Abstract
Purpose
Local failure rates after radiation therapy (RT) for locally advanced non–small-cell lung cancer (NSCLC) remain high. Consequently, RT dose intensification strategies continue to be explored, including hypofractionation, which allows for RT acceleration that could potentially improve outcomes. The maximum-tolerated dose (MTD) with dose-escalated hypofractionation has not been adequately defined.
Patients and Methods
Seventy-nine patients with NSCLC were enrolled on a prospective single-institution phase I trial of dose-escalated hypofractionated RT without concurrent chemotherapy. Escalation of dose per fraction was performed according to patients' stratified risk for radiation pneumonitis with total RT doses ranging from 57 to 85.5 Gy in 25 daily fractions over 5 weeks using intensity-modulated radiotherapy. The MTD was defined as the maximum dose with ≤ 20% risk of severe toxicity.
Results
No grade 3 pneumonitis was observed and an MTD for acute toxicity was not identified during patient accrual. However, with a longer follow-up period, grade 4 to 5 toxicity occurred in six patients and was correlated with total dose (P = .004). An MTD was identified at 63.25 Gy in 25 fractions. Late grade 4 to 5 toxicities were attributable to damage to central and perihilar structures and correlated with dose to the proximal bronchial tree.
Conclusion
Although this dose-escalation model limited the rates of clinically significant pneumonitis, dose-limiting toxicity occurred and was dominated by late radiation toxicity involving central and perihilar structures. The identified dose-response for damage to the proximal bronchial tree warrants caution in future dose-intensification protocols, especially when using hypofractionation.
INTRODUCTION
For patients with locally advanced non–small-cell lung cancer (NSCLC), radiation therapy (RT) continues to be the backbone of treatment. Randomized trials through the Radiation Therapy Oncology Group (RTOG), in the era before modern imaging and treatment planning methods, demonstrated improved local control with increasing doses of RT and established a benchmark dose of 60 Gy using conventional fractionation.1 A dose of 69.6 Gy in twice daily treatments was shown to be superior to lower doses in a phase I/II study,2 but was not significantly better in a randomized comparison with 60 Gy.3 In most of the randomized concurrent chemotherapy trials, patients were treated to doses of 60 to 66 Gy but nevertheless still experienced local failure rates of approximately 30%.4 Given these suboptimal results, strategies of treatment intensification to improve outcomes continue to be explored.
Retrospective and nonrandomized prospective data have suggested that further dose escalation in NSCLC may be associated with better outcomes.5–8 However, for patients with locally advanced disease, the benefit of dose escalation beyond 60 Gy using conventional fractionation has not been supported by level I evidence. A recent randomized study by the RTOG in patients with locally advanced NSCLC showed worse survival rates for patients receiving 74 Gy versus 60 Gy with concurrent chemotherapy.9
As an alternative to pure dose escalation, accelerated treatment schedules may allow for improved outcomes by shortening overall treatment time, as demonstrated in the Continuous Hyperfractionated Accelerated Radiotherapy trial.10 However, such highly fractionated dose-intensification schedules present extraordinary logistical challenges. Alternatively, hypofractionation can be used to shorten radiation treatment time more conveniently.11
To investigate the safety and feasibility of treatment intensification through hypofractionation in a broad patient population, we initiated a phase I clinical trial of dose-per-fraction escalation for patients with NSCLC. Given concern for increased toxicity with hypofractionated RT and concurrent chemotherapy, patients were allowed sequential but not concurrent chemotherapy. Intensity-modulated radiotherapy (IMRT) was used to minimize high doses received by normal structures. Radiobiological modeling suggested limiting treatment time to no more than 5 weeks (25 fractions),12 with dose-per-fraction (and total dose) escalation guided by normal tissue complication probability (NTCP) modeling for pneumonitis risk. Preliminary results have been presented13 and were negative for grade 3 or greater pneumonitis or grade 4 or greater esophagitis, the protocol-defined primary and secondary dose-limiting toxicities (DLTs), respectively. Now, with more mature results we report on other DLTs, particularly late effects, with supportive dose-response data.
PATIENTS AND METHODS
Eligibility Criteria
This prospective single-institution phase I study was approved by the local institutional review board and was monitored by an independent data safety committee. Patients were required to be age 18 years or older, with an Eastern Cooperative Oncology Group performance status of 0 to 2 and a histologically or cytologically proven primary lung cancer for which full-dose radiation therapy was recommended (eg, a conventional dose of at least 60 Gy in 6 weeks). Because this was a phase I trial, all stages were eligible, including patients with recurrent tumors. Exclusion criteria included concurrent chemotherapy, previous thoracic RT, previous chemotherapy with bleomycin or gemcitabine, and regular use of supplemental oxygen. The remaining eligibility criteria have been published previously.13 All patients were required to provide informed consent at the time of enrollment according to institutional and federal guidelines.
