Background: AKAP79/150 scaffolds PKA, PKC, and calcineurin into networks.
Results: Knockdown of AKAP79/150 in cardiac myocytes inhibited the recycling of the β1-AR and increased β1-AR-mediated production of cyclic AMP.
Conclusion: AKAP79/150 is involved in trafficking and signaling of myocardial β1-AR.
Significance: Our results indicate that AKAP79/150 might be cardioprotective via its key role in regulating the signaling intensity of cardiac β1-AR.
Keywords: Adrenergic Receptor, AKAP, Cyclic AMP (cAMP), Fluorescence Resonance Energy Transfer (FRET), G-protein-coupled Receptors (GPCR), Protein Kinase A (PKA), Receptor Recycling, Trafficking
Abstract
Protein kinase A-anchoring proteins (AKAPs) participate in the formation of macromolecular signaling complexes that include protein kinases, ion channels, effector enzymes, and G-protein-coupled receptors. We examined the role of AKAP79/150 (AKAP5) in trafficking and signaling of the β1-adrenergic receptor (β1-AR). shRNA-mediated down-regulation of AKAP5 in HEK-293 cells inhibited the recycling of the β1-AR. Recycling of the β1-AR in AKAP5 knockdown cells was rescued by shRNA-resistant AKAP5. However, truncated mutants of AKAP5 with deletions in the domains involved in membrane targeting or in binding to calcineurin or PKA failed to restore the recycling of the β1-AR, indicating that full-length AKAP5 was required. Furthermore, recycling of the β1-AR in rat neonatal cardiac myocytes was dependent on targeting the AKAP5-PKA complex to the C-terminal tail of the β1-AR. To analyze the role of AKAP5 more directly, recycling of the β1-AR was determined in ventricular myocytes from AKAP5−/− mice. In AKAP5−/− myocytes, the agonist-internalized β1-AR did not recycle, except when full-length AKAP5 was reintroduced. These data indicate that AKAP5 exerted specific and profound effects on β1-AR recycling in mammalian cells. Biochemical or real time FRET-based imaging of cyclic AMP revealed that deletion of AKAP5 sensitized the cardiac β1-AR signaling pathway to isoproterenol. Moreover, isoproterenol-mediated increase in contraction rate, surface area, or expression of β-myosin heavy chains was significantly greater in AKAP5−/− myocytes than in AKAP5+/+ myocytes. These results indicate a significant role for the AKAP5 scaffold in signaling and trafficking of the β1-AR in cardiac myocytes and mammalian cells.
Introduction
Protein kinase A anchoring proteins (AKAP)3 are a family of structurally diverse proteins, which share a common motif at their C terminus that binds to the regulatory subunits of the cyclic AMP-dependent protein kinase (PKA). In addition, AKAPs mediate signal integration through protein-protein binding domains that bind to other kinases, phosphatases, GPCR, effector enzymes, and channels (1, 2). By scaffolding these diverse but functionally complementary proteins, AKAPs form local signaling networks that can modulate the microenvironment, metabolism, and downstream signaling of cyclic AMP (3). Gene knockdown and overexpression methodologies identified numerous functions for individual AKAP in regulating the signaling outputs of GPCRs. siRNA-mediated knockdown of endogenous AKAP79 in HEK-293 cells increased cyclic AMP and sustained PKA activity upon agonist-mediated stimulation of endogenous β2-AR (4). Overexpression of muscle AKAP in heart cells increased PKA-catalyzed phosphorylation of ryanodine Ca2+ release channels and β-AR-induced cardiomyocyte hypertrophy (5). In addition, PKA anchored to AKAP7 (AKAP15/18) was involved in rapid regulation of myocardial L-type Ca2+ currents in response to β-AR stimulation (6).
AKAP5 is a membrane-bound AKAP that, in addition to binding PKA, binds to PKC, calcineurin (PP2B), membrane-associated guanylate kinase protein SAP97, adenylyl cyclase V/VI, and other signaling and scaffolding proteins (4, 7–9). AKAP5-anchored complexes play a key role in regulating GPCR signaling in general and β-adrenergic signaling in particular. Disruption of AKAP-PKA interactions by the st-Ht31 peptide significantly increased the duration of plasma membrane-delineated cyclic AMP (10). Global deletion of AKAP5 resulted in loss of β-adrenergic stimulated Ca2+ transients and the phosphorylation of substrates involved in Ca2+ handling. Thus, deletion of AKAP5 impaired β-AR-mediated modulation of excitation-contraction coupling in adult mouse cardiac myocytes (11). Moreover, AKAP5 was involved in targeting the β1-AR to specific membrane compartments in cardiomyocyte-like H9c2 cells (12). Furthermore, AKAP5 and its anchored PKA were involved in trafficking of β1-AR and glutamate receptors (13–15). We have determined that the AKAP5-PKA complex was targeted to the β1-AR by SAP97 (16). SAP97 is a scaffolding protein that binds simultaneously to the type 1 PSD-95/DLG/ZO1 (PDZ) domain in the C-terminal tail of the β1-AR and to AKAP79 (16). SAP97-mediated targeting of the AKAP5-PKA complex to the C terminus of the β1-AR facilitated the phosphorylation of Ser312 in the third intracellular loop of the β1-AR by this pool of PKA (13, 14, 16).
β1-ARs are critically involved in regulating renin release from the kidney and in mediating chronotropic, inotropic, and lusitropic effects of catecholamines in the heart. The effects of the association between AKAP5 and the β1-AR on trafficking and signaling of the β1-AR are unknown. Therefore, we determined the role of AKAP5 in signaling and trafficking of the β1-AR in established cells lines such as HEK-293 and in more physiologically relevant cells such as cardiac myocytes. Our results indicate that AKAP5 regulated the recycling and intensified the signaling output of the β1-AR in cardiac myocytes. Moreover, cardiomyocytes from AKAP5 null mice were hypertrophied, indicating that AKAP5 could exert a potentially important protective role in β-adrenergic-mediated cardiac hypertrophy.
EXPERIMENTAL PROCEDURES
Preparation of Neonatal Cardiac Myocytes from Rats and AKAP5 Transgenic Mice
A colony of AKAP5 heterozygous mice (AKAP5+/−) was provided by G. Stanley McKnight, University of Washington, Seattle (11). Neonatal 1–2-day-old mouse pups with two alleles of AKAP5 (AKAP5+/+) or homozygous knock-out for AKAP5 (AKAP5−/−) were used for the preparation of cardiac myocytes. Mouse ventricles were digested 5–8 times, each for 10 min in dissociation buffer supplemented with collagenase II (0.2 mg/ml), pancreatin (0.5 mg/ml), and DNase I (1.5 μg/ml). The dissociated cells were then pre-plated for 2 h to enrich for cardiac myocytes. Ventricles from 1- to 2-day-old rat Sprague-Dawley pups were digested in dissociation buffer supplemented with 60 mg/100 ml of pancreatic enzyme concentrate (Bio Case V, Thomas Laboratories, Tolleson, AZ) and then purified by a centrifugation in a Percoll step gradient as described previously (17). Rat or mouse cardiac myocytes were cultured in plating medium composed of DMEM/M-199 (80:20) supplemented with 10% horse serum and 5% fetal bovine serum. Human embryonic kidney 293 (HEK-293) cells were maintained in DMEM with 10% fetal bovine serum. Rodent cardiac myocytes were transfected with adenovirus expressing the various constructs of the β1-AR for 6 h and subsequently cultured for 24–48 h before use.
