy).
Surveillance After TMT and Salvage Strategy
Each patient was generally surveyed by the following surveillance strategy: patient visits were performed every 3 months for the first year, then every 6 months for 2 additional years, and then once a year for at least 5 years. At each visit, an imaging study (computed tomography [CT] or positron emission tomography CT [PET-CT]), and blood tests were performed. Endoscopic evaluation for lumen recurrence was performed every 6 months in the first 18 months and then once a year. First recurrences, distant metastases (with or without LRF), or LRF only were documented. Patients who had LRF with distant metastases at first occurrence were excluded from this analysis since they were not subjected to a salvage strategy. Patients with LRF for whom salvage therapy was attempted were carefully monitored for outcome. Chemoradiotherapy was the preferred approach for salvage (if the LRF occurred outside the prior field of radiation), and surgery salvage was the last choice.
The survival follow-up was carried out through our institution's Tumor Registry, electronic medical records, and/or the Social Security Database. The data were last updated in May 2012.
Statistical Analysis
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. Relapse-free time and follow-up time were calculated from the date of surgery to the event, overall survival (OS) and relapse-free survival outcomes were calculated by using Kaplan-Meier estimators, and the log-rank test was used to compare the Kaplan-Meier curves. If an event date was not available, the date of the last follow-up was used. Statistical significance was defined as P < .05. Univariable and multivariable Cox regression models were obtained. Covariates with P < .25 in the univariable analysis were entered into the multivariable analysis. Stepwise backward elimination was used to obtain a final multivariable analysis. Statistical analysis was performed by using IBM SPSS statistics 19.0 (IBM, Chicago, IL).
RESULTS
Patient and Treatment Characteristics
We analyzed 518 patients with EAC who met the selection criteria. The clinical characteristics of these patients are provided in Table 1. The median age was 61 years (range, 23 to 79 years). The median follow-up time from esophagectomy was 29 months (range, 1 to 149 months) for all patients and 55 months (range, 1 to 149 months) for those 239 patients who remain alive. At baseline staging, 79% of 518 patients had T3, 14% had T2, 2% had T4a (resectable), and 1.0% had T1 tumors. Most patients (62%) had N-positive disease, and 5% of patients had M1a disease staging. A majority of the tumors (65%) were at the esophagogastric junction, 33% were in the lower esophagus, and 2% were in the middle esophagus. The median radiation dose was 50.4 Gy (range, 39.6 to 64.8 Gy) delivered in daily fractions of 1.8 Gy by a conformal technique.
Table 1.
Pretreatment Characteristics
| Characteristic | No. of Eligible Patients (N = 518) | % |
|---|---|---|
| Age, years | ||
| Median | 61 | |
| Range | 23-79 | |
| Sex | ||
| Male | 467 | 90 |
| Female | 51 | 10 |
| Race | ||
| White | 474 | 92 |
| Other | 44 | 9 |
| Primary site | ||
| Middle third | 11 | 2 |
| Lower third | 170 | 33 |
| Gastroesophageal junction | 337 | 65 |
| Histology | ||
| Adenocarcinoma | 518 | 100 |
| Histologic grade | ||
| Well to moderate | 230 | 44 |
| Poor | 287 | 55 |
| Unspecified | 1 | 0.2 |
| Pretreatment clinical stage | ||
| X* | 21 | 4 |
| I | 2 | 0.4 |
| II | 202 | 39 |
| III | 268 | 52 |
| IVa | 25 | 5 |
NOTE. Because of rounding, the total may not add up to 100%.
X could not be finalized.
Type of Surgery
Three hundred sixty-four patients (70%) had right trans-thoracic (Ivor-Lewis) esophagectomy, 58 (11%) had transhiatal esophagectomy, 57 (11%) had minimally invasive esophagectomy, and 37 (7%) had three-field technique esophagectomy.
