Abstract
Purpose
Nivolumab, a human immunoglobulin G4–blocking antibody against the T-cell programmed death-1 checkpoint protein, has activity against metastatic melanoma. Its safety, clinical efficacy, and correlative biomarkers were assessed with or without a peptide vaccine in ipilimumab-refractory and -naive melanoma.
Patients and Methods
In this phase I study, 90 patients with unresectable stage III or IV melanoma who were ipilimumab naive and had experienced progression after at least one prior therapy (cohorts 1 to 3, 34 patients) or experienced progression after prior ipilimumab (cohorts 4 to 6, 56 patients) received nivolumab at 1, 3, or 10 mg/kg every 2 weeks for 24 weeks, then every 12 weeks for up to 2 years, with or without a multipeptide vaccine.
Results
Nivolumab with vaccine was well tolerated and safe at all doses. The RECIST 1.1 response rate for both ipilimumab-refractory and -naive patients was 25%. Median duration of response was not reached at a median of 8.1 months of follow-up. High pretreatment NY-ESO-1 and MART-1–specific CD8+ T cells were associated with progression of disease. At week 12, increased peripheral-blood T regulatory cells and decreased antigen-specific T cells were associated with progression. PD-L1 tumor staining was associated with responses to nivolumab, but negative staining did not rule out a response. Patients who experienced progression after nivolumab could respond to ipilimumab.
Conclusion
In patients with ipilimumab-refractory or -naive melanoma, nivolumab at 3 mg/kg with or without peptide vaccine was well tolerated and induced responses lasting up to 140 weeks. Responses to nivolumab in ipilimumab-refractory patients or to ipilimumab in nivolumab-refractory patients support combination or sequencing of nivolumab and ipilimumab.
INTRODUCTION
Randomized studies have established that CTLA-4 blockade with ipilimumab improves survival for patients with stage IV melanoma.1,2 Targeted therapy with vemurafenib improved survival for patients with BRAF-mutated melanoma.3,4 Although both of these drugs have received recent approval from the US Food and Drug Administration, the 5-year survival rate for patients with stage IV melanoma remains low, with less than 20% of patients surviving more than 5 years.5,6
High levels of programmed death ligand 1 (PD-L1) are expressed by melanomas and other tumors, leading to binding of the immune checkpoint molecule programmed death-1 (PD-1) on infiltrating T cells and downmodulation of antitumor immunity.7,8 PD-1–blocking antibodies were developed based on the importance of PD-1 as a checkpoint protein limiting T-cell proliferation, function, and cytokine secretion, particularly in chronic viral infections or cancer-bearing hosts.9–16 High PD-1 is found on circulating T cells and tumor-infiltrating T cells from patients with melanoma, suggesting that PD-1 abrogation may reverse cancer-associated T-cell exhaustion. Nivolumab, formerly BMS-936558/MDX-1106, is a fully human immunoglobulin G4 (IgG4) monoclonal PD-1–blocking antibody that abrogates its interaction with PD-L1 and PD-L2.
Single doses of nivolumab induced responses in patients with melanoma, colorectal cancer, and renal cell carcinoma.17 In another phase I study with nivolumab, objective responses were reported in ipilimumab-naive patients with non–small-cell lung cancer, melanoma, and renal cell cancer, with a 31% response rate in melanoma.18,19 No responses were observed in a small cohort of patients whose tumors did not express PD-L1. Blockade of PD-L1 also resulted in durable tumor regression, with a 16% response rate in patients with melanoma treated with a human IgG4 PD-L1–blocking antibody and a 28% response rate in patients treated with a chimeric IgG4 molecule.20,21 Another IgG1 PD-1 antibody is active in melanoma, and simultaneous nivolumab and ipilimumab resulted in high response rates but significant toxicity.22,23 These data highlight the potential of agents that abrogate PD-1/PD-L1 interactions in T cells.
We report the results of treatment of patients with unresectable metastatic melanoma who were ipilimumab naive (34 patients) or refractory (56 patients) and received nivolumab at 1, 3, or 10 mg/kg. Some patients also received an HLA-A*0201–restricted multipeptide vaccine. In addition to reporting on the safety, tolerability, and efficacy of this regimen, we also explored a number of immune correlates of response to this treatment and evaluated the hypothesis that PD-L1 expression in tumor tissue predicts response to nivolumab.
