Abstract
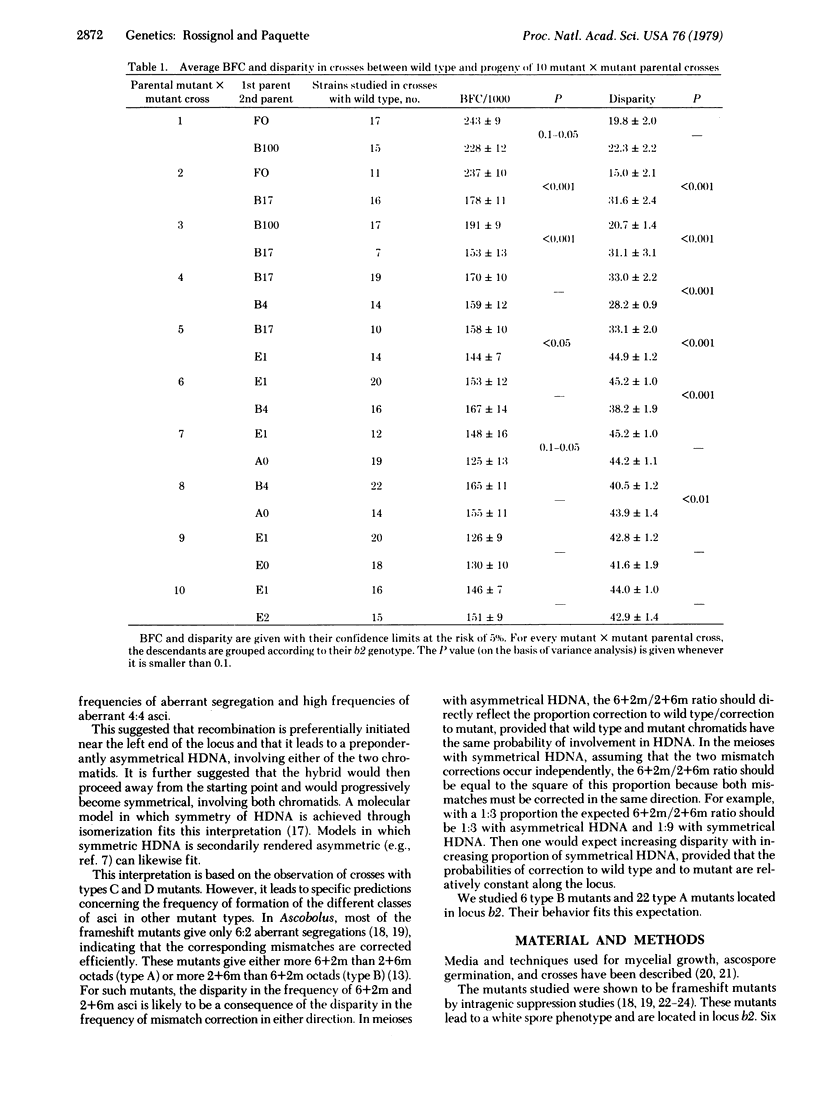
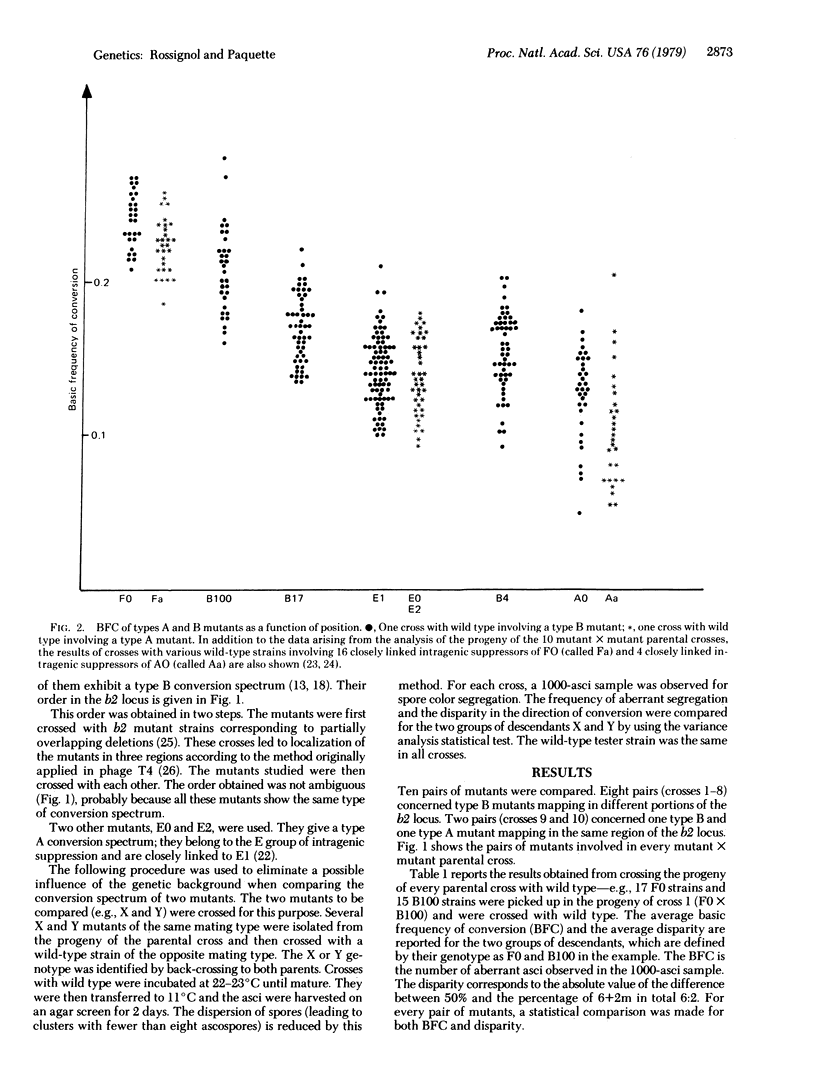
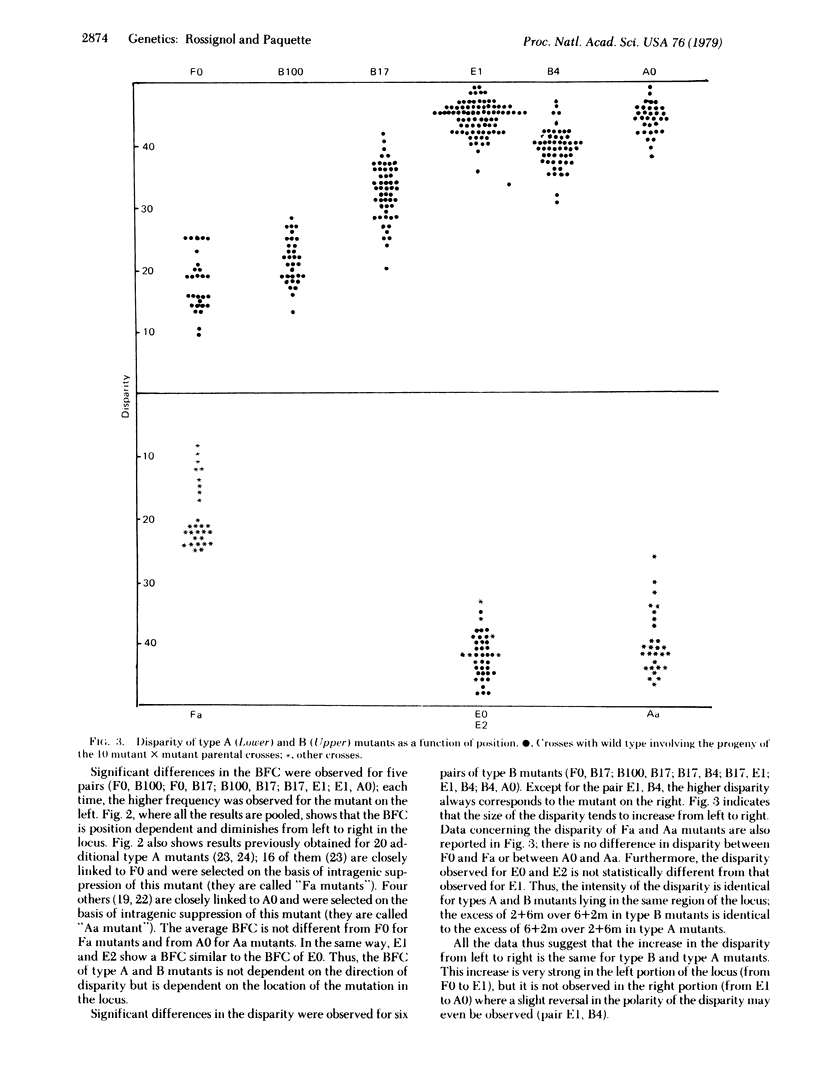
The frequency of conversion and the disparity in the direction of conversion were studied for six frameshift mutants lying in locus b2 of Ascobolus immersus and giving more 2 wild type:6 mutant (2+6m) than 6 wild type:2 mutant (6+2m) aberrant asci (type B). The frequency of conversion decreased from left to right in the locus. The disparity steadily increased from left to right and then reached a plateau. Twenty-two frameshift mutants giving more 6+2m than 2+6m aberrant asci (type A) and closely linked to three type B mutants were also studied; they showed the same frequency of conversion and the same disparity (but in the opposite direction) as the type B mutant to which they are linked. The polar variation of both the frequency of conversion and the disparity as a function of position were expected on the basis of a previous study of 15 mutants giving postmeiotic segregations and located in locus b2. This variation is assumed to reflect the existence of a preferential region for the initiation of hybrid DNA (HDNA) during recombination and a duality in the distribution of this HDNA, with preponderant asymmetrical HDNA near the starting point and preponderant symmetrical HDNA farther from it.