Treatment
Protocol treatment consisted of IMRT over a course of 25 once-per-day fractions given 5 days per week as described previously.13 Greater than 95% of fractions were delivered via helical tomotherapy. The gross tumor volume consisted of gross disease as seen on computed tomography (CT) and/or fluorodeoxyglucose positron emission tomography imaging. No elective nodal irradiation was performed. A four-dimensional computed tomography scan was used to account for tumor motion and define an internal target volume. A clinical target volume margin of 6 mm accounted for microscopic disease, with an additional 2-mm margin for set-up uncertainty to create the planning target volume (PTV). Dose was prescribed such that 95% of the PTV volume received the prescription dose.
The prescribed dose was assigned using a Bayesian dose-escalation scheme incorporating predicted risk for pneumonitis and respecting additional normal tissue constraints (Appendix [online-only]).12,13 After an initial pilot phase of five patients, subsequent patients were grouped into one of five dose-escalation bins according to rNTDmean, defined as mean fraction-normalized lung dose divided by the normalized prescription dose. At a given dose level, patients with higher rNTDmean (and therefore higher bin number) were predicted to be at higher risk for radiation pneumonitis. Dose was escalated in parallel within each bin during the trial as described in the Appendix.
The dose levels shared by all bins were as follows: 57 Gy at 2.28 Gy/fraction (bins 4 and 5 starting dose), 63.25 Gy at 2.53 Gy/fraction (bin 3 starting dose), 69.25 Gy at 2.77 Gy/fraction, 75 Gy at 3.0 Gy/fraction (bin 2 starting dose), 80.5 Gy at 3.22 Gy/fraction (bin 1 starting dose), and 85.5 Gy at 3.42 Gy/fraction. Using the linear-quadratic conversion, the estimated equivalent doses for late effects at 2 Gy per fraction for these dose levels were estimated as 60, 70, 80, 90, 100, and 110 Gy, respectively (assuming an α/β ratio of 3 Gy).12 RT was delivered using daily CT-based image guidance.
According to protocol, the maximum-tolerated dose (MTD) of each bin was defined as the maximum dose level at which a ≤ 20% risk of DLT was identified for that bin. As per the iterative Bayesian design, the study would continue to accrue to all bins with further up/down dose adjustments until the target accrual of 75 evaluable patients was met. Evaluable patients were defined as those who completed more than 80% of the prescribed RT course and returned for at least one toxicity assessment. Patients accrued to a given bin were eligible for dose escalation beyond a given dose level if at least three patients within the bin (or in bins with larger rNTDmean values) had been previously treated at that dose and received follow-up for 3 months without evidence of DLT.
Follow-Up and Data Collection
Patients were seen once per week during the course of their radiotherapy treatments and received a chest CT and follow-up examination 1 month after radiotherapy was completed. Repeat imaging and clinical follow-up was then performed every 3 to 4 months for the first 3 years and every 6 months thereafter. For patients who died in the absence of progressive disease, medical records were reviewed and/or the next of kin were contacted to ascertain the cause of death.
Definitions and Statistical Analysis
The primary end point of this study was grade 3 pneumonitis as defined by the National Cancer Institute Common Toxicity Criteria for Adverse Events version 3.0, with the additional requirement of radiographic findings consistent with radiation pneumonitis. All other toxicities were coded according to National Cancer Institute Common Toxicity Criteria for Adverse Events criteria. Late toxicities were defined as those occurring more than 90 days from the start of treatment. In the presence of uncertainty regarding the attribution of a toxicity (eg, disease progression versus treatment), the toxicity was included in the analysis if the consensus of the institutional safety monitoring committee was that the toxicity was at least possibly related to protocol treatment. DLT was defined as grade 3 or greater radiation pneumonitis, grade 4 or greater acute esophagitis, or any other grade 3 or greater toxicity. Local failure was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria.14
Local control was analyzed with death as a competing risk and cumulative incidences were compared using Gray's test.15 Risk factors for radiation pneumonitis were assessed by logistic regression using a generalized linear model. The observed rate of pneumonitis was compared with that predicted by NTCP modeling using a χ2 test for goodness of fit. Overall survival and grade 4 to 5 toxicity estimates were calculated using the Kaplan-Meier method with time-to-event defined from the start date of radiation therapy and patients censored at the time of last follow-up (or death from disease or intercurrent illness in the case of the toxicity analysis). Univariate analyses for censored toxicity data were conducted using the log-rank test for categoric variables and a Cox proportional hazards model for continuous variables.