PATIENTS AND METHODS
Patients
Ninety patients were enrolled at Moffitt Cancer Center onto this trial approved by the University of South Florida Institutional Review Board (ClinicalTrials.gov identifier: NCT01176461). Inclusion criteria included written informed consent; age ≥ 16 years; histologic diagnosis of unresectable stage III or IV melanoma with measurable disease by RECIST 1.1; progressive disease after at least one previous systemic treatment; positive tumor staining in at least 10% of tumor cells for gp100, NY-ESO-1, and/or MART-1; Eastern Cooperative Oncology Group performance status of 0 or 1; and adequate hepatic, renal, and hematologic function. Patients were prescreened for HLA-A*0201 by allele-specific polymerase chain reaction for cohorts 1 to 5. Patients with treated brain metastases were allowed if they were radiologically stable 8 weeks after treatment; patients with untreated brain metastases were allowed in cohort 6. Any number of prior therapies was allowed; treatment with prior anti–PD-1 or anti–PD-L1 was not. The 34 patients in consecutively accrued cohorts 1 to 3 were ipilimumab naive and received nivolumab with peptide vaccine, and the 56 patients in cohorts 4 to 6 (ipilimumab refractory) had progressive disease without responding to prior ipilimumab. Cohorts 4 and 5 were consecutively accrued and received nivolumab with peptide vaccine, and cohort 6 received nivolumab alone and accrued concurrently with cohorts 4 to 5 (Table 1). Before completing one cycle, in cohorts 1 to 3, two patients experienced progression, one withdrew consent, and one developed dose-limiting optic neuritis; in cohorts 4 to 6, one patient withdrew consent. All five patients had progressive disease, were included in the analysis for safety and efficacy, and were replaced per protocol. No patients were ineligible or lost to follow-up.
Table 1.
Description of Cohorts
| Cohort | No. of Patients | Dose of Nivolumab (mg/kg) | Received Prior Ipilimumab | Received Peptide Vaccine | HLA-A*0201 Positive |
|---|---|---|---|---|---|
| Cohort 1 | 10 | 1 | No | Yes | Yes |
| Cohort 2 | 13 | 3 | No | Yes | Yes |
| Cohort 3 | 11 | 10 | No | Yes | Yes |
| Cohort 4 | 10 | 3 | Yes | Yes | Yes |
| Cohort 5 | 5 | 3 | Yes | Yes | Yes |
| Cohort 6 | 41 | 3 | Yes | No | Not required |
NOTE. For cohorts 4 and 6, only grade 1 and 2 dose-limiting toxicities for ipilimumab were allowed; cohort 5 patients were required to have had grade 3 dose-limiting toxicity for ipilimumab.
Study Design and Treatment
Nivolumab was provided by Bristol-Myers Squibb (Princeton, NJ). The gp100209-217 (210M; National Service Center [NSC] No. 683472) and MART-126-35 (27L; NSC No. 709401) peptides were provided by the Cancer Therapy Evaluation Program of the National Cancer Institute. The good manufacturing practice–grade gp100280-288 (288V; NSC No. 683473) and NY-ESO-1157-165 (165V; NSC No. 717388) peptides were produced by Clinalfa (Zurich, Switzerland). All peptides were emulsified in Montanide ISA 51 VG (Seppic, Paris, France) and were included to assess the effects of PD-1 blockade on antigen-specific T-cell reactivity. The protocol was conducted under Investigation New Drug number BB 13704, with primary end points of toxicity and tolerability and secondary correlative end points.
Assessment of Response and Adverse Effects
Tumor assessments included chest, abdomen, and pelvis computed tomography (CT) and brain magnetic resonance imaging with contrast every 12 weeks. Objective response (complete response [CR] + partial response [PR]) was evaluated using RECIST 1.1 and immune-related response criteria; immune-related response criteria were only used to determine whether patients should remain on treatment in case of a mixed response. Patients were assessed with history and physical examinations every 2 weeks for up to 24 weeks and then every 12 weeks thereafter. Leukapheresis was performed before treatment, at week 12, and at week 24 in cohorts 1 to 5.24 Patients were discontinued from treatment for progression, dose-limiting nivolumab- or vaccine-related adverse events as defined in the Appendix (online only), or withdrawal of consent.
Statistical Analysis
The primary objective of this study was to assess the safety and tolerability of nivolumab with a peptide vaccine in ipilimumab-refractory or -naive patients. The secondary objectives were to evaluate the objective response rate (ORR) and changes in immunity. Toxicity rate was calculated by using the number of patients with grade 3 or greater toxicity divided by all patients. The ORR was estimated using the number of CRs and PRs at 24 weeks divided by the total number of patients evaluable for response. Patients who were observed for at least 24 weeks were evaluable for efficacy. The stable disease rate was estimated using the number of patients with stable disease (SD) for at least 24 weeks divided by the total number of evaluable patients. Progression-free survival rate was calculated as the sum of the ORR and stable disease rate. The Clopper-Pearson method was used to estimate 95% CIs for toxicity rate and ORR. Fisher's exact test was performed to investigate whether the proportion of grade 1 or 2 infusion reactions differed between cohorts 1 to 3 and 4 to 6. To evaluate change in biomarkers after 12 weeks of treatment, a two-sided signed rank test was performed. A Wilcoxon rank sum test was performed to determine whether biomarker levels at baseline or their change after 12 weeks of treatment differed between responders and nonresponders. For tetramer data, the frequency of the negative tetramer control was subtracted from each corresponding tumor-associated tetramer to ensure that the biomarkers were comparable across batches. To adjust for multiplicity for biomarker analyses, a false discovery rate of 5% was used to declare statistical significance. For other end points, an α level of .05 was used to declare statistical significance. The binomial CIs in Table 4 were calculated using the exact Clopper-Pearson method. We used SAS 9.2 (SAS Institute, Cary, NC) and Matlab 2009b (MathWorks, Natick, MA) for the statistical analyses.