Failure Patterns
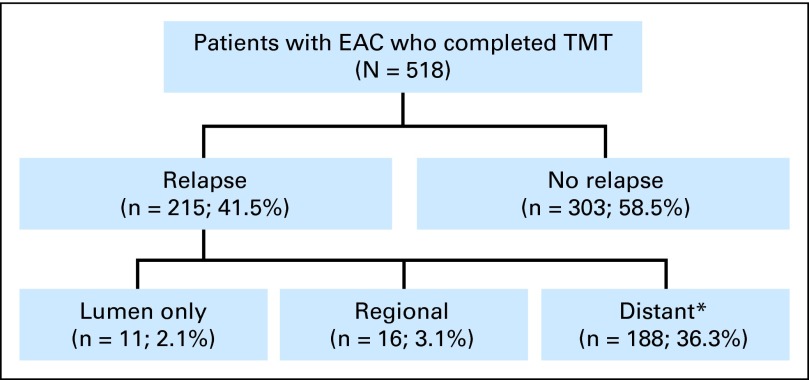
First relapses were as follows: 188 patients (36%) had distant metastases (with or without LRF) and 27 (5%) had LRF only (lumen only and/or regional relapse; Fig 1). Of 27 LRFs, 11 patients (2% of 518) had a lumen-only relapse. Of patients with lumen-only relapses, nine had them at the anastomosis site and two were not at the anastomosis site. The timings of relapses are shown Table 2. Seventeen (63% of 27) LRFs occurred within 2 years and 24 (89% of 27) occurred within 3 years of surgery.
Fig 1.
Patterns of relapse after trimodality therapy (TMT). EAC, esophageal adenocarcinoma. (*) Distant plus or minus lumen and/or regional.
Table 2.
Duration-Specific Rate of LRF From Surgery
| Tumor Location | 0-12 Months |
13-24 Months |
25-36 Months |
37-48 Months |
49+ Months |
Total Months |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Entire Cohort (%)* | LRF Only (%)† | No. | Entire Cohort (%)* | LRF Only (%)† | No. | Entire Cohort (%)* | LRF Only (%)† | No. | Entire Cohort (%)* | LRF Only (%)† | No. | Entire Cohort (%)* | LRF Only (%)† | No. | Entire Cohort (%)* | LRF Only (%)† | |
| Lumen only | 1 | 0.2 | 4 | 7 | 1.4 | 26 | 2 | 0.4 | 7 | 0 | 0.0 | 0.0 | 1 | 0.2 | 4 | 11 | 2 | 41 |
| Regional | 1 | 0.2 | 4 | 8 | 1.5 | 30 | 5 | 1.0 | 19 | 1 | 0.2 | 4 | 1 | 0.2 | 4 | 16 | 3.1 | 59 |
| Locoregional | 2 | 0.4 | 7.4 | 15 | 3 | 56 | 7 | 1.4 | 26 | 1 | 0.2 | 4 | 2 | 0.4 | 7 | 27 | 5 | 100 |
Abbreviation: LRF, locoregional failure.
For calculating percentages, the denominator was 518 patients.
For calculating percentages, the denominator was 27 patients.
Role of PET-CT
Twenty-one of 27 patients with LRF only had a PET-CT scan. Eight of 11 patients with luminal-only recurrence had a PET-CT scan, and those scans suggested recurrence in seven of eight of these patients (all had biopsy confirmation of recurrence). Thirteen of 16 patients with regional recurrence had a PET-CT scan, and all scans in these patients were diagnostic (10 patients had histologic confirmation; histologic confirmation for diagnosis was not indicated in three patients).