Antibodies, siRNA, and Additional Reagents
Cy3-labeled anti-FLAG M2, NOQ7.5.4D anti-β-myosin heavy chains (β-MHC), and EA-53 anti-α-actinin antibodies were from Sigma. Monoclonal anti-AKAP79 or SAP97 were purchased from (BD Biosciences and Enzo Life Sciences, Farmingdale, NY), respectively. Xamoterol, salmeterol, CGP-20712A, ICI-118,551, and GF 109203X were from Tocris Bioscience (Minneapolis, MN). The sequence in AKAP79 between 850 and 870 corresponding to ACCCTAGAAAGTGCACCAAAT or its inactive control (GTCTCCACGCGCAGTACATTT) was cloned into RNAi expression vectors pcDNA-6.2-GW-mir or pcDNA-6.2-GW/EmGFP-mir (Invitrogen). The sequence between 861–881 in AKAP150 (AAGAAGACAAAATCCAAACTT) was used to generate the AKAP150-specific shRNA. Plasmids expressing the shRNA were transfected into HEK-293 using the Lipofectamine 2000TM transfection reagent (Invitrogen) and into heart cells by the TransITTM LT1 reagent (Mirus Corp.). Two point mutations were introduced into positions 860 and 861 of WT AKAP79 by site-directed mutagenesis to generate an AKAP79 cDNA mutant that would escape siRNA-mediated degradation. From this mutant, the other siRNA-resistant truncated versions of AKAP79 were generated by PCR and cloned into pIRES-EGFP. pIRES-EGFP is a bicistronic vector that allows the expression of the gene of interest to be monitored at the single-cell level due to expression of hrGFP II (green) on the same transcript. Adenoviruses harboring the WT β1-AR, β1-ARΔPDZ, (S312A)β1-AR, (S312D)β1-AR, and (S312D)β1-ARΔPDZ were generated at the Vector Core of the University of Tennessee Health Sciences Center.
Confocal Recycling Microscopy Protocol
HEK-293 cells or neonatal mouse cardiomyocytes were grown on poly-l-lysine-coated or collagen-coated glass coverslips, respectively. These cells were serum-starved for at least 3 h in DMEM supplemented with 25 mm HEPES, pH 7.4. Cells were incubated with Cy3-labeled anti-FLAG M2 IgG (5–10 μg/ml) for 1 h at 37 °C and then exposed to 10 μm isoproterenol for 30 min at 37 °C to promote agonist-mediated β1-AR internalization. After 30 min, the cells were chilled and washed with ice-cold Hanks' balanced salt solution (HBSS) to halt receptor endocytosis. To promote the recycling of internal β1-AR, cultures were incubated with 100 μm of the β-AR antagonist alprenolol at 37 °C for 15, 30, or 60 min and then fixed with 4% paraformaldehyde (14, 16). Some of the alprenolol (60 min) slides were chilled and exposed to 0.5 m NaCl, 0.2 m acetic acid, pH 3.5, for 4 min on ice to remove antibody bound to extracellular β1-AR and then fixed (18–20). Slides were visualized by confocal microscopy using Cy3, DAPI, and GFP laser settings of the Olympus FluoViewTM FV-1000 confocal microscope.
Immunoprecipitations and Western Blotting
For immunoprecipitations, HEK-293 cells or cardiac myocytes were lysed in lysis buffer composed of 150 mm NaCl, 50 mm Tris, pH 8.0, 5 mm EDTA, 1% Triton X-100 with protease inhibitor mixture (Sigma). Insoluble cellular debris was removed by centrifugation at 14,000 × gav for 15 min at 4 °C. After equalizing protein concentrations across all samples, lysates were incubated with ∼3–10 μl of M2 anti-FLAG-IgG beads at 4 °C with gentle rotation overnight. In control experiments, lysates were incubated with preimmune IgG at the same concentration. The beads were washed in lysis buffer, and immune complexes were eluted with Laemmli sample buffer containing 20 mm dithiothreitol. Eluted proteins and input lysates were separated by SDS-PAGE under denaturing conditions and electroblotted to nitrocellulose. Identical gels were run and transferred for separate detection of AKAP79 or SAP97.
Determination of Cyclic AMP by Fluorescence Resonance Energy Transfer Microscopy and by ELISAs
Cytosolic CFP-EPAC-YFP (21) was a generous gift from Kees Jalink (Division of Cell Biology, The Netherlands Cancer Institute, Amsterdam). Cells were plated on glass-bottom collagen-coated 35-mm plates (MatTek Corp., Ashland, MA) and transiently transfected with CFP-EPAC-YFP for 24–48 h. Cells were washed with HBSS after transfection and imaged in the dark on a 37 °C temperature-controlled stage. Subtype-selective β-AR antagonists were applied 10 min before the perfusion of isoproterenol and maintained throughout the experiment. Images were captured every 10 s using a 60× oil immersion objective in the Olympus FluoViewTM FV-1000 microscope. Background corrections of fluorescent images were carried out by subtracting the intensity of the background (autofluorescent cells and/or no cells) from the emission intensities of fluorescent cells expressing the reporters. All FRET ratios were normalized to time 0.
For the determination of cyclic AMP by ELISA, mouse ventricular myocytes were seeded into a 24-well plate at ∼60,000 cells per well. Cells were incubated in serum-free DMEM for 2 h and then in induction buffer (HBSS with 200 μm isobutyl-1-methylxanthine). Each selective β-adrenergic antagonist was added 15 min before exposing the cells to isoproterenol. The indicated concentrations of the nonselective β-agonist isoproterenol, selective β1-AR agonist xamoterol, or selective β2-AR agonist salmeterol were added for 10 min at 37 °C into triplicate wells. Following the stimulation, the medium was aspirated, and 0.5 ml of cold 0.1 n HCl was added for 10 min, followed by determining the absolute levels of cyclic AMP (direct cAMP ELISA, Enzo Life Sciences). For each condition, one well that was treated in an identical manner was used to determine either the cell number/well or protein level/well. Mean ± S.E. values were compared between the groups by ANOVA.
Immunocytochemistry, Morphometry, and Contraction Rates of Myocytes
Mouse cardiac myocytes on laminin-coated glass coverslips were exposed to buffer or 10 μm isoproterenol for 6 h. The cells were fixed and permeabilized in 0.2% Triton X-100 in HBSS for 10 min at 4 °C (22, 23). Permeabilized myocytes were incubated with a 1:2,000 dilution of anti-sarcomeric α-actinin or β-MHC antibodies and then incubated with a 1:100 dilution of rhodamine-conjugated goat anti-mouse (EMD Biosciences, San Diego) and analyzed by immunocytochemistry. Fixed cells were incubated with FluoromountTM-DAPI for 5 min to label the nuclei. Immunofluorescent images of cells stained for α-actinin or β-MHC were acquired at fixed settings of the Olympus FV-1000 microscope. These images were analyzed by ImageJ 1.46 software (National Institutes of Health) to measure surface areas and pixel intensities of 20 cells per field in four fields. β-MHC pixel intensities were divided by surface area to determine pixel intensity/area of each cell. The means ± S.E. for pixel intensity/area for 80 cells in each group were calculated and compared by one-way ANOVA to group A (WT/vehicle) to determine significance.
Measurement of spontaneous contraction rates of cardiac myocytes was performed as described (24). In brief, 2–3 × 105 mouse ventricular cardiac myocytes were cultured on glass coverslips for 5 days to obtain a uniformly beating syncytium. The cells were placed on the temperature-regulated stage of an inverted microscope connected to a video camera. Cells were equilibrated at 37 °C for 10 min before monitoring the contraction rate. Contraction rates of cells within the syncytium were determined at 60-s intervals for 10 min before and 20 min after stimulation with isoproterenol or subtype-selective β-agonists. The data were analyzed using Prism software 5.01.
