Abstract
Background
Effective treatments for dogs with advanced stage mast cell tumors (MCT) remain a pressing need. A micellar formulation of paclitaxel (paclitaxel [micellar]) has shown promise in early-phase studies.
Hypothesis/Objectives
The objective was to demonstrate greater activity for paclitaxel (micellar) compared with lomustine. The null hypothesis was μp = μL (ie, proportion of responders for the paclitaxel [micellar] and lomustine groups, respectively).
Animals
Two hundred and fifty-two dogs with advanced stage nonresectable grade 2 or 3 MCT.
Methods
Prospective multicenter randomized double-blind positive-controlled clinical trial. The primary endpoint was confirmed overall response rate (CORR) at 14 weeks. A secondary endpoint, biologic observed response rate (BORR), also was calculated. Safety was assessed by the characterization and grading of adverse events (AE).
Results
Overall CORR (7% versus 1%; P = .048) and BORR (23% versus 10%; P = .012) were greater for paclitaxel (micellar) compared with lomustine. Paclitaxel (micellar)-treated dogs were 6.5 times more likely to have a confirmed response and 3.1 times more likely to experience a biologic observed response. The majority of AE with paclitaxel (micellar) were transient and clinically manageable. Twenty-seven dogs (33%) receiving lomustine were discontinued because of hepatopathy compared with 3 dogs (2%) receiving paclitaxel (micellar) (P < .0001; odds ratio 26.7).
Conclusions and Clinical Importance
Paclitaxel (micellar)’s activity and safety profile are superior to lomustine. The addition of an active and novel taxane to the veterinary armamentarium could fill a substantial need and, as its mechanism of action and AE profile do not overlap with currently available TKI, its availability could lead to effective combination protocols.
Keywords: Cancer, Canine, Chemotherapy, Paclitaxel, Taxane
MCT is the most common cutaneous tumor in the dog, accounting for nearly one-fifth of all skin tumors encountered in companion (pet) dogs.1 Surgical excision or surgical cytoreduction followed by radiation therapy is the treatment of choice for early stage, low, and intermediate grade MCT in dogs. However, of pressing clinical need in the current practice of veterinary oncology are effective and safe treatment options for companion dogs with macroscopic (gross) advanced stage disease not amenable to surgical excision, in regions lacking available radiation facilities, or when high-grade MCT increases the likelihood of recurrence and distant metastasis after surgery. With few exceptions, advanced stage, nonsurgical MCT is a uniformly progressive and fatal disease in dogs.
At present, no cytotoxic-class chemotherapeutics exist that are registered for treatment of solid tumors in dogs. Two small molecule tyrosine kinase inhibitors (TKI) (toceranib and masitinib) are registered for use in macroscopic MCT in dogs. Their mechanism of action primarily targets the c-kit TK growth factor receptor, a receptor that is known to be mutated and aberrantly expressed in approximately 25–30% of grade II/III MCT encountered in companion dogs.2–5 This leaves a continued need for cytotoxic chemotherapeutics that are active against MCT in dogs and the primary mechanism of action of which is not dependent on the presence or inhibition of c-kit.
The current practice of veterinary oncology relies, for the most part, on the extra-label use of chemotherapeutics registered for use in humans. The 2 most commonly used cytotoxic agents currently thought to have activity against canine MCT disease are lomustine and vinblastine.1,6–12 These agents have not been subject to rigorous GCP-standard field trials13 and their safety and activity in companion dogs therefore is considered to be anecdotal.
Taxane-class chemotherapeutics (eg, paclitaxel) are the most widely prescribed cytotoxic therapies in human oncology based on their broad-spectrum activity across several tumor histologies and their predictable safety profile.14 Taxol (Bristol-Myers Squibb, Princeton, NJ), the most widely used formulation of paclitaxel, requires Cremophor EL as an excipient to allow water solubility for parenteral delivery. Taxol has shown activity in dogs with malignant tumors; however, adverse events are common with this formulation and the majority of dogs experience allergic or anaphylactic hypersensitivity reactions to the cremophor excipient despite receiving premedication with antihistamines and corticosteroids.15 A new formulation of paclitaxel (Paccal Veta) that is made water-soluble by using a mixed micellar preparation with a surfactant based on derivatives of retinoic acid (referred to as paclitaxel [micellar] from here forward) has recently been developed.16 The excipient in paclitaxel (micellar), retinoid-derived XR-17, has not resulted in systemic toxicity in normal laboratory dogs (unpublished data). Furthermore, 32 dogs that received paclitaxel (micellar) in an open-label clinical study experienced dose-limiting neutropenia at day 4 at 175 mg/m2 whereas a mean dose of 150 mg/m2 generally was well tolerated.16 Other adverse events reported in these dogs include alopecia, transient inappetence and vomiting, diarrhea, or both.
The objective of the field trial reported here was to document the safety and efficacy of paclitaxel (micellar) in companion dogs with macroscopic advanced nonresectable MCT in the context of a GCP-compliant, randomized, controlled clinical trial (versus lomustine).
Materials and Methods
Trial Design
The study was designed as a randomized double-blind positive-controlled confirmatory clinical field study and was conducted by veterinary oncologists at sites in the United States (n = 17) and the European Union (n = 5) in compliance with good clinical practice (GCP).17 The protocol was deemed acceptable by the US Food and Drug Administration Center for Veterinary Medicine and was reviewed and approved by European Union regulatory and ethics committees. Owner informed consent was obtained in writing before initiating study-related procedures.
Dog Selection and Discontinuation
Client-owned dogs presented between October 2008 and March 2010 with nonresectable grade 2 or 3 MCT (clinical stage 2a or 3a) that were of any age, sex, or breed and weighed at least 5 kg were screened for enrollment. Dogs were excluded from enrollment for any of the following reasons: (i) pregnant, lactating, or intended for breeding; (ii) life expectancy <1 month, (iii) performance status score18 of ≥ 3; (iv) absolute neutrophil count <2.0 × 109/L, platelet count <100 × 109/L, bile acid concentrations, alanine aminotransferase (ALT) activity, or alkaline phosphatase (ALP) activity > twice the upper limit of normal (ULN), serum creatinine concentrations > ULN; (v) previous or concurrent treatment with any chemotherapeutic agent, target lesion radiation, hormonal, immunological (including antiandrogens), or biologic treatment; (vi) systemic corticosteroid treatment within 3 weeks of the study; (vii) active infection or any concurrent disease that would require additional therapy and could result in death of the dog within 3 months; or (viii) enrollment in another clinical trial.