Salvage Strategies
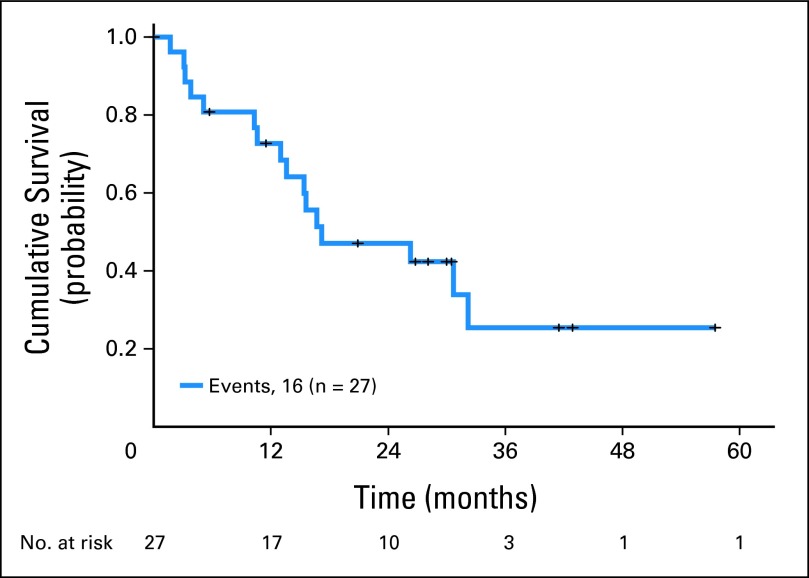
The median OS of the 27 patients with LRF (from relapse) was 17 months (95% CI, 1.1 to 33.3 months; Fig 2). As of this analysis, 16 (59% of 27) patients have died. Twelve of 27 LRF patients were eligible for salvage with chemoradiotherapy, and five patients survived beyond 2 years. Four of 27 patients required salvage surgery. All four surgeries were difficult because of fibrosis, and supercharged jejunal conduit had to be used in two patients. For two patients, readvancement of the gastric pull-up was performed to anastomose remaining stomach and esophagus, and intrathoracic latissimus dorsi muscle flap was used for anastomotic coverage. Each patient who had surgical salvage experienced considerable morbidity (two requiring re-operation). Three of four patients who experience salvage surgery survived more than 2 years but all developed distant metastases.
Fig 2.
Kaplan-Meier survival plot from diagnosis of locoregional failure for 27 patients.
Eleven patients who could not undergo any type of salvage were palliatively treated with systemic chemotherapy (six patients) or best supportive care (five patients). The estimated median OS duration for these 11 patients was 5 months (95% CI, 1.0 to 23 months) with a 3-year survival rate of 10.0%.
DISCUSSION
Surveillance following local therapy (eg, TMT for patients with EAC) is carried out routinely in the western world; however, there are considerable variations in the approach. The purpose of surveillance is to be able to offer salvage therapy to patients who develop LRF only. LRF can be potentially curable with salvage techniques.15–26 However, we did not find any reports in the literature of salvage surgery for LRF after TMT. Surveillance often involves the use of imaging techniques (routinely or as clinically indicated) and/or endoscopic evaluations (structured approach or as clinically indicated; see the National Comprehensive Cancer Network Gastroesophageal Cancer Guidelines). Although, we have not performed a cost analysis for our patients, needless to say, the surveillance enterprise in patients with EAC is associated with considerable cost. The costs vary widely in different regions of the world; therefore, a cost analysis would not be generalizable. Endoscopic procedures can be associated with complications which may, in turn, be associated with sedation, anesthesia, and multiple biopsies. In addition to cost and morbidity, two other major factors must be considered: (1) the immense anxiety and stress each surveillance visit creates for the patient and relatives and (2) our lack of knowledge about whether such a high-stress and high-cost endeavor fulfills our objective of benefiting patients. In addition, the benefit is not defined and is subject to debate. Is the benefit a cure rate of 20%, 30%, or 40% of all patients who have LRF only? Or is the benefit a cure rate of only 5%, 10%, or 20% of all patients surveyed? These are real challenges we face in the clinic on a daily basis, and success is based entirely on the definition of benefit. Another minor dimension of surveillance is that it can diagnose M1 disease so that systemic therapy can be initiated early; however, its benefit to the patient is not known.
Our results have some sobering findings. The good news is that the rate of LRF only is remarkably low (approximately 5% of 518 patients) after TMT. The lumen-only LRF is exceedingly rare (2% of all surveyed) and provides a platform for discussing the inclusion of periodic endoscopies (and cost and risks associated with it) in patients who successfully complete TMT. Our data suggest that surveillance PET-CT scans can regularly detect local and/or regional recurrences (that have been confirmed histologically) and therefore, point to another algorithm in which an upper endoscopy could be considered only when PET-CT results suggest local or regional recurrence. The bigger question is whether 37% of LRF patients who had salvage therapy and survived more than 2 years could be considered a benefit for such extensive surveillance or should we state that because only 2% (10 of 518) of all patients surveyed survived more than 2 years, our surveillance strategy does not seem beneficial to our patients. These are tough questions to consider. It is clear, however, that our results suggest that surgical salvage comes at a high price of morbidity and potential mortality, and overall results of salvage in 27 patients are not remarkable, which casts considerable doubt on current surveillance strategy. If our data provide baseline evidence for developing an informed surveillance strategy, then it may be that a more conservative algorithm might suffice.