Statistics
All data are expressed as means ± S.E., except where indicated. For comparison between two groups of data, Student's unpaired t test was used to determine significance, whereas multiple groups were compared by one-way ANOVA with Newman-Keuls post tests using Prism software (GraphPad, San Diego). Results were considered significant at p < 0.05.
RESULTS
Role of AKAP79 in Recycling of the β1-AR in HEK-293 Cells
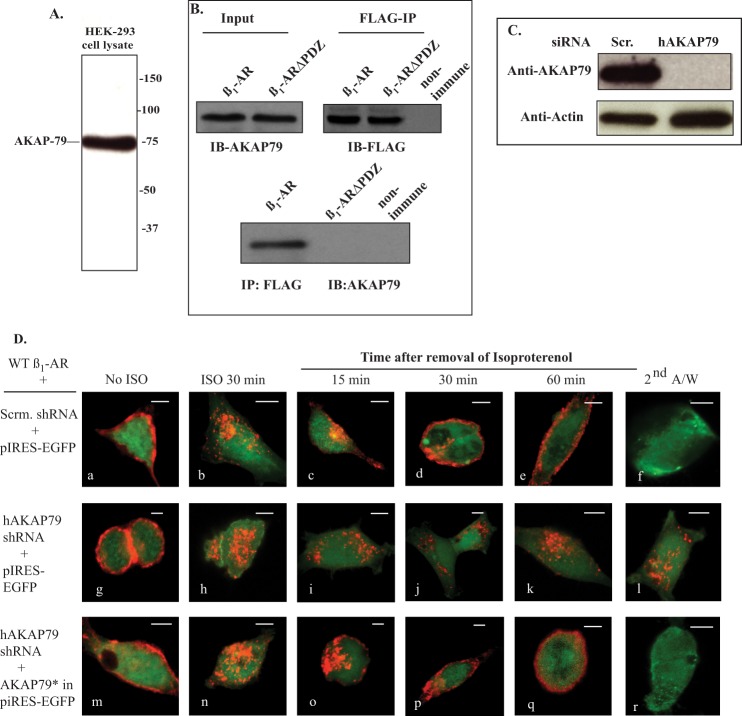
Immunoblots of cell extracts from HEK-293 cells indicated that these cells endogenously expressed AKAP79 and that immunoprecipitation (IP) of the β1-AR co-immunoprecipitated AKAP79 (Fig. 1, A and B). AKAP79 is targeted to the β1-AR by the scaffolding protein SAP97, which binds simultaneously to AKAP79 and to the type 1 PDZ at the extreme C-terminal tail of the β1-AR (16, 25, 26). To find out if the type 1 PDZ of the β1-AR was involved in its binding to AKAP79, the PDZ sequence (ESKV) between amino acids 474 and 477 in the β1-AR was inactivated by mutagenesis to alanine (β1-ARΔPDZ). HEK-293 cells stably expressing moderate levels of FLAG-tagged β1-ARΔPDZ were lysed, and FLAG-IPs were probed for co-IP of AKAP79 (Fig. 1B). These experiments showed that β1-ARΔPDZ did not co-IP SAP97, indicating that the type 1 PDZ of the β1-AR was involved in binding AKAP79.
FIGURE 1.
Role of AKAP79 in β1-AR trafficking in HEK-293 cells. A, detection of AKAP79 in HEK-293 extracts by immunoblotting (IB). B, cells stably expressing FLAG-tagged WT β1-AR or β1-ARΔPDZ were transfected with AKAP79 cDNA for 2 days. Extracts from these cells were probed for expression of AKAP79 (upper right-hand panel). Equal amounts of lysate were incubated with preimmune mouse IgG beads or with anti-FLAG IgG resin overnight. The resin was washed and eluted in Laemmli sample buffer. About 4% of the affinity-purified eluate was probed with anti-FLAG IgG to verify that equal amounts of β1-AR were immunoprecipitated (upper left-hand panel). The remainder of the eluate was probed for the co-IP of AKAP79 (lower panel). C, double-stable HEK-293 cells expressing FLAG β1-AR with either scrambled (Scr.) shRNA or AKAP79 shRNA were lysed and probed by Western blotting for expression of AKAP79. D, panels a–l, HEK-293 cells stably expressing FLAG-tagged WT β1-AR with scrambled shRNA (panels a–f) or AKAP79 shRNA (panels g–l) were transfected with empty pIRES-EGFPII vector. In panels m–r, HEK-293 cells stably expressing FLAG-tagged WT β1-AR and AKAP79 shRNA were transfected with shRNA-resistant AKAP79* in pIRES-EGFPII (panels m–r). Cells on glass slides were prelabeled for 1 h with Cy3-anti-FLAG antibody and fixed (panels a, g, and m). The rest of the slides were exposed to 10 μm isoproterenol (ISO) for 30 min and then fixed (panels b, h, and n). The rest of the slides were incubated with 100 μm alprenolol, subjected to recycling conditions for the indicated time period and then fixed. Slides that were incubated with alprenolol for 1 h were exposed to acid wash and then fixed (panels f, l, and r). The distribution of fluorescent pixels was obtained using the software of the confocal microscope, and the colors shown are pseudo colors. These experiments were repeated n = 5 times, and images were obtained from at least 20-cell fields/experiment. A/W, acid wash. Each scale bar, 5 μm.
An AKAP79 knockdown and rescue strategy was used to determine whether the expression of AKAP79 was required for trafficking of the β1-AR in HEK-293 cells. Scrambled or AKAP79 shRNAs in mir pcDNA 6.2 were expressed in HEK-293 cells that stably expressed the FLAG-tagged WT β1-AR. Endogenous AKAP79 was detected in cells expressing the scrambled shRNA, but it was selectively reduced by >95% in cells expressing AKAP79 shRNA (Fig. 1C). Next, we determined the effect of scrambled- or AKAP79-shRNA on trafficking of the WT β1-AR in HEK-293 cells (Fig. 1D). Cells stably expressing the FLAG WT β1-AR with scrambled or AKAP79 shRNA were transiently transfected with the empty mammalian expression vector p-IRES-EGFP (Fig. 1D, panels a–l). The cells were incubated with Cy3-labeled anti-FLAG M2-IgG (Fig. 1D, red) for 1 h to label the β1-AR and then visualized by confocal microscopy. Expression of the shRNA with pIRES-EGFP did not interfere with the membranous distribution of the β1-AR (Fig. 1D, panels a and g). Exposing these cells to isoproterenol resulted in rapid endocytosis of membranous β1-AR into intracellular vesicles (Fig. 1D, panels b and h). The effect of each shRNA on recycling of internal β1-AR was initiated by removal of isoproterenol followed by the addition of the β-adrenergic antagonist alprenolol to block the internalization of additional β1-AR and to promote the recycling of internal β1-AR. In cells expressing scrambled shRNA, the agonist-internalized β1-AR recycled back to the cell membrane within 60 min from the removal of isoproterenol with a t0.5 = 18 ± 5 min (Fig. 1D, panels c–e). The rate of recycling of the β1-AR in these cells was comparable with the kinetics of β1-AR recycling in native HEK-293 cells (14), indicating that the scrambled shRNA or pIRES-EGFP had no appreciable effect on the recycling kinetics of the β1-AR. However, β1-AR did not recycle in HEK-293 cells expressing AKAP79 shRNA, indicating that AKAP79 was required for recycling of the β1-AR in HEK-293 cells (Fig. 1D, panels i–k).