Table 4.
Efficacy
| Cohort | No. of Patients | Objective Response |
Response Duration (weeks) | Stable Disease ≥ 24 Weeks |
Progression-Free Survival Rate at 24 Weeks (%) | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | 95% CI (%) | No. | % | ||||
| Ipilimumab-naive patients | ||||||||
| Cohort 1 | 10 | 3 | 30 | 6.7 to 65.3 | 140+, 128+, 76+ | 2 | 20 | 50 |
| Cohort 2 | 13 | 4 | 31 | 9.1 to 61.4 | 84+, 36, 24, 24 | 1 | 8 | 39 |
| Cohort 3 | 11 | 1 | 9 | 0.2 to 41.3 | 84+ | 4 | 36 | 45 |
| Patients who received prior ipilimumab | ||||||||
| Cohort 4 | 10 | 3 | 30 | 6.7 to 65.3 | 60+, 60+, 60+ | 2 | 20 | 50 |
| Cohort 5 | 5 | 1 | 20 | 0.5 to 71.6 | 36+ | 2 | 40 | 60 |
| Cohort 6 | 38 | 10 | 26 | 13.4 to 43.1 | 48+, 36+, 36+, 36+ | 7 | 18 | 44 |
| All cohorts | 87 | 22 | 25 | 16.6 to 35.8 | 36+, 24+, 24+, 24+ 24+, 12+ | 18 | 21 | 46 |
RESULTS
Baseline Patient Characteristics
Between August 2010 and December 2012, 102 patients were screened, and 90 patients were enrolled. All patients were evaluated for toxicity, 87 were evaluated for response, and three were too early for response evaluation. In cohorts 1 to 3, 10 patients received 1 mg/kg, 13 patients received 3 mg/kg, and 11 patients received 10 mg/kg of nivolumab and were evaluable for efficacy and safety. Thirty patients completed cycle 1 and were evaluable for immune response, and four patients dropped out as a result of progression. In cohorts 4 and 5, 15 patients completed nivolumab at 3 mg/kg in cycle 1 and were evaluable for toxicity, efficacy, and immune response. In cohort 6, 41 patients were treated with nivolumab 3 mg/kg, and one patient dropped out as a result of progression; all patients were evaluable for safety and 37 were evaluable for efficacy. Median age was 62 years. Seventy percent of patients had American Joint Commission on Cancer M1c disease. Thirty patients received two or more prior therapies for metastatic disease. Seventy-four patients had primary cutaneous melanoma. Eight patients had ocular melanoma, and eight patients had an unknown primary. BRAF mutational status was known for 70 tumors, and 16 tumors were BRAF mutated. Three patients had experienced progression after a BRAF-targeted therapy before enrollment, and one of the three patients exhibited a PR on this trial. Ten patients had radiated brain metastases, and five additional patients in cohort 6 had untreated brain metastases. Patient characteristics at trial entry are listed in Table 2.
Table 2.
Demographic and Clinical Characteristics by Cohort
| Characteristic | No. of Patients |
|||||
|---|---|---|---|---|---|---|
| Cohort 1 (n = 10) | Cohort 2 (n = 13) | Cohort 3 (n = 11) | Cohort 4 (n = 10) | Cohort 5 (n = 5) | Cohort 6 (n = 41) | |
| Sex | ||||||
| Female | 5 | 7 | 4 | 3 | 1 | 12 |
| Male | 5 | 6 | 7 | 7 | 4 | 29 |
| Age, years | ||||||
| Mean | 66.2 | 61.5 | 58.5 | 57.8 | 67.4 | 62.1 |
| Range | 55-77 | 17-85 | 48-70 | 26-78 | 62-72 | 24-85 |
| BRAF genotype | ||||||
| V600E positive | 1 | 1 | 3 | 2 | 3 | |
| V600K positive | 1 | 1 | ||||
| Wild type | 2 | 5 | 5 | 4 | 4 | 36 |
| Not tested | 7 | 6 | 3 | 3 | 1 | 2 |
| No. of prior treatments | ||||||
| 1 | 9 | 12 | 9 | 4 | 2 | 22 |
| 2 | 1 | 1 | 2 | 6 | 3 | 14 |
| 3 | 4 | |||||
| 4 | 1 | |||||
| Stage | ||||||
| M1a | 0 | 0 | 2 | 0 | 0 | 2 |
| M1b | 6 | 0 | 2 | 0 | 2 | 5 |
| M1c | 4 | 13 | 7 | 10 | 2 | 30 |
| IIIc | 0 | 0 | 0 | 0 | 1 | 4 |
| Ocular primary | ||||||
| Yes | 3 | 1 | 1 | 0 | 1 | 2 |
| No | 7 | 12 | 10 | 10 | 4 | 39 |
Safety
