Abstract
Classical approaches to analysis of mitotic recombination by use of simian virus 40 (SV40) are limited in usefulness because of low frequencies of recombination. To bypass the apparent rate-limiting step in normal SV40 recombination, oligomeric SV40 was constructed in vitro by ligation of mixtures of pairs of linear DNAs carrying genetically distinct temperature-sensitive mutations. Cultured monkey cells infected with the unfractionated ligation products yielded frequencies of nonparental recombinant progeny that were increased up to 500-fold relative to cells infected with a mixture of the untreated circular molecules. Pairwise crosses were performed with tsB4, tsB8, and tsBC11 DNAs, using unfractionated oligomers constructed from linear molecules cleaved by EcoRI or BamHI. In each cross the fraction of progeny with nonparental genotypes was roughly proportional to the physical distances between the mutant sites. These results suggest a random, rather than site-specific, conversion of oligomers to monomers. Somewhat surprisingly, nonligated mixtures of linear tsB4 and tsB8 DNAs, created by EcoRI digestion, produced a 40-t to 100-fold increase in the frequency of nonparental progeny. These results indicate that intermolecular associations must occur with fairly high efficiency between these linear molecules.
Full text
PDF
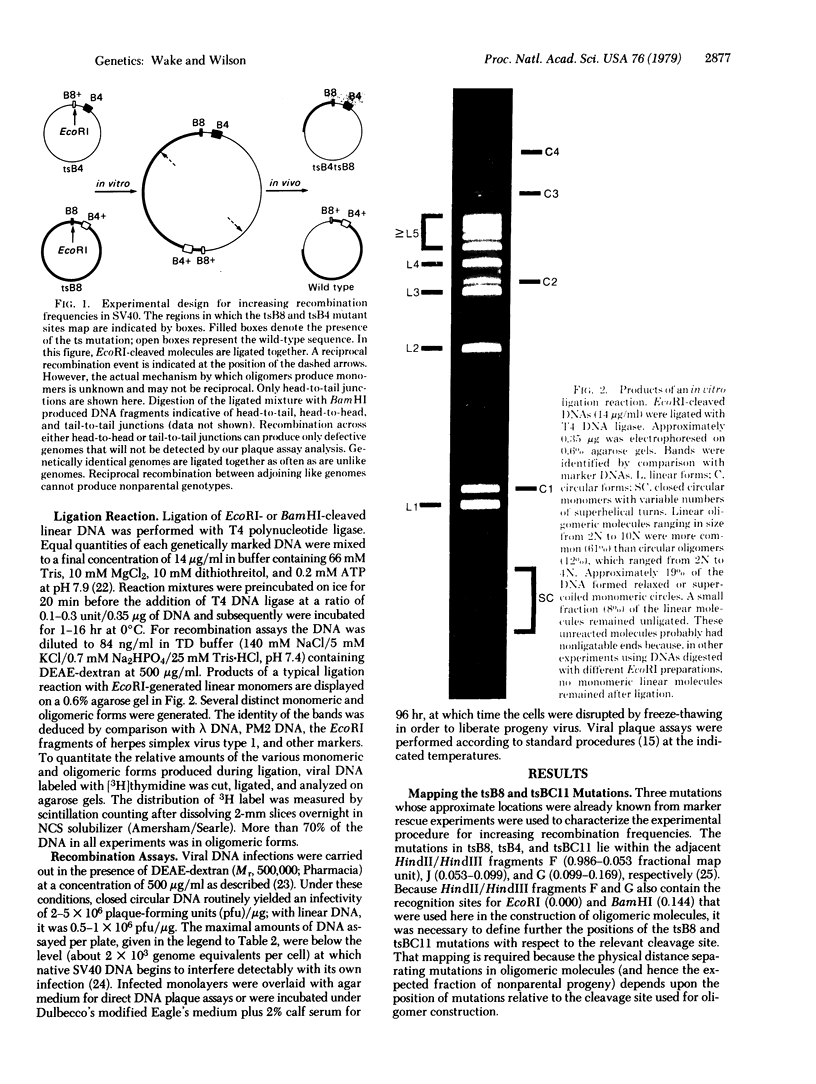

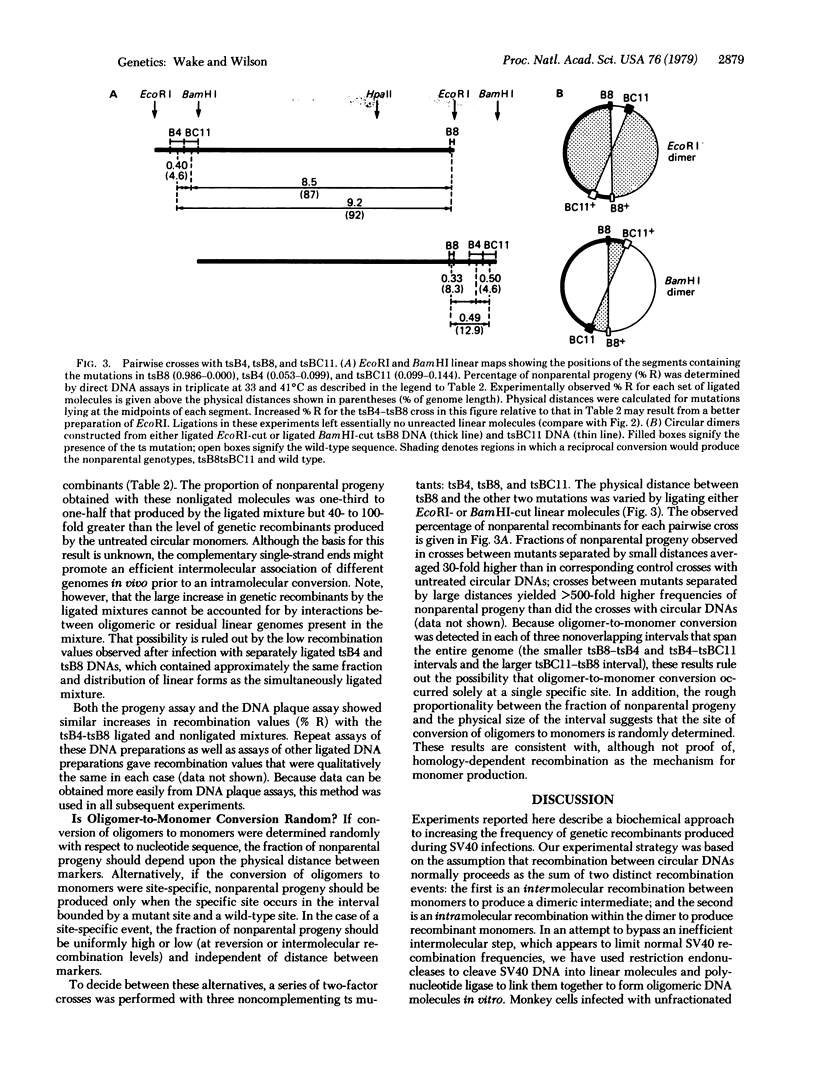

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. G., Baker R. F. Evidence for translocation of DNA sequences during sea urchin embryogenesis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5627–5630. doi: 10.1073/pnas.75.11.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger J., Warner R. C., Tessma I. Role of circular dimer DNA in the primary recombination mechanism of bacteriophage S13. Nat New Biol. 1973 Mar 7;242(114):9–12. doi: 10.1038/newbio242009a0. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Rachmeler M., Kit S. Recombination between temperature-sensitive mutants of simian virus 40. Virology. 1974 Jan;57(1):161–174. doi: 10.1016/0042-6822(74)90117-2. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Structure and formation of circular dimers of simian virus 40 DNA. J Virol. 1977 Oct;24(1):295–302. doi: 10.1128/jvi.24.1.295-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types II. Restoration of Mating Ability to Sterile Mutants in Homothallic and Heterothallic Strains. Genetics. 