A major consequence of GPCR recycling is externalization, which involves the re-insertion of internalized GPCR into the cell membrane (18–20). If the externalized β1-AR were inserted properly into the cell membrane, then Cy3-conjugated anti-FLAG IgG bound to the N-terminal FLAG epitope of the β1-AR would be oriented extracellularly. In this case, an acid wash would strip Cy3-anti-FLAG IgG from the externalized β1-AR population but not from internal β1-AR (18, 19). Cells in which the β1-AR was internalized and then allowed to recycle for 60 min were exposed to an acid wash (Fig. 1D, panels f and l). In cells expressing the scrambled shRNA, the acid wash procedure stripped >80% of the Cy3 pixels, indicating that recycled β1-AR were externalized (Fig. 1D, panel f). However, Cy3 pixel distribution in AKAP79 knockdown cells was not altered by this maneuver, indicating that the β1-AR remained inside the cell (Fig. 1D, compare panels f–l).
An shRNA-resistant AKAP79 in pIRES-EGFP (pIRES-EGFP-AKAP79*) was created by mutating two oligonucleotides in the shRNA-binding sequence of AKAP79. This construct was used to determine whether shRNA-resistant AKAP79 (AKAP79*) could rescue the recycling of the WT β1-AR in AKAP79 knockdown cells. HEK-293 cells stably expressing FLAG-β1-AR and AKAP79 shRNA were transiently transfected with pIRES-EGFP-AKAP79* (Fig. 1D, panels m–r). Images of cells that were preincubated with Cy3-labeled anti-FLAG M2 IgG (red) for 1 h revealed that expression of pIRES-EGFP-AKAP79* did not interfere with membranous distribution of the β1-AR (Fig. 1D, panel m). Exposing these cells to isoproterenol resulted in rapid endocytosis of membranous β1-AR into intracellular vesicles (Fig. 1D, panel n). Expression of AKAP79* from the pIRES-EGFP-AKAP79* vector rescued the recycling of the β1-AR, which was externalized with a t0.5 of 21 ± 6 min (Fig. 1D, panels o–r). The recycling kinetics of the β1-AR in AKAP79*-expressing cells were no different from those observed in HEK-293 cells (13).
Role of the Protein Binding Domains of AKAP79 in Binding and Recycling of the β1-AR in HEK-293 Cells
AKAP79 is a networking protein that contains several protein-protein interaction domains. AKAP79 binding partners include the following: the membrane and PKC binding domains (amino acids 1–151) in the N terminus; a protein phosphatase 2B (calcineurin) binding domain between amino acids 321 and 360, and the PKA binding domain between 360 and 427 (7–9). Using the shRNA-resistant AKAP79* template, three truncated AKAP79* mutants, (amino acids 1–360) AKAP79* (deletion of PKA binding), (amino acids Δ321–360) AKAP79* (deletion of calcineurin binding), and (amino acids 151–427) AKAP79* (deletion of PKC and membrane binding domains) were generated. Specific domain deletions of AKAP79* were cloned into p-IRES-EGFP-II and were expressed in HEK-293 cells that stably expressed FLAG-β1-AR and AKAP79 shRNA. Immunoblots of ∼5% of each cell lysate revealed that each AKAP79* deletion construct migrated with the expected mobility, indicating that they escaped degradation by the AKAP79 shRNA (Fig. 2B). Equal amounts of each lysate were incubated with 3–5 μl of anti-FLAG IgG resin and the IPs were subjected to Western blot analysis with anti-AKAP79 antibody (Fig. 2C). FLAG-β1-AR IPs co-immunoprecipitated full-length AKAP79*, (amino acids 1–360) AKAP79*, (amino acids 151–427) AKAP79*, and (amino acids Δ321–360) AKAP79*. Therefore, these deletions in AKAP79 did not abrogate its binding to the β1-AR.
FIGURE 2.
Role of the scaffolding domains of AKAP79 in recycling of the β1-AR in HEK-293 cells. A, schematic representation of AKAP79*(1–427) (full length) and its three deletion mutants, AKAP79*(1–360), AKAP79*(Δ321–360), and AKAP79*(151–427). B, HEK-293 cells stably expressing FLAG-β1-AR and AKAP79 shRNA were transiently transfected with AKAP79* or its shRNA-resistant mutants for 2 days. Lysates from these cells were immunoblotted (IB) with anti-AKAP79 antibody. C, extracts from the cells in B above were incubated with preimmune mouse IgG beads or with anti-FLAG IgG resin overnight. The resin was washed, eluted, and probed for the co-IP of AKAP79. D, AKAP79* in pIRES-EGFP or its deletion mutants were transfected into double-stable HEK-293 cells expressing FLAG β1-AR and AKAP79-shRNA. Internalization and recycling of the β1-AR in these cells were conducted as described under “Experimental Procedures” and in the legend of Fig. 1. Each scale bar represents 5 μm. E, extracts from the cells in B above were incubated with preimmune IgG resin or anti-AKAP79 IgG resin. The resin was eluted and probed for co-IP of SAP97. Input lysates represented 5% of the total extract, whereas 50% of the total volume was used for immunoprecipitation. A/W, acid wash.
Next, we investigated whether the three AKAP79* deletion mutants could substitute for full-length AKAP79 in rescuing the recycling of the β1-AR in HEK cells stably expressing FLAG-β1-AR and AKAP79 shRNA (Fig. 2D). Truncations in these AKAP79* mutants did not affect either the membranous distribution of the β1-AR or its internalization in response to isoproterenol (Fig. 2D, panels a, b, g, h, m, and n). The cells were then washed to remove the β-agonist, and β1-AR-Cy3 fluorescence was re-evaluated after 15, 30, and 60 min from the addition of alprenolol. In contrast to what we observed with full-length AKAP79* (Fig. 1D, panels o–q), the three deletion mutants of AKAP79* could not rescue the recycling of the β1-AR, and these receptors were retained by >85% internally (Fig. 2D, panels c–f, i–l, and o–r).
To find out if loss of the PKC-binding site between amino acids 1 and 75 of AKAP5 affected the trafficking of the β1-AR, the activity of PKC was inhibited with 5 μm bisindolylmaleimide (GF 109203X), which at this concentration inhibits all the isoforms of PKC (27). In cells pretreated with GF 109203X, the WT β1-AR was internalized and recycled efficiently and completely, indicating that the activity of PKC might not be required for the trafficking of the β1-AR in HEK-293 cells (data not shown).
SAP97 is involved in recycling of the agonist-internalized β1-AR (16). Thus, a major reason for failure of truncated AKAP79* mutants in rescuing the recycling of the β1-AR might be due to their inability to bind SAP97. To find out if SAP97 would bind to these AKAP79* truncation mutants, each pIRES-EGFP AKAP79* deletion mutant was transiently transfected into HEK-293 cells stably expressing AKAP79 shRNA. Lysates prepared from each of these cells were immunoprecipitated with anti-AKAP79 and then probed for the co-IP of endogenous SAP97 (Fig. 2E). IPs of AKAP79 co-immunoprecipitated SAP97 from all of these cells, indicating that the SAP97-binding site in AKAP79 was not inactivated by these mutations. These results demonstrate that the entire AKAP79 protein was necessary to rescue the recycling of the β1-AR.