Study dogs were removed from the study if any of the following occurred: (i) clinically relevant hepatopathy (ALT > twice ULN or more than twice the activity observed at baseline); (ii) progressive disease (PD); (iii) clinically relevant adverse events, treatment delay > 31 days from previous cycle or both; (iv) or withdrawal of owner consent or protocol noncompliance.
Study Treatments
Study dogs were randomly allocated (see Statistical Analysis) to treatment with a 2 : 1 ratio to receive paclitaxel (micellar)a (150 mg/m2 IV) or lomustineb (70 mg/m2 PO), respectively. An unblinded treatment administrator administered treatments on Day 0 of each of 4 consecutive 21-day cycles (Table 1). Paclitaxel (micellar) was supplied as 60 mg of lyophilized powder in 100- or 75-mL vials, which were reconstituted in 60 mL of Ringer’s acetate to a paclitaxel (micellar) concentration of 1 mg/mL. The reconstituted paclitaxel (micellar) was immediately infused IV slowly over 15 to 30 minutes. Lomustine was provided as capsules for PO administration (10 and 40 mg capsules; for individual dogs, rounding up or down to the nearest 10 mg was allowed).
Table 1.
Study treatment cycles, visits, and days.
| Cycle | Cumulative Days | Visit | Day within Cycle |
|---|---|---|---|
| Screening | −14 to −1 | V1 | |
| Treatment cycle 1 | 0 | V2 | 0 |
| V3 | 4 ± 1 | ||
| V4 | 7 ± 2 | ||
| Treatment cycle 2 | 21 | V5 | 0 ± 3 |
| V6 | 4 ± 1 | ||
| V7 | 7 ± 2 | ||
| Treatment cycle 3 | 42 | V8 | 0 ± 3 |
| V9 | 4 ± 1 | ||
| V10 | 7 ± 2 | ||
| Treatment cycle 4 | 63 | V11 | 0 ± 3 |
| V12 | 4 ± 1 | ||
| V13 | 7 ± 2 | ||
| End of study visit | 100 | V14 | 35 ± 3 |
The dosage of paclitaxel (micellar) had been established from previous studies.16,19 Lomustine was chosen as the positive control because other appropriate veterinary-registered treatments were not available when the study was initiated, anecdotal activity of lomustine in canine MCT was documented in the veterinary literature,10,11 and its 3-week treatment cycle was consistent with 3-week intervals used with paclitaxel (micellar). The rationale for and dose of lomustine were adopted from the literature and consultation with key opinion leaders in veterinary oncology.1,10,11
Randomization, calculation of study drug dosages, and administration of study drugs were performed by an unblinded treatment administrator. The treatment administrator was prohibited from making efficacy assessments. As the route of administration differed between treatment groups, a forelimb of every dog was shaved and bandaged by the treatment administrator, regardless of treatment to blind those assessing efficacy.
Dose reductions or delays were permitted by the protocol because of dose-limiting toxicity (DLT) defined as grade III or IV toxicity according to the Veterinary Comparative Oncology Group – Common Terminology Criteria for Adverse Events (VCOG-CTCAE v1.0).20 The maximal allowable duration of delay was 31 days, after which the animal was withdrawn from the study. Dose reductions for paclitaxel (micellar) were permitted in decrements of 10 mg/m2, and lomustine by at least 10 mg per dog.
No concomitant anticancer therapies (chemotherapy, immunotherapy, hormonal cancer treatment, systemic corticosteroids, radiation treatment, experimental cancer therapies or NSAID) were permitted during the study. In the presence of severe neutropenia or fever, antibiotics were administered at the discretion of the investigator. Maropitant (2 mg/kg PO or 1 mg/kg SQ) was recommended as an antiemetic if emesis was observed, but was not used prophylactically.
Clinical Assessments
Treatment and assessment events are summarized in Tables 1 and 2. An investigator blinded to treatment always made efficacy assessments, and data collected from unblinded and blinded personnel were recorded in 2 separate study binders. After obtaining written owner consent, the investigator performed a screening examination within 14 days before the start of the first treatment cycle. Screening examinations included a medical history and physical examination (including body temperature and weight). Concurrent disease or conditions that might influence tumor progression or treatment were noted, and demographic information was recorded. Concomitant medications were recorded at all visits. Physical examinations were performed, and performance status assessed18 at all visits. The owner was asked to specifically record episodes of perceived nausea, vomiting, and diarrhea in the intervals between all visits.
Table 2.
Study events by visit.
| Event | Visit 1 | Visits 2, 5, 8, 11 | Visits 3, 6, 9, 12 | Visits 4, 7, 10 | Visit 13 | Visit 14 |
|---|---|---|---|---|---|---|
| Owner consent | X | |||||
| Demographics, med. history | X | |||||
| Concomitant meds. | X | X | X | X | X | X |
| Phys. exam, vital signs | X | X | X | X | X | X |
| Target/nontarget lesions | X | |||||
| Diagnosis | X | |||||
| Tumor grading | X | |||||
| Tumor staging | X | X | X | |||
| Inclusion/exclusion | X | |||||
| Hematology | X | X | X | X | X | X |
| Clinical chemistry | X | X | X | X | X | |
| Urinalysis | X | X | X | X | X | |
| Performance status score | X | X | X | X | X | X |
| RECISTa | X | X | X | |||
| Study drug administration | Xb | |||||
| Adverse event reporting | X | X | X | X | X |
Response evaluation criteria in solid tumors (RECIST v1.0). Tumor measurements were made at Visits 5, 8 and 11 to determine progression.
Study drugs administered after all scheduled assessments were made at Visits 2, 5, 8 and 11.