For analysis of radiation dosimetry, the variable DXcc was defined as the largest dose (in Gy) such that X cm3 of a structure received greater than or equal to that dose. VX was defined as the volume percentage of a structure that received greater than or equal to dose X (in Gy). Dmax was defined as the maximum voxel dose within an organ. NTCP curves as a function of dose were calculated using point estimates at 2 years generated from the Cox proportional hazards model while varying the dose parameter. The NTCP curves were checked by plotting point estimates of toxicity incidences for dose quartiles centered at the quartile means. All statistical analyses were performed using R version 2.13.1 with the cmprsk package (The R Foundation for Statistical Computing, www.r-project.org).
RESULTS
Patients
A total of 79 patients were enrolled onto the trial from 2004 to 2010 and a summary of patient and treatment characteristics is listed in Table 1. After five patients were treated on the pilot study at a dose of 57 Gy without DLT, dose escalation proceeded as per protocol. Dose escalation was limited in 44 patients (56%), who had to be assigned to lower dose levels because of their inability to meet normal tissue constraints, usually in the esophagus. There was a trend for better performance status to be correlated with higher prescribed dose (P = .054). Larger PTV size was associated with higher bin number (P = .001) and lower prescribed dose (P = .003). The median delivered dose was 57 Gy (range, 22.8 to 85.5 Gy) in 25 fractions. Patients not eligible for per-protocol analysis included two who did not receive at least 80% of the prescribed course of radiotherapy and two patients treated to a prescribed 57 Gy but who were lost to follow-up after completing treatment. None of the excluded patients had grade 3 or greater toxicity attributable to treatment.
Table 1.
Patient and Treatment Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Total patients | 79 | 100 |
| Age, years | ||
| < 50 | 6 | 9 |
| 50-69 | 47 | 59 |
| ≥ 70 | 26 | 32 |
| Sex | ||
| Female | 33 | 42 |
| Male | 46 | 58 |
| Stage | ||
| I/II | 7 | 9 |
| IIIA | 21 | 27 |
| IIIB | 35 | 44 |
| IV | 10 | 13 |
| Recurrent | 6 | 8 |
| Histology | ||
| Adenocarcinoma | 24 | 30 |
| Squamous cell carcinoma | 26 | 33 |
| NSCLC, NOS | 22 | 28 |
| Other NSCLC | 7 | 9 |
| Bin assignment, rNTDmean | ||
| Pilot (NA) | 5 | 6 |
| 1 (0.00-0.119) | 6 | 7 |
| 2 (0.12-0.179) | 8 | 10 |
| 3 (0.18-0.239) | 27 | 34 |
| 4 (0.24-0.309) | 29 | 37 |
| 5 (0.31-0.410) | 4 | 5 |
| Performance status | ||
| 0 | 48 | 61 |
| 1 | 30 | 38 |
| Not recorded | 1 | 1 |
| Prescribed radiation dose, Gy | ||
| 57 | 47 | 59 |
| 63.25 | 11 | 14 |
| 69.25 | 3 | 4 |
| 75 | 12 | 16 |
| 80.5 | 4 | 5 |
| 85.5 | 2 | 3 |
| Chemotherapy | ||
| Neoadjuvant | 17 | 21 |
| Adjuvant | 33 | 41 |
| Both | 3 | 4 |
| None | 25 | 32 |
| Other* | 1 | 1 |
Abbreviations: NA, not applicable; NSCLC, non–small-cell lung cancer; NOS, not otherwise specified; rNTDmean, mean normalized tissue (lung) dose divided by normalized prescription dose.
Patient received erlotinib before, during, and after radiation.
Survival and Local Control
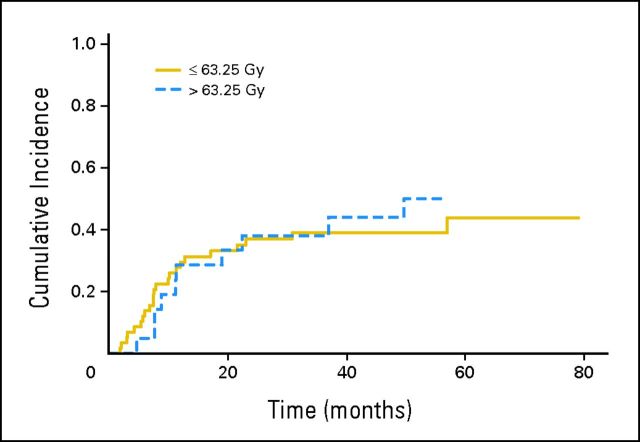
With a median follow-up period of 17.0 months (range, 2.3 to 79 months) from the time of treatment, the median overall survival rate was 16.0 months with a 3-year overall survival of 29%. For patients with a performance status of 0 who received sequential chemotherapy, median survival was 23.6 months. When this article was written, 15 patients were alive with a median follow-up of 49 months. As shown in Figure 1, there was no significant difference in local control in patients treated to 69.25 Gy and higher compared with those treated at the 57 and 63.25 Gy dose levels (P = .81).