Keywords: meiosis, octads, genetic markers, polarity, hybrid DNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Culbertson M. R., Charnas L., Johnson M. T., Fink G. R. Frameshifts and frameshift suppressors in Saccharomyces cerevisiae. Genetics. 1977 Aug;86(4):745–764. doi: 10.1093/genetics/86.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S., Yu-Sun C. C. Gene Conversion in the Pasadena Strain of ASCOBOLUS IMMERSUS. Genetics. 1967 Jan;55(1):39–47. doi: 10.1093/genetics/55.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S. Postmeiotic segregation in Saccharomyces. Mol Gen Genet. 1971;111(3):297–299. doi: 10.1007/BF00433113. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Girard J., Rossignol J. L. The suppression of gene conversion and intragenic crossing over in Ascobolus immersus: evidence for modifiers acting in the heterozygous state. Genetics. 1974 Feb;76(2):221–243. doi: 10.1093/genetics/76.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblon G. Mechanism of gene conversion in Ascobolus immersus. II. The relationships between the genetic alterations in b 1 or b 2 mutants and their conversion spectrum. Mol Gen Genet. 1972;116(4):322–335. doi: 10.1007/BF00270089. [DOI] [PubMed] [Google Scholar]
- Leblon G., Paquette N. Intragenic suppression at the b2 locus in Ascobolus immersus. I. Identification of three distinct groups of suppression. Genetics. 1978 Nov;90(3):475–488. doi: 10.1093/genetics/90.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblon G., Rossignol J. L. Mechanism of gene conversion in Ascobolus immersus. 3. The interaction of heteroallelas in the conversion process. Mol Gen Genet. 1973 Apr 12;122(2):165–182. doi: 10.1007/BF00435189. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive L. S. ABERRANT TETRADS IN SORDARIA FIMICOLA. Proc Natl Acad Sci U S A. 1959 May;45(5):727–732. doi: 10.1073/pnas.45.5.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszewski A. Gene conversion: observations on the DNA hybrid models. Genet Res. 1970 Feb;15(1):55–64. doi: 10.1017/s0016672300001373. [DOI] [PubMed] [Google Scholar]
- RIZET G., ENGELMANN N., LEFORT C., LISSOUBA P., MOUSSEAU J. [On an Ascomycete of interest for the study of certain aspects of the problem of gene structure]. C R Hebd Seances Acad Sci. 1960 Mar 14;250:2050–2052. [PubMed] [Google Scholar]
- Rommelaere J., Miller-Faurès A. Detection by density equilibrium centrifugation of recombinant-like DNA molecules in somatic mammalian cells. J Mol Biol. 1975 Oct 15;98(1):195–218. doi: 10.1016/s0022-2836(75)80109-4. [DOI] [PubMed] [Google Scholar]
- Rossignol J. L. Existence of homogeneous categories of mutants exhibiting various conversion patterns in gene 75 of Ascobolus immersus. Genetics. 1969 Dec;63(4):795–805. doi: 10.1093/genetics/63.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITEHOUSE H. L. A THEORY OF CROSSING-OVER BY MEANS OF HYBRID DEOXYRIBONUCLEIC ACID. Nature. 1963 Sep 14;199:1034–1040. doi: 10.1038/1991034a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. E., Jr, Radman M. A mechanism for initiation of genetic recombination. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3619–3622. doi: 10.1073/pnas.72.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]