Each dog’s MCT was evaluated during the study, per RECIST (v1.0)21 which required at least 1 measurable lesion. Target lesions were selected on the basis of their size (lesions with the longest diameter) and their suitability for repeatable measuring. A sum of the longest diameter for all target lesions was calculated and reported as the baseline sum of the longest diameter. Biopsy and histopathology were required to confirm the diagnosis of all target lesions (within 6 months before or at the screening visit) and tumors were graded according to Patnaik.22 Lymph node presence or absence of mast cells was confirmed by fine needle aspiration and cytology. All measurable primary lesions (up to a maximum of 5) representative of the skin and if applicable a maximum of 5 regional lymph node lesions were to be identified and measured as target lesions (a maximum of 10 total target lesions). Calipers were used to measure target lesions and a digital camera was used to document the measurements. The measurements were performed by the blinded investigator throughout the study. All other lesions (or sites of disease) were classified as nontarget lesions. Measurements of all nontarget lesions were not required, but presence or absence of each was noted at Visits 13 and 14. Tumor staging was performed according to the WHO classification system23 by abdominal ultrasound examination, lymph node palpation, and fine needle aspiration of enlarged lymph nodes. Thoracic radio-graphs were recommended, but not required, if the target lesion was located cranial to the insertion of the diaphragm. Tumor staging was repeated at Visits 13 and 14.
Blood and urine samples were collected at most visits (Table 2) for routine hematology (CBC), serum biochemistry (including alanine aminotransferase [ALT], alkaline phosphatase [ALP] and aspartate aminotransferase [AST] activities), and urinanalyses.
Efficacy Assessments
The study’s primary a priori regulatory endpoint was confirmed overall response rate (CORR) from tumor assessments according to RECIST (v1.0).21 Response outcome was categorized as complete response (CR; disappearance of all target lesions); partial response (PR; ≥ 30% decrease in the sum of the longest diameters [LD] compared with baseline); progressive disease (PD; ≥ 20% increase in the sum of the LD compared with the smallest measured sum at any visit); and stable disease (SD; any change not qualifying as CR, PR or PD). CORR (yes or no) for each study dog was defined as complete response (CR) or partial response (PR) of target and nontarget lesions and no new lesions at Visit 13, and the overall response were confirmed at Visit 14 (only responses confirmed at Visit 14 were eligible to be counted). Dogs were considered as responders at Visit 14 if they satisfied at least 1 of the following 3 treatment outcomes: (i) target and nontarget lesions observed with CR, and no new lesions; (ii) target lesions observed with CR, and nontarget lesions observed with PR or SD, and no new lesions; (iii) target lesions observed with PR, and nontarget lesions observed with non-progressive disease, and no new lesions. All other dogs were considered nonresponders.
A secondary efficacy endpoint, biologic observed response rate (BORR), often referred to as Clinical Benefit, which combines the stable disease (SD) rate with the CR and PR rate, also was assessed at Visit 13 and confirmed at Visit 14.
Exploratory Comparison of Activity
At the completion of study, more conventional assessments of clinical activity were assessed including best overall response rate (BESTORR), defined as PR or CR across all measurement time points24 and progression-free survival (PFS) rate at 6 weeks were calculated post hoc in dogs receiving either treatment to allow comparison of activity with those of TKI and unregistered (off-label) cytotoxic agents used in dogs with MCT and reported in the veterinary literature using these end-points.
Safety Assessments
Abnormal clinical examination findings, clinically relevant laboratory abnormalities, or other clinically relevant observations were reported as adverse events. Adverse events (AE) were recorded spontaneously at any time point during the study and were defined as any undesirable event, expected or not, occurring in a dog during the study, whether considered as having a causal relation to study treatment or not. Adverse events were classified according to the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA) and graded according to VCOG-CTCAE (v1.0).20,25
Statistical Analysis
Randomization was conducted with a 2:1 treatment allocation with a web-based centralized randomization program.c Tumor grade (grade 2 or 3) and stage (stage 2 or 3) were used as stratification factors. The number of dogs in each stratum was not predetermined and was dependent upon actual accrual. In particular, there was no requirement for the strata to have equal sizes. According to the protocol, 243 dogs were to be randomized to the study. The study was originally powered (80%) to detect a 20 percentage unit difference between treatments assuming an initial lomustine response rate of 50% (target of 225 dogs, with 252 dogs actually randomized). However, because of the unexpectedly low response rate, enrollment numbers were subsequently increased relative to the original power estimate. A treatment administrator handled the centralized computer randomization system at each study site. Each randomized dog was allocated a sequential randomization number by the central randomization system. The randomization system produced an electronic copy stating the date and time of randomization and the dog’s randomized study treatment.
The objective of this study was to demonstrate a statistically greater CORR after 4 treatment cycles according to RECIST v1.0 for paclitaxel (micellar) compared with lomustine. The null hypothesis was μp = μL (ie, the proportion of confirmed overall responders for the paclitaxel [micellar] and lomustine groups, respectively). CORR was analyzed with exact logistic regression that included the main effect of treatment group and the stratification variables: tumor grade and tumor stage. Attributable to an unexpectedly low responder rate in this study, the interaction between treatment and stratification variables could not be included in the statistical models. The 95% confidence intervals and odds ratios were calculated. These same analyses also were used for the secondary endpoint of BORR assessments.
Group comparisons for other variables were considered exploratory; nonetheless treatment groups were compared with Fischer exact or Chi-square tests for categorical variables, Cochran-Mantel-Haenszel tests controlling for the stratification factors (tumor grade and tumor stage) for ordinal variables, and Mann Whitney U-tests for continuous variables. Wilcoxon matched-pair signed rank test was used for changes from baseline for each treatment group, separately, for continuous variables. Kaplan–Meier methodology was used to investigate treatment differences in discontinuation rate. All statistical tests were conducted with SAS,d and were 2-sided with a significance level of 5%.
Results
Demographic Information
Of the 252 randomized dogs (Table 3), 249 dogs with a mean ± SD age of 8.7 ± 2.8 years (range, 1 month–14.6 years) and weight of 28 ± 13 (range, 5–70) kg received at least 1 dose of study treatment. Female dogs (56%) outnumbered male dogs (44%) and most dogs (89%) were neutered. Dogs from 49 breeds were represented: the most common breeds were mixed breed (24%), Labrador Retriever (19%), Boxer (10%), Golden Retriever (9%), and Pug (4%). Dogs with grade 2 tumors (68%) were more prevalent in the study compared with those with grade 3 tumors (32%), and most dogs (86%) had advanced stage (stage 3a) tumors. Mean age and weight and distribution of sex, neuter status, breed, and tumor grade or stage were not different between treatment groups (Table 4).
Table 3.