1977 Mar;85(3):373–393. doi: 10.1093/genetics/85.3.373b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Byrne J. C., Martin M. A. Biologic activity of oligomeric forms of SV40 DNA. Virology. 1978 Jun 15;87(2):239–246. doi: 10.1016/0042-6822(78)90129-0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. DNA replication in SV40-infected cells. V. Circular and catenated oligomers of SV40 DNA. Virology. 1971 Jun;44(3):480–493. doi: 10.1016/0042-6822(71)90361-8. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Martin M. A., Howley P. M., Byrne J. C., Garon C. F. Characterization of supercoiled oligomeric SV40 DNA molecules in productively infected cells. Virology. 1976 May;71(1):28–40. doi: 10.1016/0042-6822(76)90091-x. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Burch J. W., Ruddle F. H. Cotransfer of thymidine kinase and galactokinase genes by chromosome-mediated gene transfer. Proc Natl Acad Sci U S A. 1978 Feb;75(2):914–918. doi: 10.1073/pnas.75.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Tai H. T., Smith C. A., Sharp P. A., Vinograd J. Sequence heterogeneity in closed simian virus 40 deoxyribonucleic acid. J Virol. 1972 Feb;9(2):317–325. doi: 10.1128/jvi.9.2.317-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Dohan C., Jr, Reznikoff C. Inactivating and mutagenic effects of nitrosoguanidine on simian virus 40. Proc Natl Acad Sci U S A. 1970 Jul;66(3):745–752. doi: 10.1073/pnas.66.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B., Jacquemin-Sablon A., Live T. R., Fareed G. C., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. VI. Further purification and properties of polynucleotide ligase from Escherichia coli infected with bacteriophage T4. J Biol Chem. 1968 Sep 10;243(17):4543–4555. [PubMed] [Google Scholar]
- Wilson J. H., DePamphilis M., Berg P. Simian virus 40-permissive cell interactions: selection and characterization of spontaneously arising monkey cells that are resistant to simian virus 40 infection. J Virol. 1976 Nov;20(2):391–399. doi: 10.1128/jvi.20.2.391-399.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H. Genetic analysis of host range mutant viruses suggests an uncoating defect in simian virus 40-resistant monkey cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3503–3507. doi: 10.1073/pnas.74.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H. Interference in SV40 DNA infections: a possible basis for cellular competence. Virology. 1978 Dec;91(2):380–388. doi: 10.1016/0042-6822(78)90385-9. [DOI] [PubMed] [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]