Treatment-related adverse events are listed in Table 3 by cohort. The most common adverse events were fatigue in all cohorts and injection site reaction from vaccine in cohorts 1 to 5. Most events were mild to moderate in severity and easily managed by supportive treatment. In cohorts 1 to 3, one dose-limiting toxicity (DLT), grade 3 bilateral optic neuritis (at 3 mg/kg in cohort 2), resolved to baseline with a 60-mg prednisone taper over 4 weeks and topical corticosteroids. Two other patients in cohorts 1 to 3 discontinued treatment secondary to toxicity beyond the DLT period of 12 weeks. One patient had grade 3 fevers in cycle 2 that required 4 weeks of a prednisone taper from 60 mg for resolution, and one patient had grade 3 pneumonitis after completion of two cycles of therapy requiring a prednisone taper from 120 mg over 2 months for resolution. Dose-limiting colitis was not seen in this trial. In 56 patients in ipilimumab-refractory cohorts 4 to 6, one DLT (grade 3 rash) was observed in cohort 6 that resolved completely with a 6-week prednisone taper from 60 mg. One episode of grade 3 pneumonitis was observed in cohort 5, requiring prednisone tapers from 120 mg lasting 3 to 4 months for complete resolution, after the DLT period of 12 weeks. Both patients fully recovered to baseline. No other grade 3 immune-related adverse events were seen in cohorts 4 to 6. More grade 1 or 2 infusion reactions were observed in cohorts 4 to 6 (nine of 56 patients, 16%) than in cohorts 1 to 3 (one of 34 patients, 3%), although this was not statistically significant (P = .08). No patient discontinued nivolumab as a result of an infusion reaction, and no treatment-related deaths were observed.
Table 3.
Treatment-Related AEs by Cohort
| Treatment-Related AE | No. of Patients |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort 1 (1 mg/kg); HLA-A2 Positive, Ipilimumab Naive (n = 10) |
Cohort 2 (3 mg/kg); HLA-A2 Positive, Ipilimumab Naive (n = 13) |
Cohort 3 (10 mg/kg); HLA-A2 Positive, Ipilimumab Naive (n = 11) |
Cohort 4 (3 mg/kg); HLA-A2 Positive, Ipilimumab Refractory (n = 10) |
Cohort 5 (3 mg/kg); HLA-A2 Positive, Ipilimumab Refractory (grade 3 AE; n = 5) |
Cohort 6 (3 mg/kg); No Vaccine, HLA Unrestricted, Ipilimumab Refractory (n = 41) |
|||||||
| All AEs | Grade 3/4 | All AEs | Grade 3/4 | All AEs | Grade 3/4 | All AEs | Grade 3/4 | All AEs | Grade 3/4 | All AEs | Grade 3/4 | |
| Fatigue | 9 | 0 | 13 | 0 | 7 | 0 | 4 | 0 | 2 | 0 | 23 | 0 |
| Nausea | 4 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 3 | 0 |
| Diarrhea | 6 | 0 | 2 | 0 | 5 | 0 | 1 | 0 | 1 | 0 | 9 | 0 |
| Pruritus | 5 | 0 | 1 | 0 | 6 | 0 | 6 | 0 | 1 | 0 | 13 | 0 |
| Injection site reaction | 9 | 0 | 10 | 0 | 11 | 0 | 5 | 0 | 4 | 0 | 0 | 0 |
| Dry mouth | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 |
| Mouth sores | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rash | 4 | 0 | 1 | 0 | 4 | 0 | 6 | 0 | 4 | 0 | 17 | 2 |
| Pain, injection site | 4 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | NA | NA |
| Arthralgias | 2 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 0 | 6 | 0 |
| Chills | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 0 |
| Vomiting | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Periorbital edema | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Headache | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| Optic neuritis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fevers | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| Vitiligo | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Interstitial pneumonitis | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Hypothyroidism | 0 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 3 | 0 |
| Hypopituitarism | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Flatulence | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Night sweats | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Thyroiditis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Infusion reaction | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 3 | 0 | 3 | 0 |
| 95% CI, % | NA | 0.2 to 35 | NA | 0.5 to 71.6 | 0.1 to 12.9 | 0.7 to 9.4 | ||||||
Abbreviations: AE, adverse event; NA, not applicable.