Fig 1.
Cumulative incidence of local failure according to total dose delivered (P = .81), with death as a competing risk.
Toxicity
Of the 75 patients evaluable for per-protocol toxicity analysis, there were no instances of grade 3 or higher acute or late esophageal toxicities; 48% of patients (n = 36) experienced grade 2 acute esophagitis and 28% (n = 21) had grade 2 late esophageal toxicity. One patient developed a grade 2 sensory brachial plexopathy 1 year after completing RT to a dose of 57 Gy to a malignant right axillary lymph node and a right upper-lobe tumor invading the chest wall.
There were no incidents of grade 3 or greater radiation pneumonitis according to protocol definitions; 12 of the patients (16%) experienced grade 2 pneumonitis. This was not significantly different from the 17% risk predicted by the NTCP model (P = .73). The PTV volume, residual lung volume receiving at least 5 Gy (V5), V10, and mean residual lung dose were all significant predictors of grade 2 pneumonitis on univariate analysis, but total dose was not (Appendix).
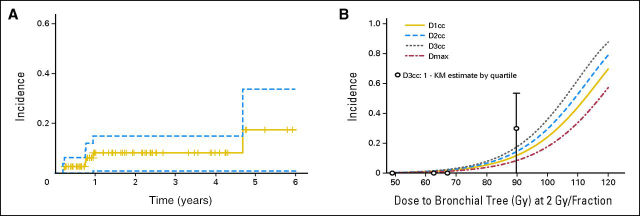
There were six incidents of grade 4 to 5 toxicity identified that were felt to be possibly or likely related to RT (Table 2 and Fig 2A). One patient treated to a dose of 63.25 Gy died as a result of progressive hypoxemia with bilateral lung infiltrates in the absence of disease progression 1 month after RT. A diagnosis of herpes simplex virus and cytomegalovirus pneumonitis was made by bronchoalveolar lavage. A second patient died of a lung abscess 1.6 months after completing RT to a dose of 75 Gy. Three other patients treated to doses of 75 to 85.5 Gy died as a result of massive hemoptysis 8 or more months after treatment. One of these patients had an endobronchial stent placed before RT. A postmortem examination revealed an arteriole that had bled into a necrotic perihilar lung cavity corresponding to the PTV and adjacent to the previously placed stent. The other two patients were without radiographic evidence of disease recurrence at the time of death. One patient experienced a grade 4 bronchocavitary fistula 10 months after receiving a dose of 75 Gy. A stent was placed, which was followed by development of a distal tracheoesophageal fistula.
Table 2.
Patients With Grade 4-5 Toxicity
| Age (years) | Sex | Stage | Bin | Dose (Gy)* | Grade | Interval (months)† | Toxicity |
|---|---|---|---|---|---|---|---|
| 69 | M | IIIB | 3 | 63.25 | 5 | 1.2 | HSV/CMV pneumonitis; history of pre-RT low-dose methotrexate |
| 66 | F | IIA | 1 | 85.5 | 5 | 55 | Fatal hemoptysis |
| 58 | M | IIIB | 3 | 75 | 5 | 7.9 | Fatal hemoptysis |
| 63 | M | IIIB | 1 | 75 | 5 | 1.6 | Lung abscess |
| 62 | M | IIIA | 3 | 75 | 5 | 8.1 | Fatal hemoptysis and abscess |
| 61 | F | IV | 3 | 75 | 4 | 10.3 | Lung abscess, bronchocavitary fistula, tracheoesophageal fistula |
Abbreviations: CMV, cytomegalovirus; HSV, herpes simplex virus; RT, radiation therapy.
All treatments prescribed over a course of 25 fractions.
From completion of radiation therapy.
Fig 2.