Characterization of the Trafficking of the β1-AR in Rat Neonatal Ventricular Myocytes
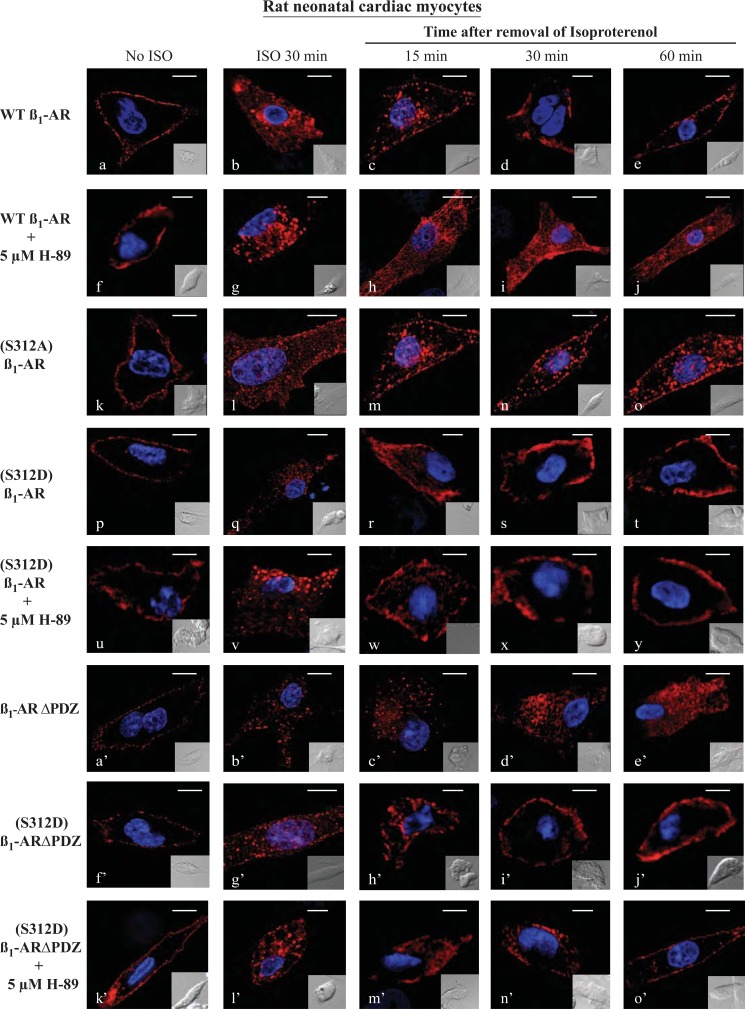
The pharmacological effects of the β1-AR are manifest mainly in the heart as increased chronotropy and inotropy. AKAP5 and SAP97 were involved in targeting the β1-AR to the membrane of cardiomyocyte-like H9c2 cells (12). Therefore, it is crucial to determine whether findings in HEK-293 cells were reproducible in a more physiologically relevant setting such as cardiac cells. Consequently, β1-AR recycling experiments were repeated in neonatal rat ventricular cardiac myocytes (NRVCM). NRVCM-expressing FLAG-tagged β1-ARs were stained with Cy3-labeled anti-FLAG IgG, fixed, and mounted in medium containing DAPI to stain the nuclei (Fig. 3, a–e). Each image in Fig. 3 represents Cy3-stained β1-AR (pseudo red color) along with superimposed DAPI staining (pseudo violet color) of cardiac myocytes, although the insets show Nomarski optical images of the NRVCM. In unstimulated NRVCM, β1-AR as indexed by Cy-3 staining was localized on the plasma membrane (Fig. 3). Similar results to those shown in Fig. 3 were obtained when NRVCM were fixed and then permeabilized with 0.2% Triton X-100 for 10 min at 4 °C (18), prior to their incubation with the Cy3-labeled anti-FLAG antibody (data not shown). Exposing NRVCM to isoproterenol promoted the internalization of β1-AR into the cytosol of rat cardiac myocytes (Fig. 3). Replacing isoproterenol with alprenolol promoted the recycling of internal β1-AR back to the membrane (Fig. 3, c–e). The t0.5 for the recycling of β1-AR in NRVCM was 17 ± 4 min, which is comparable with its recycling kinetics in HEK-293 cells (14).
FIGURE 3.
Characterization of β1-AR trafficking in rat neonatal ventricular myocytes. Neonatal rat cardiac myocytes were cultured on collagen-coated coverslips and then infected with 100 multiplicities of infection of adenovirus harboring FLAG-tagged WT β1-AR or the other β1-AR constructs. Cells were prelabeled for 1 h with Cy3-anti-FLAG antibody and fixed (a, f, k, p, u, a′, f′, and k′). The rest of the slides were exposed to isoproterenol (ISO) for 30 min then fixed (b, g, l, q, v, b′, g′, and l′). The remaining slides were subjected to recycling conditions for the indicated time period and then fixed and visualized by confocal microscopy using the Olympus FluoViewTM FV-1000 confocal microscope (n = 3 experiments and at least 40 images per experiment were analyzed). Each scale bar, 5 μm.
In Gardner et al. (14), we determined that trafficking of the β1-AR in HEK-293 cells was dependent on PKA and its target serine at position 312 in the third intracellular loop of the β1-AR. Thus, when the recycling experiment was conducted in cells pretreated with the PKA inhibitor H-89, recycling of the agonist-internalized β1-AR in NRVCM was inhibited (Fig. 3, h–j). Similarly, mutating the serine at position 312 in the β1-AR to alanine ((S312A)β1-AR) also inhibited the recycling of agonist-internalized (S312A)β1-AR in NRVCM (Fig. 3, m–o). However, the phosphoserine 312 mimic of the β1-AR ((S312D)β1-AR) was internalized in response to isoproterenol, and internal (S312D)β1-AR recycled normally and efficiently in control or H-89-treated NRVCM (Fig. 3, r–t and w–y). These results indicate that phosphorylation of Ser312 promoted the positive effect of PKA on β1-AR recycling in NRVCM.
A mechanism for AKAP5-mediated regulation of β1-AR trafficking in HEK-293 cells involved targeting of the PKA-AKAP5 complex to the type 1 PDZ in the C-terminal tail of the β1-AR (16). Therefore, inactivation of the β1-AR PDZ (β1-ARΔPDZ) inhibited the interaction between AKAP5 and the β1-AR (Fig. 1B) and recycling of β1-ARΔPDZ (16). To find out if the type 1 PDZ of the β1-AR was also involved in trafficking of the β1-AR in cardiac myocytes, trafficking of β1-ARΔPDZ in NRVCM was determined (Fig. 3, a′–e′). β1-ARΔPDZ was internalized in NRVCM in response to isoproterenol, but internal β1-ARΔPDZ did not recycle (Fig. 3, c′–e′). Finally, we investigated the relationship between the type 1 PDZ, PKA, and Ser312 in regulating the trafficking of the β1-AR in NRVCM. Results from HEK-293 cells indicated that a pool of AKAP5-PKA was anchored to the β1-AR PDZ. Activation of the β1-AR would then promote the phosphorylation of Ser312 in the third intracellular loop by the PDZ-targeted pool of PKA (13). In this view, phospho-Ser312 would promote the recycling of the β1-AR even when the PDZ or PKA was inactivated. To find out if phospho-Ser312 could support the recycling of the β1-AR with an inactivated PDZ, recycling of the β1-AR mutant with a phospho-Ser312 mimic and an inactivated PDZ ((S312D)β1-ARΔPDZ) was conducted in NRVCM (Fig. 3, f′–j′). The (S312D)β1-ARΔPDZ was expressed on the surface of NRVCM, and the receptor was internalized in response to isoproterenol (Fig. 3, f′ and g′). Internal (S312D)β1-ARΔPDZ recycled rapidly and efficiently with a t0.5 of 21 ± 6 min (Fig. 3, h′–j′). Pretreatment of NRVCM expressing the (S312D)β1-ARΔPDZ mutant with H-89 did not affect the membranous distribution of the mutant receptor or its internalization in response to isoproterenol (Fig. 3, k′ and l′). Moreover, internal (S312D)β1-ARΔPDZ recycled efficiently and completely in H-89-treated NRVCM cells (Fig. 3, m′ and n′). These results show that similar mechanisms were governing the trafficking of the β1-AR in HEK-293 cells and in NRVCM.