Summary of study dog disposition and reason for study discontinuation (n and %).
| Disposition | Total | Paclitaxel (Micellar) |
Lomustine |
|---|---|---|---|
| Randomized | 252 | 169 | 83 |
| Treated | 249 | 168 | 81 |
| Treated with all 4 cyclesa |
90 | 71 (42%)*a | 19 (24%)† |
| Completeda | 76 | 60 (36%)* | 16 (20%)† |
| Discontinued (for reason below) |
173 | 108 | 65 |
| Progressive disease | 114 | 88 (81%)b | 26 (40%) |
| Hepatopathy | 30 | 3 (3%) | 27 (42%) |
| Death or euthanasia | 16 | 10 (9%) | 6 (9%) |
| Other adverse event | 3 | 3 (3%) | 0 |
| Other reasonc | 10 | 4 (4%) | 6 (9%) |
Treatments differ significantly (P < .05).
With respect to the number of treated dogs.
With respect to the number of discontinued dogs.
Protocol noncompliance, withdrawn owner consent, or reason not recorded.
Table 4.
Demographic and baseline characteristics.
| Demographic | Category/ Unit |
Paclitaxel (Micellar) N = 168 |
Lomustine N = 81 |
|---|---|---|---|
| Sex, n (%) | Male | 77 (46%) | 33 (41%) |
| Female | 91 (54%) | 48 (59%) | |
| Neutered, n (%) | No | 17 (10%) | 11 (14%) |
| Yes | 151 (90%) | 70 (86%) | |
| Age, mean ± SD | Months | 103 ± 33 | 106 ± 32 |
| Weight, mean ± SD |
Kg | 28 ± 13 | 28 ± 13 |
| Tumor grade, n (%) |
2 | 114 (68%) | 56 (69%) |
| 3 | 54 (32%) | 25 (31%) | |
| Tumor stage, n (%) |
1a | 1 (1%) | 0 |
| 2a | 23 (14%) | 11 (14%) | |
| 3a | 144 (86%) | 70 (86%) |
One paclitaxel (micellar) dog with a stage 1a tumor was inadvertently enrolled in the study. Megestrol acetate, a prohibited concomitant treatment, was inadvertently administered to 19 dogs by 1 investigator as part of that clinic’s normal protocol for appetite simulation for cases with persistent or severe anorexia. These 20 dogs were excluded from a per-protocol (PP, n = 229) analysis of efficacy. The results regarding the primary efficacy analysis obtained from the PP compared with the intention to treat (ITT, n = 252) populations were numerically and directionally similar, but the PP analysis lacked statistical power to demonstrate a statistically significant result. Nonetheless, according to the a priori statistical analysis plan and published statistical regulatory guidance for conducting superiority clinical trials,26 the ITT population was finally used for making inference on efficacy and safety.
More paclitaxel (micellar) dogs received all 4 cycles of treatment and completed the study compared with lomustine dogs (Table 3 and Fig 1). The most common reason for discontinuation of paclitaxel (micellar) was progressive disease, whereas lomustine was most commonly discontinued because of hepatopathy or progressive disease. The death rate, including euthanasia (9%), was similar between treatments.
Fig 1.
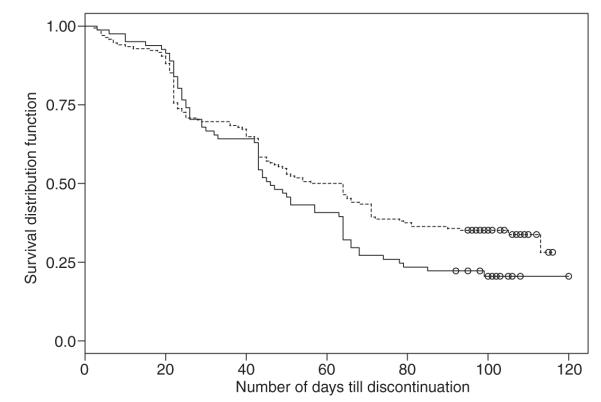
Kaplan–Meier schematic of dogs discontinuing from the study that received paclitaxel (micellar) [dotted line] or lomustine [solid line]. The y-axis represents the proportion of dogs remaining over time (days; x-axis). Censored values are indicated with open circles.
Clinical Efficacy
Overall CORR, the a priori primary endpoint, was significantly greater (7 versus 1%; P = .048) for paclitaxel (micellar) compared with lomustine (Table 5). Paclitaxel (micellar)-treated dogs were 6.5 times more likely, compared with lomustine-treated dogs, to have a confirmed response (CR or PR) at 14 weeks (Visit 14, 35 days after 4 cycles of treatment). When dogs with a response of SD were included in supplemenary analysis, BORR (Clinical Benefit) was significantly greater (23 versus 10%; P = .012) for paclitaxel (micellar) compared with lomustine (Table 5). Paclitaxel (micellar)-treated dogs were 3.1 times more likely, compared with lomustine-treated dogs, to have a confirmed BORR (CR, PR, or SD) at 14 weeks.
Table 5.
Summary of overall response rate (n and% of responders) and distribution of RECIST (v1.0) responses.
| Paclitaxel (Micellar) N = 168 |
Lomustine N = 81 |
|||
|---|---|---|---|---|
| Responders | CORR * |
BORR
(CB) ‡ |
CORR * |
BORR
(CB) ‡ |
| n (%) | 12 (7%)a | 39 (23%)c | 1 (1%)b | 8 (10%)d |
| 95% CI | 3–11% | 17–30 | 0–4% | 3–16% |
| odds ratio | 6.479 | 3.097 | ||
| RECIST (v1.0) overall response | ||||
| N | 60 | 16 | ||
| complete (CR) |
2 (3%) | 0 | ||
| partial (PR) | 10 (17%) | 1 (6%) | ||
| stable (SD) | 27 (45%) | 7 (44%) | ||
| progressive (PD) |
21 (35%) | 8 (50%) | ||
Confirmed Overall Response Rate: Dogs rated by the veterinarian as CR or PR at both Visit 13 and Visit 14 (7 and 35 days, respectively after the 4th treatment cycle).
Confirmed Biologic Overall Response Rate (Clinical Benefit): dogs rated by the veterinarian as CR, PR, or SD at both Visit 13 and Visit 14 (7 and 35 days, respectively, after the 4th treatment cycle).
Treatments differ significantly (P < .05).
Treatments differ significantly (P < .05).
Exploratory Comparison of Activity
The BESTORR and the 6-week PFS rate for paclitaxel (micellar), calculated post hoc, was 23 and 68%, respectively, and for lomustine was 23 and 66%, respectively.