(A) Incidence (1 − Kaplan-Meier [KM] estimate) of any grade 4 or 5 toxicity in patients censored at the time of death or last clinical follow-up. Dashed lines represent the 95% CI. (B) Two-year probabilities of late grade 4 or 5 toxicity according to dose-per-fraction normalized dose (EQD2) to the proximal bronchial tree and estimated using a Cox proportional hazards model. Open circles represent the 1 − KM estimate (± 95% CI) for quartiles of EQD2 D3cc (centered at the quartile mean). DXcc, maximum dose D such that X cm3 of the structure received a dose ≥ D; Dmax, maximum dose to any voxel within structure.
On univariate analysis, higher delivered dose was significantly associated with the development of any grade 4 to 5 toxicity (hazard ratio, 1.13; 95% CI, 1.04 to 1.23; P = .0036). The 2-year incidence of these toxicities was 31% (± 13%) in patients treated to 75 Gy or higher compared with 1.8% (± 1.7%) of patients treated to lower doses (P < .001). Analysis of additional variables is listed in Table 3.
Table 3.
Univariate Analysis of Any Grade 4-5 Toxicity
| Variable | No. of Events | % | P |
|---|---|---|---|
| Dose received, Gy | < .001 | ||
| ≤ 69.25 | 1 of 57 | 2 | |
| 75-85.5 | 5 of 18 | 28 | |
| Age, years | .41 | ||
| ≤ 60 | 1 of 24 | 4 | |
| > 60 | 5 of 51 | 10 | |
| Performance status | .054 | ||
| 0 | 6 of 46* | 13 | |
| 1 | 0 of 28* | 0 | |
| Radiation treatment time, days† | .16 | ||
| Bin assignment, rNTDmean | .06 | ||
| Pilot (NA) | 0 of 4 | 0 | |
| 1 (0.00-0.119) | 2 of 6 | 33 | |
| 2 (0.12-0.179) | 0 of 8 | 0 | |
| 3 (0.18-0.239) | 4 of 27 | 15 | |
| 4 (0.24-0.309) | 0 of 26 | 0 | |
| 5 (0.31-0.410) | 0 of 4 | 0 | |
| PTV, cm3† | .9 | ||
| Chemotherapy | .23 | ||
| Any | 3 of 54 | 6 | |
| None | 3 of 21 | 14 |
Abbreviations: NA, not applicable; PTV, planning target volume; rNTDmean, mean normalized tissue (lung) dose divided by normalized prescription dose.
Total No. of patients adds up to only 74 because of missing data.
Analyzed as a continuous variable.
As grade 4 to 5 toxicities seemed to be dependent on total dose and not bin number, the decision was made to report an MTD for all patients rather than for each bin. An MTD was identified at 63.25 Gy and was largely determined by late toxicities in patients receiving ≥ 75 Gy. Only three patients were treated to 69.25 Gy, so the safety of this dose level cannot be determined with the MTD definition of ≤ 20% DLT.
Given similarities in the late grade 4 and 5 toxicities seen in our study, an exploratory analysis was performed with the hypothesis that these toxicities could be linked to normal tissue dosimetry. Complete dosimetric data could not be obtained in 11 patients, none of whom experienced significant toxicity. Parameters related to lung, heart, and esophageal doses were not significant, whereas doses given to small volumes of the proximal bronchial tree were significant (Table 4). Of these, D3cc seemed to be the most significant (P = .004), while Dmax, D1 cm3, and D2 cm3 were significant to lesser degrees. For the parameters found to be significant on univariate analysis, the 2-year probability of late grade 4 to 5 toxicity was estimated as a function of equivalent dose delivered at 2 Gy per fraction (EQD2) using an α/β ratio of 3 Gy (Fig 2B). The EQD2 doses predicting a 5% complication rate at 2-years for these parameters ranged from 75 Gy (D3cc) to 83 Gy (Dmax).
Table 4.
Univariate Analysis of Late Grade 4-5 Toxicity (all patients)
| Variable | P |
|---|---|
| Lung | |
| Mean | .63 |
| V5 | .48 |
| V20 | .66 |
| V30 | .37 |
| PTV, cm3 | .41 |
| Bronchial tree dose* | |
| Max | .020 |
| Mean | .072 |
| D1cc | .012 |
| D2cc | .007 |
| D3cc | .004 |
| Heart dose | |
| Mean | .16 |
| V5 | .44 |
| V50 | .43 |
| Esophageal dose | |
| Max | .66 |
| Mean | .74 |
| D1cc | .72 |
| D2cc | .80 |
| D3cc | .80 |
Abbreviations: DXcc, maximum dose D such that X cm3 of the structure received a dose ≥ D, where D is measured in Gy; max, maximum; PTV, planning target volume; VX, percent volume receiving a dose of ≥ X Gy.