Clinical Activity
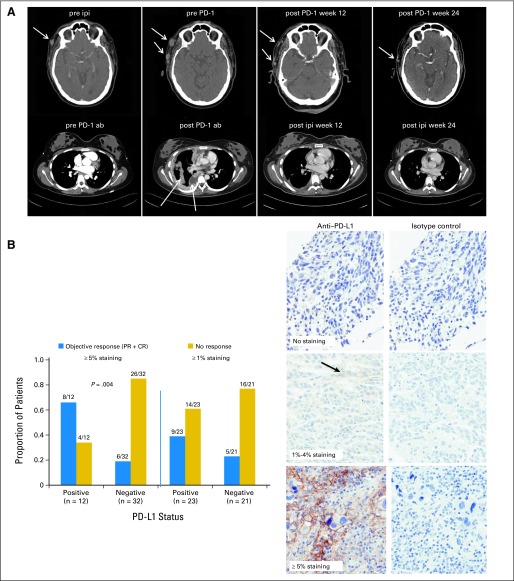
Median follow-up times were 20.3 months for patients in cohorts 1 to 3, 6.8 months for cohorts 4 to 6, and 8.1 months for all patients. Confirmed responses and durations of response for ipilimumab-naive patients in cohorts 1 to 3 (n = 34) are listed in Table 4. Two patients (6%) had CRs, six patients (18%) had PRs, seven patients (21%) had SD at 24 weeks, and 19 patients (55%) had progressive disease. The confirmed CR+PR rate for cohorts 1 to 3 was 24%, with a disease control rate (CR+PR+SD) of 45%. In responders, at a median follow-up of 21.2 months, median duration of response was not reached (range, 24 to 140+ weeks). Responses and durations of response for evaluable ipilimumab-refractory patients in cohorts 4 to 6 (n = 53) are listed in Table 4. Fourteen patients had PRs (26%, with 13 confirmed), 11 had SD at 24 weeks (21%), and 28 (53%) experienced progression. The CR+PR rate was 26%, with a disease control rate of 47%. In responders, at a median follow-up of 8.4 months, the median duration of response was not reached (range, 12+ to 60+ weeks). For all 87 evaluable patients, the CR+PR rate was 25%, with a disease control rate of 46%. In Figure 1A, representative CT scans in the top row, left to right, are shown of a patient before ipilimumab, after ipilimumab (progression with multiple neck lesions at the arrow), after the first cycle of nivolumab at week 12 (regression), and after the second cycle at week 24 (free of disease).
Fig 1.
(A) The upper row shows computed tomography (CT) scan images of the neck of a patient in cohort 4 who received ipilimumab at 3 mg/kg (pre ipi) and who experienced progression at week 12 with multiple subcutaneous lesions shown at the arrows (pre PD-1). Regression of disease occurred after six doses of nivolumab at 3 mg/kg at week 12 (post PD-1 week 12) and further regression at week 24 (post PD-1 week 24). The lower row shows CT scan images of the thorax of a patient in cohort 2 who received nivolumab 1 mg/kg (pre PD-1 ab) at baseline and who experienced progression at week 12 with increased pleural disease shown at the arrows (post PD-1 ab). There was significant regression of pleural and lung disease after four doses of ipilimumab at 3 mg/kg off protocol at week 12 (post ipi week 12) and further regression at week 24 (post ipi week 24). (B) Bar graphs indicating proportion of patients that are responders (blue bars) or nonresponders (yellow bars) using positive staining cutoffs of 1% or 5% tumor cells with membranous staining of 100 tumor cells counted to define programmed death-ligand 1 (PD-L1) status. Examples of zero staining and ≥ 1% and ≥ 5% positive staining of tumor cells are also shown in the near right column of three micrographs, with corresponding control images with isotype control staining shown in the far right column of three micrographs. Results for a total of 44 patients with available specimens are shown. CR, complete response; PD-1, programmed death-1; PR, partial response.
Immunohistochemical Staining for PD-L1
Forty-four patients in cohorts 1 to 6 had pretreatment tumors available for PD-L1 staining; 12 (27%) of 44 patients were positive when defined as greater than 5% membranous staining, and 23 (52%) of 44 patients were positive when defined as greater than 1% staining. The ORR (CR+PR) was 67% (eight of 12 patients) in the 5% positive group, whereas for patients with negative staining, the ORR was 19% (six of 32 patients). The ORR was 39% (nine of 23 patients) in the 1% positive group, whereas for patients with negative staining, it was 23% (five of 21 patients; Fig 1B, left). Representative stains of negative, ≥ 1% positive, and ≥ 5% positive specimens are shown in Figure 1B, with isotype control-stained images on the right. These data show a significant association between PD-L1 staining and response using an automated assay at the 5% membranous staining level (P = .004). Because patients with negative staining by either definition could respond to nivolumab, PD-L1 staining could not be used to accurately select patients for nivolumab treatment.
Immune Biomarkers
Thirty-seven patients in cohorts 1 to 5 had pre- and postleukapheresis specimens, and 48 patients had preleukapheresis specimens available for analyses of T-cell biomarkers. At baseline, antigen-specific CD8+ T cells that bind tetramers for NY-ESO-1157-165 and MART-126-35 were significantly lower in responders and stable patients compared with nonresponders (P < .001 and P = .003, respectively; Fig 2A and 2B). CD8+ T cells binding tetramers for MART-126-35 increased in responders and stable patients at 12 weeks but decreased significantly in nonresponders (P = .005; Fig 2C). The difference in the change of MART-126-35 T cells between responders and stable patients and nonresponders was not statistically significant after adjusting for multiple comparison (P = .002, q = 0.098). A representative gating strategy for tetramer staining analysis is shown in Appendix Figure A1 (online only).14 Regulatory T cells (Tregs; defined as CD4+CD25+CD127lowFoxP3+) decreased in responders and stable patients and significantly increased in nonresponders at 12 weeks (P = .01; Fig 2D).