Effect of AKAP5 on Trafficking of Cardiac β1-AR
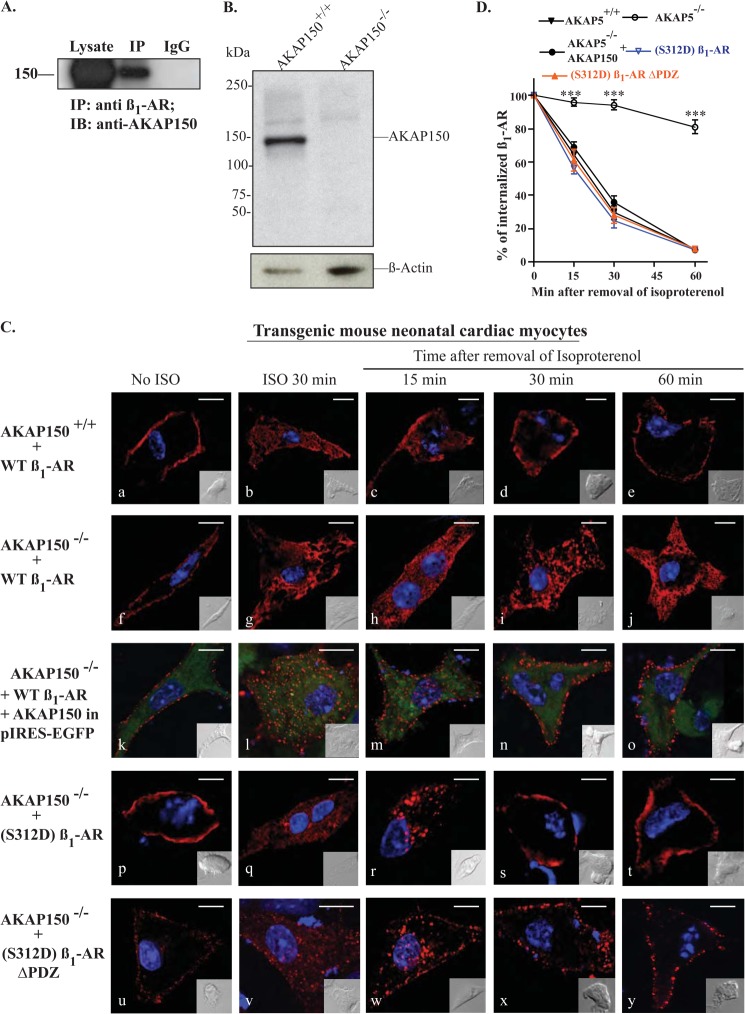
To determine whether AKAP150, which is the rodent homolog of AKAP79, played a role in the trafficking of the β1-AR in cardiac myocytes, we first determined whether the β1-AR would interact with endogenous AKAP150 in these cells. NRVCM were infected with adenovirus-expressing FLAG-tagged β1-AR for 6 h, and subsequently cultured for 48 h before lysis. Cell lysates were incubated with anti-FLAG IgG or preimmune IgG beads, and the eluates were subjected to Western blotting and probed with the anti-AKAP150 antibody (Fig. 4A). FLAG IPs co-immunoprecipitated AKAP150, although preimmune IgG IPs did not, which was similar to the results we obtained earlier in HEK-293 cells (13).
FIGURE 4.
Characterization of β1-AR trafficking in AKAP5+/+versus AKAP5−/− mouse neonatal ventricular myocytes. A, cell lysates prepared from rat neonatal cardiomyocytes were incubated with preimmune rat IgG beads or with anti-β1-AR antibody overnight and then with protein-G-agarose for 2 h. The resin was washed, eluted, and probed for the co-IP of AKAP150. IB, immunoblot. B, cardiac extracts prepared from AKAP5+/+ or AKAP5−/− mice were probed for expression of endogenous AKAP150 and β-actin by immunoblotting. C, internalization and recycling of the β1-AR in mouse neonatal cardiac myocytes. Mouse neonatal ventricular cardiac myocytes were cultured on laminin-coated coverslips and then infected with 100 multiplicities of infection of adenovirus harboring FLAG-tagged WT β1-AR or the other β1-AR constructs. In addition, the cells in panels k–o were co-transfected with AKAP150 in pIRES-EGFP. Cells were prelabeled for 1 h with Cy3-anti-FLAG antibody and fixed (panels a, f, k, p, and u). The rest of the slides were exposed to isoproterenol (ISO) for 30 min then fixed (panels b, g, l, q, and v). The remaining slides were subjected to recycling conditions for the indicated time period and then fixed. Distribution of fluorescent pixels is shown in pseudo colors as described in the legends of Figs. 1 and 3. D, pixels inside a 300-nm boundary in isoproterenol-treated cells (panels b, g, l, q, and v) were set arbitrarily to 100% to indicate 100% internalization, and the ratios in alprenolol-treated cells were calculated by the FluoViewTM FV-1000 software and plotted as % for each time period. The means ± S.E. for each time point were derived from 20 images/per experiment, and each experiment was replicated three times. The t0.5 for recycling was calculated by fitting the relevant data to a single exponential function. ***, p < 0.001 when compared with recycling in AKAP5−/− myocytes by one-way ANOVA with post hoc tests. Each scale bar, 5 μm.
To determine with more precision the role of AKAP5 in trafficking of cardiac β1-AR, cardiac myocytes were prepared from neonatal AKAP5+/+ and from AKAP5−/− mice, which do not express AKAP150 (Fig. 4B). Neonatal mouse ventricular cardiac myocytes (NMVCM) prepared from AKAP5+/+ or AKAP5−/− mice were plated on collagen-coated coverslips and then infected with adenovirus-expressing FLAG-tagged WT β1-AR (Fig. 4C). The β1-AR was localized to the cell membrane of unstimulated NMVCM (Fig. 4C, panels a and f). Stimulation of β1-AR with isoproterenol promoted the internalization of the majority of Cy3-labeled β1-AR (Fig. 4C, panels b and g). In myocytes prepared from AKAP5+/+, β1-AR recycled rapidly and efficiently when isoproterenol was replaced with alprenolol (Fig. 4C, panels c–e). However, internal β1-AR in cardiac myocytes prepared from AKAP5−/− mice did not recycle (Fig. 4C, panels h–j). The estimated t0.5 for recycling of the β1-AR in AKAP5+/+ myocytes was 19 ± 6 min, which is similar to the recycling kinetics of the WT β1-AR in HEK-293 cells (Fig. 4D). However, there was no evidence of β1-AR recycling in AKAP5−/− myocytes (n = 16).
To determine whether AKAP150 would restore the recycling phenotype of the β1-AR in NMVCM from AKAP5−/− mice, AKAP5−/− myocytes were transfected with WT β1-AR expressing adenovirus for 6 h and then overnight with the AKAP150 expressing p-IRES-EGFP plasmid (Fig. 4C, panels k–o). The recycling assay was carried out as described earlier, but trafficking of the β1-AR was determined in cells that expressed AKAP150 (i.e. GFP-expressing cells). Images in Fig. 4C (panels k–o) show combined Cy-3, EGFP, and DAPI fluorescence, whereas EGFP fluorescence indexed AKAP5 expression, and Cy-3 staining revealed the distribution of the β1-AR. Expression of AKAP150 in AKAP5−/− NMVCM did not affect membranous distribution of β1-AR or their internalization in response to isoproterenol (Fig. 4C, panels k and l, respectively). Recycling of β1-AR in AKAP5−/− myocytes was restored in cells that expressed AKAP150-pIRES-EGFP (Fig. 4C, panels m–o). The effect of AKAP150 on the recycling of β1-AR in AKAP5−/− cardiomyocytes was remarkable, although β1-AR recycled with a t0.5 of 16 ± 4 min (Fig. 4D). These results indicate that trafficking kinetics of the β1-AR in mouse cardiac myocytes and in HEK-293 cells were comparable.