Clinical Safety
Clinically relevant AE in both treatment groups, with respect to laboratory results and physical examination or vital sign abnormalities, were observed in 167 (of 168) paclitaxel (micellar) dogs and 80 (of 81) lomustine dogs (summarized in Table 6). Most non-hematologic AE were graded as nonsevere (grade <3). Hematologic (in particular neutropenia) and gastrointestinal (emesis, anorexia, and diarrhea) events were the most common reported AE in paclitaxel (micellar)-treated dogs. Hematologic and hepatic events were the most common reported AE in lomustine-treated dogs.
Table 6.
Incidence of dogs with clinically significant adverse events (AE) and those qualifying as severe (VCOG ≥ 3).
| Paclitaxel (Micellar) |
Lomustine |
|||
|---|---|---|---|---|
| N = 168 |
N = 81 |
|||
| All AE (%) |
VCOG ≥ 3 (%) |
All AE (%) |
VCOG ≥ 3 (%) |
|
| Neutropenia | 82 | 73 | 93 | 86 |
| Emesis | 79 | 20 | 51 | 6 |
| Hepatopathy | 19 | 5 | 49 | 33 |
| Anorexia | 76 | 10 | 48 | 1 |
| Diarrhea | 70 | 9 | 46 | 4 |
| Lethargy | 69 | 7 | 47 | 5 |
| Alopecia | 39 | 3 | 2 | 0 |
| Dehydration | 26 | 10 | 14 | 7 |
| Dermatitis | 24 | 2 | 10 | 0 |
| Pyrexia | 13 | 2 | 17 | 7 |
| Edema | 14 | 2 | 4 | 1 |
| Lameness | 12 | 5 | 10 | 5 |
| Anemia | 8 | 2 | 6 | 2 |
| Loss of body condition |
7 | 0 | 1 | 0 |
| Thrombocytopenia | 7 | 0 | 9 | 2 |
| Leukopenia (nonneutrophil) |
4 | 2 | 9 | 5 |
Relative to baseline results, neutrophil count was consistently lowest on Day 4 of each cycle for both treatment groups, and had generally returned to baseline by Day 0 of the following cycle (Fig 2). Most neutropenia events in paclitaxel (micellar)-treated dogs were because of grade 3 and 4, transient, clinically silent neutropenia; only 6 cases (4%) were accompanied by grade 3 or 4 pyrexia and only 2 (1%) resulted in treatment discontinuation.
Fig 2.
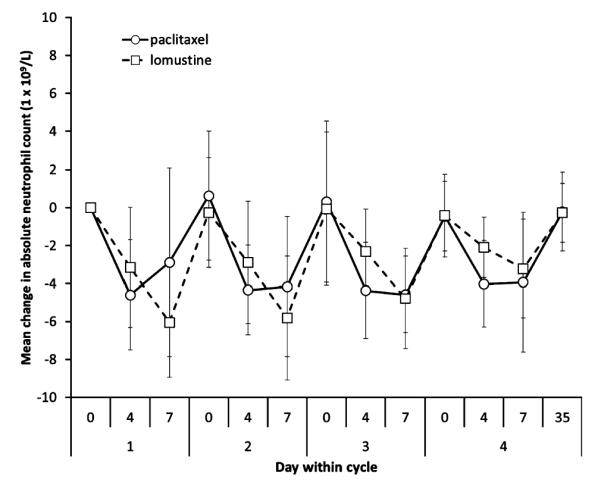
Mean (± SD) change (from baseline) for neutrophil count over time by treatment group.
Increases in hepatic enzyme activity (ALT, AST, ALP), relative to baseline, were greater for lomustine compared with paclitaxel (micellar) dogs. Twenty-seven dogs (33%) in the lomustine group were discontinued because of hepatopathy compared with 3 (2%) in the paclitaxel (micellar) group (P < .0001; odds ratio 26.7). The majority of lomustine dogs that developed clinically relevant hepatopathy (as measured by grade 3 increases in ALT activity) leading to discontinuation did so at Visit 8 (just before the 3rd cycle of treatment).
The incidence of perceived nausea, vomiting, and diarrhea generally was highest on Day 4 of each cycle (Table 7). More paclitaxel (micellar) dogs were observed with these 3 events on Day 4 of each cycle, compared with lomustine dogs. The incidence of these events (on Day 4 of each cycle) decreased with time.
Table 7.
Incidence (%) of dogs with an investigator-rated performance status score of “normal” and owner-observed reports of nausea, vomiting, and diarrhea.
| Cycle | 1 |
2 |
3 |
4 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days within Cycle | 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | 0 | 4 | 7 | 35 |
| Normal | |||||||||||||
| paclitaxel | 88 | 51 | 72 | 74 | 65 | 77 | 76 | 63 | 70 | 76 | 67 | 71 | 73 |
| lomustine | 89 | 88 | 76 | 86 | 85 | 84 | 86 | 78 | 84 | 81 | 71 | 67 | 61 |
| Nausea | |||||||||||||
| paclitaxel | 75 | 30 | 13 | 53 | 14 | 10 | 45 | 20 | 7 | 50 | 9 | 2 | |
| lomustine | 26 | 16 | 13 | 15 | 13 | 6 | 3 | 0 | 0 | 7 | 0 | 5 | |
| Vomiting | |||||||||||||
| paclitaxel | 50 | 5 | 7 | 24 | 5 | 10 | 10 | 1 | 0 | 7 | 0 | 2 | |
| lomustine | 12 | 7 | 13 | 2 | 6 | 15 | 7 | 0 | 3 | 0 | 0 | 0 | |
| Diarrhea | |||||||||||||
| paclitaxel | 27 | 10 | 6 | 22 | 4 | 2 | 15 | 8 | 1 | 12 | 1 | 3 | |
| lomustine | 12 | 5 | 9 | 2 | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 10 | |
The incidence of physical examination abnormalities was relatively low during the study. Mean body weight (approximately 28 kg), body temperature (approximately 39°C), and mean change in body temperature from baseline were similar between treatment groups and over time. Mean change in body weight, relative to baseline, decreased over time for both groups and was greatest (− 1.3 kg) for paclitaxel (micellar) dogs on Visit 9 (Day 4 of Cycle 3). The percentage of paclitaxel (micellar) dogs with a performance status score of ‘normal’ was lowest on Day 4 after treatment and highest by Day 0 of the following treatment cycle (Table 7). The percentage for normal paclitaxel (micellar) dogs tended to be numerically lower than for lomustine dogs throughout the study.