Fig 2.
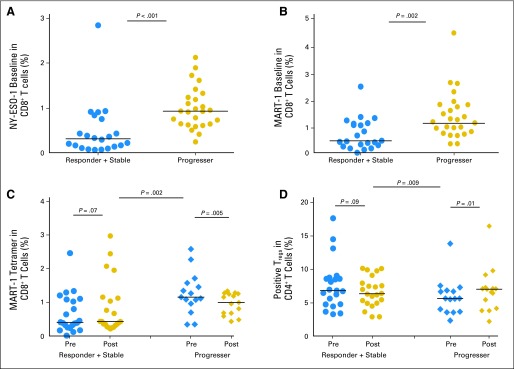
Baseline tetramer staining of CD8+ (A) NY-ESO-1157-165– and (B) MART-126-35–specific T cells as a percentage of total CD8 T cells on the ordinate, and response plus stable disease versus progression on the abscissa for 45 patients. (C) Changes from baseline to week 12 in tetramer staining of MART-126-35–specific T cells as a percentage of total CD8 T cells on the ordinate, with responder plus stable disease versus progression on the abscissa for 37 patients. (D) Dot plots showing CD4+/CD25+/CD127lowFoxP3+ T regulatory cells (Tregs) as a percentage of total CD4 T cells on the ordinate, with responder plus stable disease versus progression on the abscissa.
Treatment With CTLA-4 Blockade After Progression on Nivolumab
An unplanned analysis was performed on patients who received ipilimumab after progression in cohorts 1 to 3. Twelve of the 18 nonresponders subsequently received ipilimumab at 3 mg/kg for a planned four doses. Two patients had PRs (48 weeks and 84+ weeks). In Figure 1A, representative CT scans in the bottom row, left to right, are shown of a patient before nivolumab, after nivolumab (progression in pleural disease, see arrow), after four doses of ipilimumab at week 12 (regression), and at week 24. Two mixed responses were observed, and eight patients had progressive disease. Two of the 12 patients required corticosteroids and infliximab for dose-limiting colitis with ipilimumab.
DISCUSSION
This study demonstrates the clinical activity of nivolumab with or without a multipeptide vaccine in ipilimumab-refractory or -naive metastatic melanoma. One quarter of patients had objective responses in this pretreated population, albeit in a heterogenous population at different doses of nivolumab. Durability of response is noteworthy, with all but four RECIST responders of 22 remaining on study. Five patients stopped treatment secondary to toxicity. All patients successfully returned to baseline status with systemic corticosteroids. Three of those patients experienced progression, and two sustained a partial response without further therapy, suggesting no clear association of corticosteroid-requiring immune-related adverse events with response. Disease regression was observed as early as 5 weeks after initiating treatment. Responses were seen in patients with both BRAF wild-type and BRAF V600E-mutated melanomas and in those with untreated brain metastases. The addition of the peptide vaccine did not impact clinical efficacy with PD-1 blockade, although it provided a tool for immune monitoring of T-cell responses.
All dose levels were safe and tolerable, with only one DLT at 3 mg/kg with vaccine (cohort 2). Nivolumab, with and without peptide vaccination, was safe at the doses and schedule used in ipilimumab-refractory or -naive patients. Our data suggest that treatment with nivolumab was well tolerated in patients who experienced progression after ipilimumab. Notably, in five patients in cohort 5 who had prior grade 3 immune-related adverse events with ipilimumab, none of the DLTs were recapitulated after nivolumab.
A number of pretreatment and pharmacodynamic immune biomarkers were associated with response or progression in this study. Increased Treg frequency at week 12 was associated with progression. These data are consistent with reports of high levels of Tregs in patients with melanoma who experience progression after other forms of immunotherapy.20 CD4+CD25+FoxP3+ Tregs derived from peripheral blood suppress the generation of melanoma-specific cytotoxic T lymphocytes, and CTLA-4 expression on Tregs is important for their suppressive activity.25–27 PD-1 blockade has been shown to promote melanoma antigen-specific CTL generation and attenuate the inhibitory effect of Tregs.27,15 High pretreatment tetramer staining on CD8+ T cells for NY-ESO-1 or MART-1 antigens was associated with progression. We hypothesize that the high baseline levels of tumor antigen–reactive T cells observed in patients who experienced progression may be dysfunctional and unable to respond to PD-1 abrogation as a result of expression of other inhibitory checkpoint proteins.28–32 Assessment of the tetramer-positive T cells from responders and those who experienced progression is ongoing. An association was observed between pretreatment tumor expression of PD-L1 and response using a cutoff of more than 5% membranous staining (P = .004), but not with a cutoff of more than 1%. The utility of this biomarker for patient selection was unclear because both ipilimumab-refractory and -naive patients with negative staining still responded to nivolumab. Our data differ from those of Topalian et al,18 but we used a different antibody and an automated staining system. PD-L1 expression co-localizes with infiltrating lymphocytes and may be associated with secretion of interferon gamma, so its induction may depend on the dynamic ratio of effector to regulatory cells within tumors that may vary over time.33 Ipilimumab can induce an influx of CD8 T cells, which might augment PD-L1 expression,34 but tumors from ipilimumab-refractory patients in our study did not have a higher frequency of PD-L1 expression than those from ipilimumab-naive patients. The utility of PD-L1 staining as a predictive biomarker needs to be further explored in large randomized studies.