A major finding concerning the mechanism of the AKAP5-PKA complex in regulating the trafficking of the β1-AR in HEK-293 cells was its critical role in phosphorylating Ser312 (16). The role of phospho-Ser312 in recycling of the β1-AR in an AKAP5 null environment was investigated (Fig. 4C, panels p–t). In AKAP5−/− cardiomyocytes, (S312D)β1-AR was expressed at the cell membrane, and the receptor was internalized in response to isoproterenol (Fig. 4C, panels p and q, respectively). Internal (S312D)β1-AR recycled efficiently and completely in AKAP5−/− myocytes, indicating that the Ser312 to Asp312 mutation overcame the effect of knocking out AKAP5. To verify that the phosphoserine 312 mimic was indeed capable of surmounting the combined deficits of AKAP79 and the β1-AR PDZ, recycling of the double mutant (S312D)β1-ARΔPDZ was determined in AKAP5−/− cardiomyocytes (Fig. 4C, panels u–y). (S312D)β1-ARΔPDZ was expressed on the cell surface of AKAP5−/− myocytes and was internalized in response to isoproterenol (Fig. 4C, panels u and v). After substituting isoproterenol with alprenolol, internal (S312D)β1-ARΔPDZ recycled efficiently with a t0.5 of 21 ± 7 min (n = 6). In addition, (S312D)β1-ARΔPDZ recycled efficiently in AKAP5−/− cardiomyocytes in which PKA was inhibited by H-89 (data not shown). Thus, the data in Figs. 1–4 demonstrated that the mechanism of AKAP5-mediated regulation of β1-AR recycling was shared between mammalian cells and involved the phosphorylation of Ser312 by the PKA-AKAP complex tethered to the PDZ domain of the β1-AR (16).
Role of AKAP5 in Spatiotemporal Dynamics of Cyclic AMP Signals Elicited by Isoproterenol in Cardiac Myocytes Derived from AKAP5 Transgenic Mice
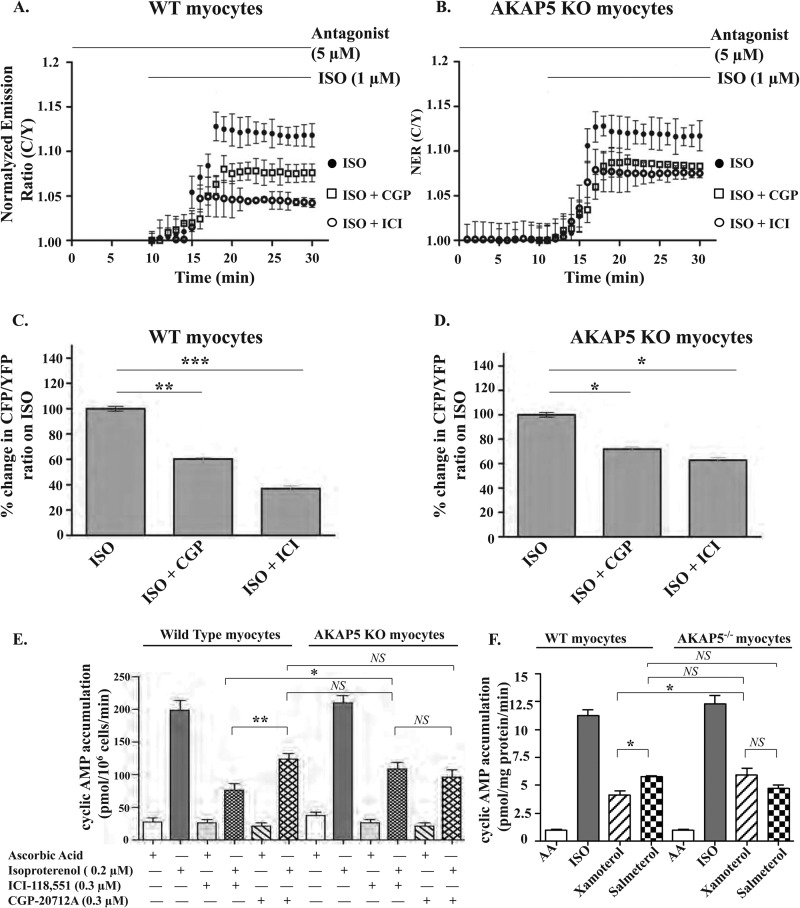
Sympathetic activation of myocardial β-AR increases heart rate and the force of myocardial contractions by increasing the intracellular levels of cyclic AMP. Therefore, it is of great interest to determine the effect of AKAP5 on cyclic AMP dynamics in cardiac cells because cyclic AMP directly activates PKA, which is intimately involved in regulating the recycling of the β1-AR (16). Neonatal cardiac myocytes express β1- and β2-AR, and the activation of both of these receptors leads to a rapid rise in intracellular cyclic AMP levels. To investigate how AKAP5 affects the two β-AR subtypes in regulating cyclic AMP dynamics in NMVCM, we used the cyclic AMP sensor CFP-EPAC-YFP (21). Cardiac myocytes from AKAP5+/+ mice were super-fused with the nonselective β-AR agonist isoproterenol, which produced a moderate increase of 12 ± 4% in C-EPAC-Y FRET ratios (Fig. 5A). Next we analyzed the contribution of each subtype of the β-AR to cyclic AMP levels by repeating these experiments in cells preincubated with 1 μm of the selective β1-AR antagonist CGP-20712 to index the β2-AR component or with 1 μm of the selective β2-AR antagonist ICI-118,551 to index the β1-AR component. Stimulation of β1- or β2-AR in AKAP5+/+ myocytes increased the amplitude of C/Y fluorescence ratios by 3.4 ± 1 and 7.5 ± 1%, respectively (Fig. 5, A and C). In AKAP5−/− myocytes, isoproterenol increased C/Y fluorescence ratios by 11 ± 2%, which was similar to its effect on cyclic AMP in AKAP5+/+ myocytes and in agreement with the results of Nichols et al. (11). Similarly, isoproterenol + CGP (β2-AR component) induced an 8.7 ± 1% increase in maximal C/Y fluorescence ratios, which was not significantly different from their effect on cyclic AMP levels in AKAP5+/+ myocytes (compare isoproterenol + CGP in Fig. 5, C and D). However, isoproterenol + ICI (β1-AR component) increased the C/Y fluorescence ratios by 7.6 ± 1% in cardiac myocytes derived from AKAP5−/− mice, which was ∼2-fold higher than the β1-AR component in AKAP5+/+ myocytes (p < 0.05). These results show that knock-out of endogenous AKAP5 increased myocardial signaling intensity of β1-AR as indexed by changes in C/Y fluorescence ratios of the cyclic AMP sensor.
FIGURE 5.