What can we glean from the literature? The literature is rather sparse on this subject, leaving us with much work to do to solve this difficult problem. Meguid et al10 reported recurrence in 84 (31%) of 267 patients with EC after TMT; of these 84, 23% had LRF and 77% had distant metastases. That study included 208 patients (78%) with EAC, 55 (21%) with squamous cell histology, and four (1.5%) with carcinoma not otherwise specified. Koshy et al9 reported relapse patterns in 112 patients with EAC and in 52 patients with squamous cell carcinoma. A smaller fraction of patients (39%) had LRF and a majority (68%) had distant metastases. Both these studies have a higher rate of LFR compared with our study, which represents the largest cohort of patients. Neither these studies nor any others have directly addressed the value of surveillance or the benefit from salvage strategies. Our results raise more questions than answers. To the best of our knowledge, our report is the first to discuss salvage after TMT.
The preferred salvage approach is not crisply defined. However, our group used the pragmatic approach of using surgical salvage as the last resort because of its anticipated higher morbidity/mortality rate over the preferred chemoradiotherapy salvage. We acknowledge that chemoradiotherapy salvage depends on the location of the LRF in relation to the prior radiation field.
Our data also bring out another point that will have to be considered for developing better surveillance strategies in the future. Most occurrences of LRF only (89%) were documented within the first 3 years after surgery. This finding may have implications on surveillance of all patients beyond 3 years.
Weaknesses of our analysis are that it is a retrospective review, it is a single high-volume center experience, and the data may not necessarily be generalizable. An ideal study would be a prospective randomized study comparing an aggressive surveillance approach (for example, one described in this report) versus a conservative approach (for example, studies performed only when a patient becomes symptomatic), with the end points of defining patient benefit and performing cost comparisons. However, we do not foresee the launch of such a study. Our data have strengths that can contribute to the refinement of surveillance strategies of the future: (1) we studied a large cohort of patients, (2) we used a uniform surveillance strategy, (3) we used a rather aggressive surveillance strategy (had we used a conservative strategy, it would have invited considerable discussion), (4) we have a single high-volume experience demonstrating that first and only LRF after TMT is a rare event, and (5) we observed that the salvage strategies result in unimpressive results (only a few patients with LRF survived > 2 years and only 10 patients benefited when 518 patients were surveyed (which raises cost-benefit issues).
In conclusion, our data show that LRF only (in which salvage strategies can potentially be implemented) is uncommon after completion of TMT in patients with EAC, and salvage strategies produced unimpressive results. Our data also shed light on which tests could be high yielding in the context of the timing of surveillance. Our data can contribute to the development of an evidence-based surveillance strategy.
Acknowledgment
We acknowledge the invaluable contributions of Zhongxing Liao, MD, Garrett Walsh, MD, Ara A. Vaporciyan, MD, William A. Ross, MD, Reza J. Mehran, MD, James Welsh, MD, and Stephen H. Lin, MD, all at The University of Texas MD Anderson Cancer Center.