Ipilimumab-refractory patients had a clinically meaningful response rate and tolerated nivolumab safely, supporting further testing of nivolumab at 3 mg/kg in patients who experienced progression after receiving ipilimumab, even with grade 3 immune-related adverse events. The peptide vaccination did not add to the clinical activity of nivolumab, although induction of antigen-specific T-cell responses may be associated with clinical response.35 The peripheral-blood biomarkers explored herein require further study. Determination of the optimal sequencing or simultaneous combination of these two immune checkpoint inhibitory therapies will be required to maximize patient benefit.
Supplementary Material
Acknowledgment
The outstanding assistance of the Cancer Therapy Evaluation Program of the National Cancer Institute is acknowledged, particularly Dr Howard Streicher. We are grateful to Joyce Lampasona, Rasa Hamilton, and Amber Isley who provided exemplary administrative support, to Kate Shapland and the Moffitt Flow Cytometry Core for their tireless dedication, and to Drs James Mulé, Christian Poehlein, David Feltquate, and Arvin Yang who read and commented on the final article.
Appendix
Description of Cohorts and Treatment
Cohort 1 was treated at 1 mg/kg; cohorts 2, 4, 5, and 6 were treated at 3 mg/kg; and cohort 3 was treated at 10 mg/kg of nivolumab. Patients on cohorts 4 and 6 were ipilimumab refractory and could have had grade 1 or 2 toxicity, but patients on cohort 5 could have had grade 3 dose-limiting toxicity (DLT) after ipilimumab without requiring infliximab. Patients on cohorts 4 and 5 were HLA-A*0201 positive and received peptide vaccine; cohort 6 was an extension group totaling 41 patients who were not required to be HLA-A*0201 positive and did not receive peptide vaccine, as shown in Table 1. Each treatment cycle (12 weeks) comprised six doses of nivolumab and peptide vaccine administered every 2 weeks. Patients received two cycles with response assessments at 12 and 24 weeks. After 24 weeks, nivolumab alone was administered for those with stable or regressing disease every 12 weeks for up to 2 years.
Definition of Tolerability
Groups of 10 patients were planned for cohorts 1 to 5, and in those cohorts, DLT seen in two or more of the first six patients or three or more of 10 patients was defined as beyond the maximum-tolerated dose for that dose and cohort and would be felt to be intolerable.
Toxicity
Adverse events were graded and recorded according to Common Terminology Criteria for Adverse Events, version 4.0. DLT was defined as any grade 3 or 4 adverse event or grade 2 or greater ocular adverse event within 12 weeks of treatment. Grade 3 infusion reactions were exempt from the DLT definition. All grade 3 immune-related adverse events that resolved to grade 1 or less within 28 days were exempt from DLT definition with the exception of pancreatitis; ocular, hepatic, and endocrine toxicities; and colitis.
Flow Cytometry
Peripheral-blood mononuclear cells were collected by leukapheresis as previously described.24 Functional and phenotypic markers of T cells were evaluated by flow cytometry using antibodies from BD Biosciences (San Jose, CA), except where indicated. Peripheral-blood mononuclear cells were stained with Live/Dead violet dye (Invitrogen, Carlsbad, CA) to gate on live cells. After this, cells were assessed for expression of CD45, CD3, CD4, CD8, PD-1, and CTLA-4. Nivolumab was found in control experiments to not interfere with binding of the PD-1–specific antibody (MIH4 from eBioscience, San Diego, CA) to the PD-1 receptor on T cells. T regulatory cells (Tregs) were characterized by staining for T cells that were CD4+CD25+CD127lowFoxP3+ (eBioscience). For tetramer analyses, a dump gate was used that excluded CD14+, CD19+, and CD56+ cells.24 Antigen-specific CD8+ T cells were assessed with tetramers staining for gp210 (wild type and 210M), MART-1 (wild type and 27L), and NY-ESO-1 (wild type and 165V; Beckman Coulter, Brea, CA). A negative tetramer control was included for every sample. Data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed with Flowjo software (TreeStar, Ashland, OR). Data were analyzed by the principal investigator, J.S.W., and biostatisticians X.Z. and Y.A.C.