Approximately 42% of dogs in both treatment groups required a dose reduction at 1 or more cycles during the study; slightly fewer paclitaxel (micellar) dogs (9%) required a dose delay compared with lomustine (14%). The mean dose of paclitaxel (micellar) per dog was numerically lower for Cycles 2 through 4 (130 ± 43 mg) compared with Cycle 1 (134 ± 46 mg), with an average dose change of −12% in dogs over 4 cycles of treatment. The mean dose of lomustine per dog decreased with cycle from 62 ± 21 to 53 ± 19 mg. The average dose change over time was similar between paclitaxel (micellar) (−12%) and lomustine (− 10%).
The most commonly (31–76% of dogs) administered concomitant medications were (in descending order) diphenhydramine, famotidine, maropitant, electrolytes, metronidazole, amoxicillin, enrofloxacin, and metoclopramide. Other therapies concurrently administered during the study were megestrol, tramadol, ampicillin, sucralfate, butorphanol, cephalexin, ondansetron, ciprofloxacin, sulfa drugs, loperamide, omeprazole, atipamezole, ivermectin, dexmedetomidine, marbofloxacin, glucosamine, methionine, mirtazapine, fipronil, multivitamins, filgrastim, and levothyroxine.
Discussion
Paclitaxel (micellar) is active against macroscopic advanced nonresectable MCT in dogs and has a safety margin consistent with cytotoxic chemotherapeutic class agents. Both activity and safety were superior to lomustine. Addition of an active and novel taxane to the veterinary armamentarium could fill a substantial need in the field. Its mechanism of action and dose-limiting AE profile do not substantially overlap with currently available TKI, and as such its availability could lead to effective combination protocols, which are the current treatment paradigm in medical oncology.
The 7% CORR at 14 weeks for dogs receiving paclitaxel (micellar), although numerically modest, was nevertheless statistically significant (P = 0.048) compared with the positive comparator (lomustine), a drug described in the literature and understood by veterinarians to be active in MCT. Although CORR is a reasonable choice for a regulatory endpoint for a cancer drug, it is not a sufficient measure of clinical benefit and does not adequately portray the therapeutic benefit of an agent in the context in which it is to be used. Direct clinical benefit also should be interrogated as it relates to (i) the BORR, as the inclusion of stabilization of disease, along with the CR and PR rate, over a 14-week period can reasonably be assumed to translate into clinical benefit; (ii) comparable documented efficacy observed with therapeutic agents that have been granted regulatory authorization for this indication; (iii) comparable documented efficacy and expectations observed with unregistered cytotoxic agents currently used off-label by the veterinary oncology community for this indication.
When one considers BORR, the rate of 23% for paclitaxel (micellar)-treated dogs and 10% for lomustine dogs at the 14-week confirmation point (P = .012) again confirms superior clinical benefit over the “active” lomustine comparator. The rationale for inclusion of SD along with CR/PR, now routinely considered in response evaluations20 is that nonprogression of tumors over 14 weeks translates into clinical benefit in a disease that if progressive, results in morbidity and ultimate mortality in the patient. Therefore, approximately one-quarter of companion dogs with advanced, macroscopic MCT disease experienced Clinical Benefit from paclitaxel (micellar) for at least 14 weeks.
When comparing efficacy observed with currently registered therapeutic agents, 2 TKI agents currently are approved for use in dogs with MCT (toceranib and masitinib). Paclitaxel (micellar) has a spectrum advantage over TKI in that its mechanism of action is independent of aberrant c-kit function (present in only 25–30% of gradeII/III MCT where TKI treatment has its greatest benefit)2,3 and, therefore, paclitaxel (micellar) with measurable efficacy regardless of c-kit status would fill an important need. Regarding toceranib, the published BORR at 6 weeks was documented to be 60%2 which also translates into the 6-week progression-free rate. The 6-week BORR for paclitaxel (micellar), calculated from raw data in the current trial, compares favorably at 68%. Furthermore, although a placebo (no treatment) group was not included in the current trial, the 68% 6-week PFS rate (32% progression) is substantially better than the placebo (no treatment) groups in 2 previous GCP-compliant registration trials for advanced mast cell disease.2,3 In these previous studies, over 50% of dogs experienced PD within 3 weeks and over 70% within 6 weeks of placebo treatment, a circumstance that can reasonably be associated with the ultimate death of the patient.
In addition, when comparing efficacy with unregistered cytotoxic agents currently used off-label by the veterinary oncology community, paclitaxel (micellar) was superior to the positive lomustine control used in this trial. Moreover, the only 2 published studies assessing single-agent conventional-dose weekly vinblastine (2.0 mg/m2) without prednisone reported BESTORR of 12%.10,27 The BESTORR of 23% for paclitaxel (micellar) determined in the exploratory posthoc calculations used in a similarly advanced MCT bearing population (without prednisone) compares favorably. These interpolative and comparative data further demonstrate the clinical benefit of paclitaxel (micellar).
Unexpectedly, lomustine greatly underperformed in this field trial when considering anecdotal activity reports in the veterinary literature.10 Several explanations exist, including (i) GCP assessments are more stringent compared with anecdotal (non-GCP) assessments and often document less robust activity; (ii) the high discontinuation rate because of hepatotoxicity and; (iii) the use of less stringent end-points in previously published non-GCP reports of lomustine use in dogs with MCT. This later point is particularly germane, in that most prior reports utilize best overall response rate (BESTORR), defined as the CR/PR rate at any time point during study, without the use of a final confirmatory time point, as was the case in this study. This allows response assessment to be performed before development of hepatotoxicity. Therefore, most prior studies reported activity by 6 weeks, whereas the 33% discontinuation rate (typically occurring before the 3rd treatment cycle) in the study reported here resulted in removal from trial before the 14-week confirmation of assessment visit. This serves to further illustrate the importance of GCP trial management and equality of end-point measures when evaluating and comparing agents.