Proximal bronchial tree defined as per Radiation Therapy Oncology Group 0813.
DISCUSSION
This study demonstrated that without concurrent chemotherapy, hypofractionated RT up to 63.25 Gy in 25 fractions (and possibly up to 69.25 Gy) is well-tolerated when strict normal tissue constraints are respected. No grade ≥ 3 radiation pneumonitis was observed. However, other grade 4 to 5 toxicities, including late effects, were manifest and found to correlate with total prescribed dose. We attributed late grade 4 to 5 toxicities to cause damage to central and perihilar structures, specifically the proximal bronchial tree and surrounding vasculature. In support of this, we identified a relationship between severe late toxicity and the dose given to small volumes of the proximal bronchial tree, with maximum dose, D1cc, D2cc, and D3cc all significant on univariate analysis.
The potential for significant damage to central structures has been described in patients undergoing stereotactic body radiotherapy (SBRT) for early-stage NSCLC,16 with subsequent protocols restricting eligibility based on location.17 Although current protocols exist that allow SBRT for central tumors, our results indicate that late central toxicities can still limit dose-escalation strategies, even when a more fractionated approach is used. The rate of central toxicity seen in our cohort was somewhat surprising given the higher EQD2 doses in commonly used SBRT fractionation schemes for central lung lesions (eg, 50 Gy in five fractions as per the starting dose in RTOG 0813; EQD2 = 130 Gy for late effects). However, the size limitations in SBRT may naturally limit the volume of the bronchial tree receiving high doses. All patients who experienced grade 4 to 5 late toxicities in our cohort had gross tumor encasing or abutting a mainstem or proximal lobar bronchus. In addition, local invasion by the more advanced tumors in our study may have compromised the radiation tolerance of adjacent normal structures.
The risk for radiation pneumonitis in our study was well controlled following a procedure similar to that used by other groups investigating dose escalation.18 However, our results are a reminder that the spectrum of toxicities from dose-intensified thoracic radiotherapy can be broad. In addition to pneumonitis and pulmonary fibrosis, patients treated to high biologic doses are at risk for bronchial stenosis, fatal hemoptysis,19 and disruption of the fibrocartilagenous tracheobronchial tree.20 These are toxicities that might not always be captured with high fidelity using conventional prospective reporting mechanisms or they may be misdiagnosed by outside physicians as disease progression despite no radiographic evidence of such. This theme has been alluded to in a recent editorial regarding the results of RTOG 0617, in which the higher number of deaths in the 74 Gy versus 60 Gy arm was felt to be attributable to RT-related normal tissue damage, even though there were no reported differences in toxicity rates between the two groups.21
These results also have implications for clinical trials in which dose is escalated to maintain a predicted rate of isotoxicity based on radiobiologic modeling of a single organ or tissue at risk. For example, the experimental arm in the currently open study RTOG 1106 uses hypofractionation to dose-escalate up to 85.5 Gy in 6 weeks with concurrent chemotherapy as long as mean lung doses of 20 Gy are achieved. In our study, the NTCP model for lung toxicity was accurate in predicting grade 2 radiation pneumonitis but did not control for late central toxicity, which was identified as the driving etiology behind dose-limiting adverse events. Accurate modeling of other toxicities is therefore needed to safely allow dose escalation using this paradigm.
For patients with NSCLC treated with IMRT, hypofractionated RT at doses of 57 to 63.25 Gy in 25 fractions was well-tolerated in the absence of concurrent chemotherapy and with strict normal tissue constraints. Grade 4 and 5 late toxicities were attributed to damage in central and perihilar structures, as supported by a significant correlation with dose delivered to the proximal bronchial tree. In addition to controlling for pneumonitis risk, radiation dose-escalation studies in lung cancer should require strict limits to doses received by the proximal bronchial tree.
Supplementary Material
Acknowledgment
We thank Idarto Tanumihardjo for his assistance in facilitating data retrieval for the dosimetric analysis, Jack Fowler, PhD, DSc, for developing the dose-escalation model, and Hazim Jaradat, PhD, and Emiliee Soisson, PhD, for dosimetric/physics support.
Appendix
Bin assignment.