Footnotes
Supported by the Caporella, Dallas, Sultan, Park, Smith, Frazier, Oaks, Vanstekelenberg, and Cantu families; by the Schechter Private Foundation, Rivercreek Foundation, Kevin Fund, Myer Fund, Dio Fund, and Milrod Fund; by multidisciplinary grants from The University of Texas MD Anderson Cancer Center, Houston, TX; and by Grants No. CA129906 and CA172741 from the National Cancer Institute (J.A.A.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jaffer A. Ajani
Financial support: Jaffer A. Ajani
Administrative support: Jaffer A. Ajani
Provision of study materials or patients: Mariela A. Blum, Ritsuko Komaki, Jeffrey H. Lee, Manoop S. Bhutani, Brian Weston, Heath D. Skinner, David C. Rice, Stephen G. Swisher, Wayne L. Hofstetter, Jaffer A. Ajani
Collection and assembly of data: All authors
Data analysis and interpretation: Kazuki Sudo, Takashi Taketa, Arlene M. Correa, Wayne L. Hofstetter, Jaffer A. Ajani
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012. [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: Are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 4.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–1187. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: Role of obesity and diet. J Natl Cancer Inst. 1995;87:104–109. doi: 10.1093/jnci/87.2.104. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 8.Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002;123:175–183. doi: 10.1067/mtc.2002.119070. [DOI] [PubMed] [Google Scholar]
- 9.Koshy M, Greenwald BD, Hausner P, et al. Outcomes after trimodality therapy for esophageal cancer: The impact of histology on failure patterns. Am J Clin Oncol. 2011;34:259–264. doi: 10.1097/COC.0b013e3181e841ce. [DOI] [PubMed] [Google Scholar]
- 10.Meguid RA, Hooker CM, Taylor JT, et al. Recurrence after neoadjuvant chemoradiation and surgery for esophageal cancer: Does the pattern of recurrence differ for patients with complete response and those with partial or no response? J Thorac Cardiovasc Surg. 2009;138:1309–1317. doi: 10.1016/j.jtcvs.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. ed 7. New York, NY: Springer; 2010. [Google Scholar]
- 12.Greene FL, Page DI, Fleming ID, et al. AJCC Cancer Staging Manual. ed 6. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- 13.Ajani JA, Correa AM, Hofstetter WL, et al. Clinical parameters model for predicting pathologic complete response following preoperative chemoradiation in patients with esophageal cancer. Ann Oncol. 2012;23:2638–2642. doi: 10.1093/annonc/mds210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajani JA, Correa AM, Walsh GL, et al. Trimodality therapy without a platinum compound for localized carcinoma of the esophagus and gastroesophageal junction. Cancer. 2010;116:1656–1663. doi: 10.1002/cncr.24935. [DOI] [PubMed] [Google Scholar]
- 15.Mullen JT, Rodriguez-Bigas MA, Chang GJ, et al. Results of surgical salvage after failed chemoradiation therapy for epidermoid carcinoma of the anal canal. Ann Surg Oncol. 2007;14:478–483. doi: 10.1245/s10434-006-9221-7. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara H. Salvage surgery for esophageal carcinoma after definitive chemoradiation therapy. Ann Thorac Cardiovasc Surg. 2007;13:293–295. [PubMed] [Google Scholar]
- 17.Marks JL, Hofstetter W, Correa AM, et al. Salvage esophagectomy after failed definitive chemoradiation for esophageal adenocarcinoma. Ann Thorac Surg. 2012;94:1126–1132. doi: 10.1016/j.athoracsur.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 18.Yoo C, Park JH, Yoon DH, et al. Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg. 2012;94:1862–1868. doi: 10.1016/j.athoracsur.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 19.Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg. 2007;94:1059–1066. doi: 10.1002/bjs.5865. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill S, Danbury C, Kitching A. Salvage oesophagectomy after local failure of definitive chemoradiotherapy (Br J Surg 2007; 94: 1059-1066) Br J Surg. 2007;94:1572. doi: 10.1002/bjs.6088. [DOI] [PubMed] [Google Scholar]
- 21.Meunier B, Raoul J, Le Prisé E, et al. Salvage esophagectomy after unsuccessful curative chemoradiotherapy for squamous cell cancer of the esophagus. Dig Surg. 1998;15:224–226. doi: 10.1159/000018618. [DOI] [PubMed] [Google Scholar]
- 22.Oki E, Morita M, Kakeji Y, et al. Salvage esophagectomy after definitive chemoradiotherapy for esophageal cancer. Dis Esophagus. 2007;20:301–304. doi: 10.1111/j.1442-2050.2007.00677.x. [DOI] [PubMed] [Google Scholar]
- 23.Hokamura N. [Salvage surgery in esophageal cancer treatment: Its review in this decade and promises for the next decade] [in Japanese] Gan To Kagaku Ryoho. 2011;38:179–183. [PubMed] [Google Scholar]
- 24.Nishimura M, Daiko H, Yoshida J, et al. Salvage esophagectomy following definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg. 2007;55:461–464. doi: 10.1007/s11748-007-0157-z. [DOI] [PubMed] [Google Scholar]
- 25.Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 2009;100:442–446. doi: 10.1002/jso.21353. [DOI] [PubMed] [Google Scholar]
- 26.Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2009;137:49–54. doi: 10.1016/j.jtcvs.2008.05.016. [DOI] [PubMed] [Google Scholar]