Regarding the type and temporal nature of AE experienced and their likely impact on patient well-being, of the 85% grade 3 and 17% grade 4 incidence observed with paclitaxel (micellar), most were because of transient grade 3 and 4 neutropenia (73% grade 3 and 11% grade 4). With paclitaxel (micellar), chemotherapy-induced neutropenia (CIN) nadirs occurred early, were transient, and in most cases recovered in time to allow subsequent treatment cycles to occur (only 1% resulted in treatment discontinuation). CIN events in dogs generally were devoid of clinical signs and most dogs in the field trial remained afebrile (only 4% had serious or severe pyrexia). As such, although CIN is technically serious and life threatening, the majority of dogs maintained good quality of life.
When CIN is excluded from the AE incidence rate, the remainder of severe (12%) and serious (6%) AE primarily were confined to the gastrointestinal (GI) tract. Effects on the GI tract generally were transient and recovery occurred in sufficient time to allow treatment continuation. Unlike CIN, gastrointestinal adverse events (eg, perceived nausea, vomiting, anorexia, and diarrhea) have substantially more potential to affect quality of life. That severe and serious GI AE occur in the minority of cases provides less of a counterbalance to benefit and also must be considered in the context of our current ability to apply proactive and reactive treatment measures that would prevent or alleviate their impact on quality of life. This field trial, by its very nature, was designed only to react to AE as they developed rather than to be proactive by use of prophylactic antiemetic and antidiarrheal agents. Prophylactic care is the current standard of care in veterinary patients undergoing cytotoxic chemotherapy.28 Therefore, the incidence of severe or serious AE with quality of life consequences is likely overstated. Importantly, many of the GI AE reported in this trial reflect those occurring during the natural history of advanced MCT. As such, the assessment of risk must first account for the baseline events that are associated with MCT progression. The nature of these events is tied to the systemic inflammatory biology of MCT and can affect many organ systems. Studies leading to registration of toceranib include an informative placebo population; during the initial 6-week study phase, AE occurred in 80% of the dogs that received placebo (16% severe).2
Overall, although the safety margin for paclitaxel (micellar) is low and the incidence of serious AE is high, the vast majority of AE are transient, manageable, and recovery occurs in time for subsequent cycles. Only 3 (2%) dogs died or were euthanized because of conditions likely to be related to these events. No unique AE were documented for paclitaxel (micellar) when compared with similar cytotoxic agents; therefore use will not require additional veterinary or client education or interventions, and the comfort level is similar to other agents currently employed. Furthermore, replacement of cremophor excipient with the much safer excipient (XR-17) in paclitaxel (micellar) is a clear benefit to the currently available, off-label usage of other formulations of paclitaxel.
Limitations of the current trial include the use of a priori activity endpoints that did not include a temporal measure, such as PFS. That being said, the raw data were available to calculate a 6-week PFS rate and BESTORR that allowed exploratory comparisons with other registered and off-label agents currently used in veterinary oncology practice. In addition, the high discontinuation rate experienced in the lomustine-treatment group because of hepatotoxicity led to a substantial population unable to continue to the 14-week conformation assessment. However, this replicates practical use of lomustine where AE require consideration when continuing treatment. It is possible that if sufficient discontinuation time were allowed for liver enzyme activity to normalize in those patients, the resumption of lomustine treatment at decreased dose or prolonged intertreatment intervals would have resulted in some clinical benefit to the population, but this trial was not designed for that purpose. Furthermore, recent work by Skorupski and others has documented that denamarin partially abrogates hepatic AE in dogs receiving lomustine and the addition of this protectant may allow more consistent continuation of lomustine in practice.29 Finally, after the original publication of VCOG-CTCAE v1.020 which was used in this protocol design, the updated v1.130 has established a grade 3 AE as > 4× the ULN for ALT and the observed activity would not have been classified as dose limiting under the new guidelines.
In summary, the preponderance of data indicate that paclitaxel (micellar) is active against macroscopic advanced nonresectable MCT in dogs and carries a safety margin consistent with cytotoxic chemotherapeutic class agents. Both activity and safety profile were superior to the lomustine comparator. Exploratory analyses allowed comparison of safety and activity to other currently available treatments, however, more clinically relevant than direct comparisons, the availability of paclitaxel (micellar) with its nonoverlapping mechanisms of action and adverse event profile relative to currently registered TKI, should ultimately lead to investigations of combination treatments, which, as is the current paradigm in medical oncology, may result in more active and durable treatment protocols.
Acknowledgments
None.
The study was funded by Oasmia Pharmaceutical AB, Uppsala, Sweden.
Conflict of Interest: Dr Vail periodically receives honoraria from Oasmia for his consultancy work in oncology. Dr von Euler is a part-time employee with Oasmia. Drs Rusk’s and Khanna’s employment and consultancy with Animal Clinical Investigation as the study’s Medical Director and Study Coordinator, respectively, were funded as contract research activities by Oasmia.