Immunohistochemistry
Immunohistochemical staining of paraffin-embedded sections for gp100, MART-1, and NY-ESO-1 was performed on archived formalin-fixed paraffin-embedded pretreatment tumor specimens as previously described.19 The immunohistochemistry assay for programmed death-ligand 1 (PD-L1) incorporated an anti–PD-L1 rabbit monoclonal antibody (clone 28-8), which was developed on an automated platform by Dako North America (Carpinteria, CA). Consecutive sections were stained for PD-L1 and a negative control reagent to control for nonspecific staining. All sections were independently read by two pathologists, with final scores confirmed through an adjudication process. A sample was deemed PD-L1 positive for membranous staining by two alternative definitions: if ≥ 5% or 1% of tumor cells, in a minimum of 100 evaluable tumor cells, had observable PD-L1–positive staining at any intensity. Specificity of the 28-8 antibody clone was assessed by western blotting against recombinant PD-L1 protein and lysates from PD-L1–expressing cell lines, as well as cell lines that do not express PD-L1. In addition, immunohistochemistry assays with antigen competition and assessing of the staining pattern in a panel of 30 normal human tissues demonstrated the expected pattern for specific PD-L1 binding.
Fig A1.
Gating strategy and representative tetramer staining profile for NY-ESO-1157-165 antigen-specific T cells. Percent positive staining as defined in Patients and Methods by flow cytometry is shown on the ordinates, and mean fluorescence intensity is shown on the abscissas. Initial gating was on forward scatter (FSC) and side scatter (SSC) and then on CD45+ T lymphocytes; dead cells were then gated out, as were CD4+, CD14+, CD19+, and CD56+ cells, with gating then on CD3+CD8+ T cells that were tetramer specific. “A” and “W” represent two channels on the flow cytometer. APC-A, allophycocyanin-A; FITC-A, fluorescein isothyocyanate–A; PerCP, peridinin-chlorophyll proteins.
Footnotes
Supported by National Cancer Institute Grant No. RO1 CA 129594-01A2, the Donald A. Adam Comprehensive Melanoma Research Center, and Bristol-Myers Squibb.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT01176461.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Christine E. Horak, Bristol-Myers Squibb (C); H. David Inzunza, Bristol-Myers Squibb (C) Consultant or Advisory Role: Jeffrey S. Weber, Bristol-Myers Squibb (C), Merck (C), Genentech (C); Ragini Reiney Kudchadkar, Bristol-Myers Squibb (C), Genentech (C) Stock Ownership: Christine E. Horak, Bristol-Myers Squibb Honoraria: Jeffrey S. Weber, Bristol-Myers Squibb, Merck, Genentech; Ragini Reiney Kudchadkar, Bristol-Myers Squibb Research Funding: Jeffrey S. Weber, Bristol-Myers Squibb, Merck, Genentech Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey S. Weber, Jodi Kroeger
Collection and assembly of data: Jeffrey S. Weber, Ragini Reiney Kudchadkar, Bin Yu, Donna Gallenstein, Christine E. Horak, H. David Inzunza, Alberto J. Martinez, Geoffrey Gibney, Jodi Kroeger, Cabell Eysmans, Amod A. Sarnaik
Data analysis and interpretation: Jeffrey S. Weber, Ragini Reiney Kudchadkar, Bin Yu, Christine E. Horak, Xiuhua Zhao, Wenshi Wang, Geoffrey Gibney, Jodi Kroeger, Amod A. Sarnaik, Y. Ann Chen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 6.Lebbe C, Weber JS, Maio M, et al. Five-year survival rates for patients (pts) with metastatic melanoma (mm) treated with ipilimumab (ipi) in phase II trials. 37th European Society for Medical Oncology 2012 Congress; September 28-October 2, 2012; Vienna, Austria. (abstr 1116) [Google Scholar]
- 7.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–4836. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7–H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwai Y, Terwaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 14.Wong RM, Scotland RR, Lau RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Lau R, Yu D, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter L, Fouser LA, Jussif J, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 17.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2453. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sznol M, Kluger HM, Hodi FS, et al. Survival and long-term follow-up of safety and response in patients (pts) with advanced melanoma (MEL) in a phase I trial of nivolumab (anti-PD-1; BMS-936558;ONO-4538) J Clin Oncol. 2013;31(suppl):549s. abstr CRA9006. [Google Scholar]
- 20.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid O, Sosman JA, Lawrence DP, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) J Clin Oncol. 2013;31(suppl):550s. abstr 9010. [Google Scholar]
- 22.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamid O, Solomon JC, Scotland R, et al. Alum with interleukin-12 augments immunity to melanoma peptide vaccine: Correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res. 2007;13:215–222. doi: 10.1158/1078-0432.CCR-06-1450. [DOI] [PubMed] [Google Scholar]
- 25.Yao X, Ahmadzadeh M, Lu YC, et al. Levels of peripheral CD4(+)FoxP3(+) regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood. 2012;119:5688–5696. doi: 10.1182/blood-2011-10-386482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Read S, Greenwald R, Izcue A, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2011;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fourcade J, Sun Z, Pagliano O, et al. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JY, Zhang Z, Wang X, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressor but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 32.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarhini A, Edington H, Butterfield LH, et al. Neoadjuvant ipilimumab in locally/regionally advanced melanoma: Clinical outcome and biomarker analysis. J Clin Oncol. 2012;(suppl 30):30. abstr 76. [Google Scholar]
- 35.Sarnaik AA, Yu B, Yu D, et al. Extended dose ipilimumab with a peptide vaccine: Immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi: 10.1158/1078-0432.CCR-10-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.