Patients underwent four-dimensional computed tomography simulation and target volume segmentation as described in Patients and Methods. Normal structures were also segmented, including bilateral lungs (as a single structure), esophagus, and spinal cord. A 25-fraction preliminary radiation plan was optimized and used to calculate a 2-Gy per fraction equivalent mean lung dose normalized to the prescription dose (rNTDmean). This conversion was performed with α/β = 3 Gy for each volume element and has been described previously.12
Assignment to one of five dose-escalation groups (ie, bins) was performed according to rNTDmean as listed in Appendix Table A1. Patients with higher rNTDmean were predicted to be at higher risk for pneumonitis for a given prescription dose12 (Kwa SL et al: Radiation pneumonitis as a function of mean lung dose: An analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys 42:1-9, 1998). Once a patient was categorized into the appropriate bin, a dose level was chosen following the dose escalation rules outlined in the Dose Escalation section.
Dose escalation.
An initial pilot phase of five patients treated to a dose of 57 Gy in 25 fractions was followed by a dose escalation study consisting of a set of five linked phase I trials, one for each of the bins. Dose to the planning target volume was escalated within each bin according to a Bayesian continual reassessment method (CRM) with the maximum-tolerated dose (MTD) defined as the maximum dose at which there was ≤ 20% dose-limiting toxicity (DLT). As a conservative measure, starting doses in each bin began at the dose level just below the level at which a 20% risk for grade 2 radiation pneumonitis was predicted (or at 57 Gy in the higher bins; Appendix Table A2). After every five patients had received follow-up for 3 months with radiographic and clinical evaluation, a posterior model for DLT was used as a prior model for the next cohort of patients who were to be treated at the re-estimated MTD. Using this approach, patients in a given bin were eligible for dose escalation beyond a given dose level if at least 3 patients within the bin (or in bins with larger rNTDmean values) had been treated at that dose and received follow-up for 3 months without evidence of DLT.
Once the continual reassessment method protocol identified the dose level for a new patient, the patient-specific radiation treatment plan was optimized at that dose level. If normal tissue constraints specified by the protocol were not met, the patient was dropped to a lower dose level. The spinal cord dose was restricted to fraction-normalized tissue dose (NTD) of 50 Gy and the esophageal dose was initially restricted to an NTD of 64 Gy, both with normalization to 2-Gy per fraction equivalents. However, the initial esophageal constraint proved to be too restrictive and therefore was changed to no more than 5 cm3 of esophagus receiving an NTD in excess of 64 Gy. In addition, the effective volume (Veff) of the esophagus was required to be less than 30%.
Table A1.
Bin Definitions
| Bin | rNTDmean |
|---|---|
| 1 | 0.00-0.119 |
| 2 | 0.12-0.179 |
| 3 | 0.18-0.239 |
| 4 | 0.24-0.309 |
| 5 | 0.31-0.41 |
Abbreviation: rNTDmean, mean normalized tissue (lung) dose divided by normalized prescription dose.
Table A2.
Dose Assignment Schema
| Dose (Gy)* | EQD2 (Gy) | Estimated % NTCP Risk for Grade 2 Pneumonitis |
||||
|---|---|---|---|---|---|---|
| Bin 1 | Bin 2 | Bin 3 | Bin 4 | Bin 5 | ||
| 57 | 60 | 0-11 | 11-12 | 12-15 | 15-22† | 22-40† |
| 63.25 | 70 | 0-11 | 11-13 | 13-16† | 16-26 | 40-50 |
| 69.25 | 80 | 0-12 | 12-15 | 15-22 | 22-40 | 40-65 |
| 75 | 90 | 0-12 | 12-16† | 16-26 | 26-50 | 50-80 |
| 80.5 | 100 | 0-13† | 13-21 | 21-38 | 38-61 | 61-80+ |
| 85.5 | 110 | 10-21 | 15-23 | 26-50 | High | High |
Abbreviations: EQD2, equivalent dose for late effects (α/β = 3 Gy) if delivered in 2–Gy fractions; NTCP, normal tissue complication probability.
Prescription dose delivered over 25 fractions.
Indicate starting dose level for each bin.
Table A3.
Univariate Analysis of Grade 2 Radiation Pneumonitis
| Variable | Coefficient | P |
|---|---|---|
| Chemotherapy, any | 0.77 | .35 |
| Chemotherapy, adjuvant | 1.49 | .052 |
| Age | 0.013 | .69 |
| Bin | 0.12 | .66 |
| Total dose delivered | −0.05 | .30 |
| PTV, cm3 | 0.004 | .028* |
| Lung dosimetry | ||
| V5 | 4.4 | .026 |
| V10 | 4.9 | .016 |
| V20 | 3.7 | .37 |
| V30 | 11.4 | .14 |
| Mean | 0.23 | .027* |
Abbreviations: PTV, planning target volume; VX, fraction of volume that received a dose of ≥ X Gy.
Note that PTV volume and mean lung dose were significantly correlated (P < .001).