Abbreviations
- AE
adverse event
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BESTORR
best overall response rate
- BORR
biologic observed response rate
- CIN
chemotherapy-induced neutropenia
- CORR
confirmed overall response rate
- CR
complete response
- DLT
dose-limiting toxicity
- GCP
good clinical practice
- GI
gastrointestinal
- AE
adverse event
- MCT
mast cell tumors
- MTD
maximally tolerated dose
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- SD
stable disease
- TKI
tyrosine kinase inhibitor
- ULN
upper limit of normal
- VCOG-CTCAE
Veterinary Comparative Oncology Group Common Terminology Criteria for Adverse Events
Footnotes
Paccal Vet solution for infusion, 1 mg/mL; Oasmia Pharmaceutical AB, Uppsala, Sweden
CeeNU, 10, 40, and 100 mg oral capsules; Bristol-Myers Squibb, Princeton, NJ
International Drug Development Institute, Louvain-la-Neuve, Belgium
SAS v9.1.3; SAS Institute Inc, Cary, NC
References
- 1.Thamm DH, Vail DM. Mast cell tumors. In: Withrow SW, Vail DM, editors. Small Animal Clinical Oncology. 4th ed Saunders/Elsevier; St. Louis, MO: 2007. pp. 402–424. [Google Scholar]
- 2.London CA, Malpas PB, Wood-Follis SL, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin Cancer Res. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 3.Hahn KA, Ogilvie G, Rusk T, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. J Vet Intern Med. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 4.Downing S, Chien MB, Kass PH, et al. Prevalence and importance of internal tandem duplications in exons 11 and 12 of c-kit in mast cell tumors of dogs. Am J Vet Res. 2002;63:1718–1723. doi: 10.2460/ajvr.2002.63.1718. [DOI] [PubMed] [Google Scholar]
- 5.Letard S, Yang Y, Hanssens K, et al. Gain-of-function mutations in the extracellular domain of KIT are common in canine mast cell tumors. Mol Cancer Res. 2008;6:1137–1145. doi: 10.1158/1541-7786.MCR-08-0067. [DOI] [PubMed] [Google Scholar]
- 6.Davies DR, Wyatt KM, Jardine JE, et al. Vinblastine and prednisolone as adjunctive therapy for canine cutaneous mast cell tumors. J Am Anim Hosp Assoc. 2004;40:124–130. doi: 10.5326/0400124. [DOI] [PubMed] [Google Scholar]
- 7.Thamm DH, Turek MM, Vail DM. Outcome and prognostic factors following adjuvant prednisone/vinblastine chemotherapy for high-risk canine mast cell tumour: 61 cases. J Vet Med Sci. 2006;68:581–587. doi: 10.1292/jvms.68.581. [DOI] [PubMed] [Google Scholar]
- 8.Thamm DH, Mauldin EA, Vail DM. Prednisone and vinblastine chemotherapy for canine mast cell tumor: A retrospective study of 41 cases. J Vet Int Med. 1999;13:491–497. doi: 10.1892/0891-6640(1999)013<0491:pavcfc>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Rassnick KM, Bailey DB, Flory AB, et al. Efficacy of vinblastine for treatment of canine mast cell tumours. J Vet Intern Med. 2008;22:1390–1396. doi: 10.1111/j.1939-1676.2008.0195.x. [DOI] [PubMed] [Google Scholar]
- 10.Rassnik KM, Moore AS, Williams LE, et al. Treatment of canine mast cell tumors with CCNU (lomustine) J Vet Intern Med. 1999;13:601–605. doi: 10.1892/0891-6640(1999)013<0601:tocmct>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Rassnick KM, Bailey DB, Russell DS, et al. A phase II study to evaluate the toxicity and efficacy of alternating CCNU and high-dose vinblastine and prednisone (CVP) for treatment of dogs with high-grade, metastatic or nonresectable mast cell tumors. Vet Comp Onc. 2010;8:209–220. doi: 10.1111/j.1476-5829.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 12.Vickery KR, Wilson H, Vail DM, Thamm DH. Dose-escalating vinblastine for the treatment of canine mast cell tumors. Vet Comp Oncol. 2008;6:111–119. doi: 10.1111/j.1476-5829.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson D, Garden C, Hutchinson D, Garden C, editors. 12 Golden GCP Rules for Veterinary Studies. Canary Ltd; Glasgow, Surrey, UK: 2001. [Google Scholar]
- 14.Rowinsky EK. Antimicrotubule agents. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy. 4th ed Lippincott Williams & Wilkins; Philadelphia, PA: 2006. pp. 253–267. [Google Scholar]
- 15.Poirier VJ, Hershey AE, Burgess KE, et al. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J Vet Intern Med. 2004;18:219–222. doi: 10.1892/0891-6640(2004)18<219:eatopt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.von Euler H, Pivera P, Nyman H, et al. A dose-finding study with a water-soluble formulation of paclitaxel (Paccal® Vet) for the treatment of malignant high grade solid tumors in dogs. Vet Comp Oncol. 2011 doi: 10.1111/j.1476-5829.2011.00314.x. in press. [DOI] [PubMed] [Google Scholar]
- 17.VICH GL9 [Accessed December 16, 2011];International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. Available at: http://www.vichsec.org/pdf/2000/Gl09_st7.pdf.
- 18.Theilen GH. Performance status score. In: Theilen GH, Madewell BR, editors. Veterinary Cancer Medicine. 2nd ed Lea & Febiger; Philadelphia, PA: 1987. p. 59. [Google Scholar]
- 19.Rivera P, et al. Efficacy and toxicity of a new formulation of paclitaxel (Paclical® Vet) in a phase I+II study for the treatment of malignant tumours in dogs. Proceedings of the ECVIMCA Annual Congress; Budapest, Hungary. 2007. [Google Scholar]
- 20.VCOG-CTCAE Veterinary cooperative oncology group – Common terminology criteria for adverse events following chemotherapy or biologic antineoplastic therapy in dogs and cats v1.0. Vet Compar Oncol. 2004;2:194–213. [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST) J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Patnaik AK. Canine cutaneous mast cell tumor: Morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–474. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 23.Owen LN. TNM Classification of Tumors in Domestic Animals. 1st ed vol. 34. World Health Organization; Geneva: 1980. [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.VeDDRA [Accessed December 16, 2011];Veterinary dictionary for drug regulatory activities. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/07/WC500094802.pdf.
- 26.EMA [Accessed December 16, 2011];Guideline on statistical principles for veterinary clinical trials. 2010 EMA/CVMP/EWP/81976/2010 Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500097081.pdf.
- 27.Henry CJ, Downing S, Rosenthal RC, et al. Evaluation of a novel immunomodulator composed of human chorionic gonadotropin and bacillus Calmette-Guerin for treatment of canine mast cell tumours in clinically affected dogs. Am J Vet Res. 2007;68:1246–1251. doi: 10.2460/ajvr.68.11.1246. [DOI] [PubMed] [Google Scholar]
- 28.Rau SE, Barber LG, Burgess KE. Efficacy of maropitant in the prevention of delayed vomiting associated with administration of doxorubicin to dogs. J Vet Intern Med. 2010;24:1452–1457. doi: 10.1111/j.1939-1676.2010.0611.x. [DOI] [PubMed] [Google Scholar]
- 29.Skorupski KA, Hammond GM, Irish AM, et al. Prospective randomized clinical trial assessing the efficacy of Denamarin for prevention of CCNU-induced hepatopathy in tumor-bearing dogs. J Vet Intern Med. 2011;25:838–845. doi: 10.1111/j.1939-1676.2011.0743.x. [DOI] [PubMed] [Google Scholar]
- 30.VCOG Consensus Statement Veterinary cooperative oncology group – Common terminology criteria for adverse events following chemotherapy or biologic antineoplastic therapy in dogs and cats v1.1. Vet Compar Oncol. 2011 doi: 10.1111/vco.283. e-pub ahead of print, 20JUL2011.DOI:10.1111/j.1476-5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]