Footnotes
Supported by Grant No. NCI P01 CA88960-01-05 from the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00214123.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Minesh P. Mehta, Pharmacyclics (C); Deepak Khuntia, Varian Medical Systems (C) Consultant or Advisory Role: Minesh P. Mehta, Accuray TomoTherapy (C), Elekta (C); Deepak Khuntia, Radion (C), ProCertus BioPharm (C), Varian Medical Systems (C) Stock Ownership: Minesh P. Mehta, Accuray TomoTherapy; Deepak Khuntia, Varian Medical Systems Honoraria: Minesh P. Mehta, Accuray TomoTherapy; Deepak Khuntia, Accuray TomoTherapy; Ranjini Tolakanahalli, Accuray TomoTherapy Research Funding: Anne M. Traynor, Novelos Therapeutics, Novartis, GlaxoSmithKline; Wolfgang A. Tomé, Accuray TomoTherapy Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Minesh P. Mehta, Wolfgang A. Tomé, Richard J. Chappell
Provision of study materials or patients: Anne M. Traynor
Collection and assembly of data: Donald M. Cannon, Jarrod B. Adkison, Anne M. Traynor, Ranjini Tolakanahalli, Pranshu Mohindra, George M. Cannon
Data analysis and interpretation: Donald M. Cannon, Minesh P. Mehta, Jarrod B. Adkison, Deepak Khuntia, Anne M. Traynor, Wolfgang A. Tomé, Richard J. Chappell, Ranjini Tolakanahalli, Søren M. Bentzen, George M. Cannon
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy: Report by the Radiation Therapy Oncology Group. Cancer. 1987;59:1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Cox JD, Azarnia N, Byhardt RW, et al. A randomized phase I/II trial of hyperfractionated radiation therapy with total doses of 60.0 Gy to 79.2 Gy: Possible survival benefit with greater than or equal to 69.6 Gy in favorable patients with Radiation Therapy Oncology Group stage III non-small-cell lung carcinoma—Report of Radiation Therapy Oncology Group 83-11. J Clin Oncol. 1990;8:1543–1555. doi: 10.1200/JCO.1990.8.9.1543. [DOI] [PubMed] [Google Scholar]
- 3.Sause W, Kolesar P, Taylor S, IV, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117:358–364. doi: 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 4.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 5.Belderbos JS, Heemsbergen WD, De Jaeger K, et al. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Willner J, Baier K, Caragiani E, et al. Dose, volume, and tumor control prediction in primary radiotherapy of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;52:382–389. doi: 10.1016/s0360-3016(01)01823-5. [DOI] [PubMed] [Google Scholar]
- 7.Rengan R, Rosenzweig KE, Venkatraman E, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:741–747. doi: 10.1016/j.ijrobp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: Long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JD, Paulus R, Komaki R, et al. A randomized phase III comparison of standard-dose (60 Gy) versus high-dose (74 Gy) conformal chemoradiotherapy with or without cetuximab for stage III non–small-cell lung cancer: Results on radiation dose in RTOG 0617. J Clin Oncol. 2013;(suppl):31. abstr 7501. [Google Scholar]
- 10.Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial–CHART Steering committee. Radiother Oncol. 1999;52:137–148. doi: 10.1016/s0167-8140(99)00087-0. [DOI] [PubMed] [Google Scholar]
- 11.Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28:202–206. doi: 10.1200/JCO.2009.25.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta M, Scrimger R, Mackie R, et al. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;49:23–33. doi: 10.1016/s0360-3016(00)01374-2. [DOI] [PubMed] [Google Scholar]
- 13.Adkison JB, Khuntia D, Bentzen SM, et al. Dose escalated, hypofractionated radiotherapy using helical tomotherapy for inoperable non-small cell lung cancer: Preliminary results of a risk-stratified phase I dose escalation study. Technol Cancer Res Treat. 2008;7:441–447. doi: 10.1177/153303460800700605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 17.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): Predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 19.Lee CB, Stinchcombe TE, Moore DT, et al. Late complications of high-dose (≥ 66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009;4:74–79. doi: 10.1097/JTO.0b013e3181915028. [DOI] [PubMed] [Google Scholar]
- 20.Aerni MR, Parambil JG, Allen MS, et al. Nontraumatic disruption of the fibrocartilaginous trachea: Causes and clinical outcomes. Chest. 2006;130:1143–1149. doi: 10.1378/chest.130.4.1143. [DOI] [PubMed] [Google Scholar]
- 21.Cox JD. Are the results of RTOG 0617 mysterious? Int J Radiat Oncol Biol Phys. 2012;82:1042–1044. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.