Role of AKAP5 in cytosolic cyclic AMP signaling induced by isoproterenol in neonatal mouse cardiomyocytes. Mouse neonatal cardiac myocytes from AKAP5+/+ (A and C) and AKAP5−/− (B and D) on glass-bottom dishes were infected with adenovirus harboring the CFP-EPAC-YFP construct. A and B, time profiles of normalized C/Y FRET ratios in response to 1 μm isoproterenol (ISO) or in response to isoproterenol in myocytes preincubated with 5 μm of either the β1-AR antagonist CGP 20711 or 5 μm of the β2-AR antagonist ICI-181,551 (ICI). Traces represent normalized FRET ratios from different cells. C and D, maximal changes in C/Y FRET ratios from 20 independent cells were plotted as means ± S.E. and then compared among different groups. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 when compared with controls by one-way ANOVA. E, mouse cardiac myocytes from AKAP5+/+ or AKAP5−/− mice were exposed to 200 μm isobutyl-1-methylxanthine and to the indicated concentrations of CGP or ICI for 15 min. Then the cells were exposed to buffer or isoproterenol for 10 min at 37 °C. The amount of cyclic AMP/106 cells was determined by ELISA. F, cardiac myocytes from AKAP5+/+ or AKAP5−/− mice preincubated with isobutyl-1-methylxanthine were exposed to the indicated concentrations of β-agonist for 10 min at 37 °C. The amount of cyclic AMP/mg of protein was determined by ELISA. The data were plotted as means ± S.E. and then compared among different groups. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 when compared with the indicated condition by one-way ANOVA with Newman-Keuls post tests. AA, ascorbic acid. NS, not significant.
We also determined the effect of AKAP5 on β-AR-mediated regulation of cyclic AMP in NMVCM by ELISAs (Fig. 5E). Exposing NMVCM prepared from AKAP5+/+ or AKAP5−/− mice to 200 nm isoproterenol for 10 min resulted in a comparable 8-fold increase in cyclic AMP accumulation over basal levels (p > 0.05, n = 6 each in triplicate). In AKAP5+/+ NMVCM, ICI and CGP inhibited the accumulation of cyclic AMP by 61 ± 8 and 43 ± 7%, respectively. The effect of CGP versus ICI on this parameter in AKAP5+/+ NMVCM was significant (p < 0.05 by one-way ANOVA). In AKAP5−/− NMVCM, ICI and CGP inhibited the accumulation of cyclic AMP by 52 ± 8 and by 58 ± 8%, respectively (p > 0.05). In essence, accumulation of cyclic AMP by the β1-AR component was ∼34% higher in AKAP5−/− myocytes as compared with its level in AKAP5+/+ myocytes, and this increase was significant (p < 0.05). In AKAP5+/+ NMVCM, the β2-AR component was significantly higher than the β1-AR component. However, the contribution of each β-AR subtype to cyclic AMP was not significantly different in AKAP5−/− NMVCM (Fig. 5E).
In another series of experiments, subtype-selective β-AR agonists were used to determine the contribution of the β1-AR versus the β2-AR to cyclic AMP accumulation in NMVCM prepared from AKAP5+/+ or from AKAP5−/− mice (Fig. 5F). These assays involved the use of the lowest maximally effective doses of isoproterenol (nonselective β-agonist), xamoterol (selective β1-AR partial agonist), and salmeterol (selective β2-AR agonist) in stimulating cyclic AMP in the hearts of mice (28, 29). Forskolin (5 μm) increased the accumulation of cyclic AMP from ∼1.1 ± 0.1 to 41 ± 5 pmol/mg protein/min in AKAP5+/+ or AKAP5−/− NMVCM. Similarly, the effect of isoproterenol on cyclic AMP in NMVCM prepared from neonatal AKAP5+/+ or AKAP5−/− mice was comparable (∼11 ± 1 pmol/mg protein/min). In AKAP5+/+ NMVCM, xamoterol and salmeterol increased the accumulation of cyclic AMP to 4.2 ± 0.8 and to 5.8 ± 0.6 pmol/mg protein/min, respectively (p < 0.05). In AKAP5−/− NMVCM, xamoterol and salmeterol increased the accumulation of cyclic AMP to 5.9 ± 1.5 and to 4.7 ± 0.7 pmol/mg protein/min, respectively (p > 0.05). Therefore, the effect of xamoterol on accumulation of cyclic AMP in AKAP5−/− NMVCM was significantly higher (∼40%) than its effect in AKAP5+/+ NMVCM (p < 0.05). However, the effect salmeterol on accumulation of cyclic AMP in AKAP5+/+ NMVCM was not significantly different from its effect in AKAP5−/− NMVCM (Fig. 5F). Thus, data obtained by the cyclic AMP sensor or by ELISAs indicated that production of cyclic AMP by the β1-AR signaling pathway was significantly increased in AKAP5−/− NMVCM.
Role of AKAP5 in Isoproterenol-mediated Effects on Cardiac Myocyte Contractility and Size
Enhanced stimulation of myocardial β1-AR results in chronotropy and hypertrophy of cultured cardiac myocytes in vitro and in animals in vivo (30, 31). Cardiomyocytes from AKAP5−/− mice displayed enhanced β1-AR signaling and aberrant β1-AR recycling. Therefore, an assessment of the role of AKAP5 on those cardiac parameters that are directly affected by the β1-AR and its signaling pathway is warranted because modest increases in β1-AR expression in transgenic mice resulted in deleterious effects on the heart (32). In addition, there are very few studies that have assessed the effect of genetic ablation of AKAP5 in neonatal cardiac myocytes. Therefore, experiments were designed to provide critical new information on the role of AKAP5 in modulating the effects of β-AR signaling on cardiomyocyte biology.
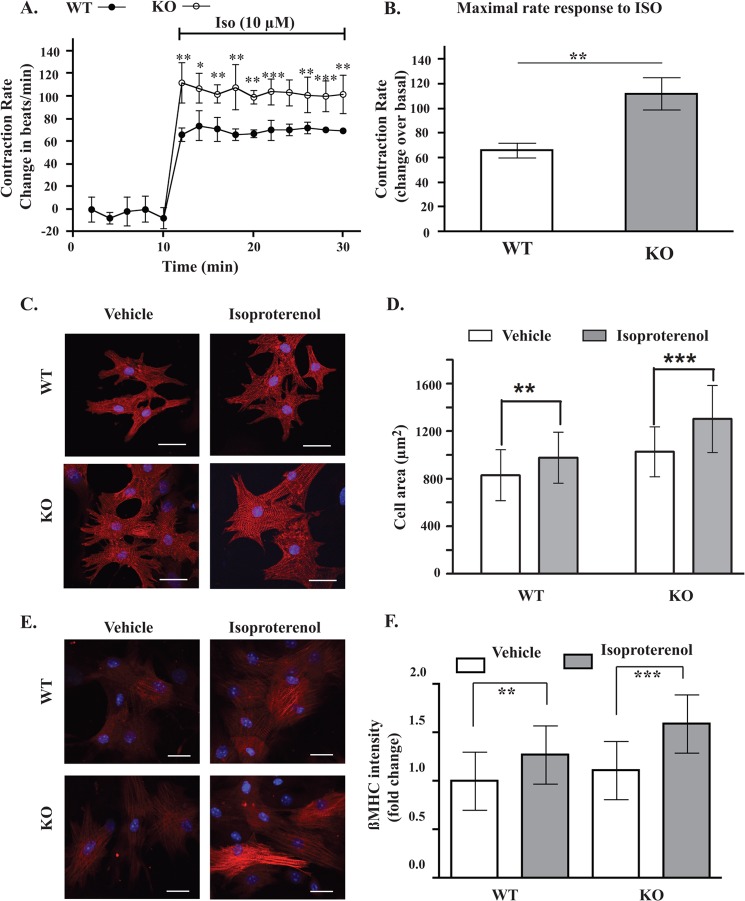
An important function of myocardial β1-AR is to control the rate of cardiomyocyte contractions (30). To find out if AKAP5 was involved, the effect of isoproterenol on contractions of cardiac myocytes prepared from AKAP5+/+ mice was compared with its effect on contractions of cardiac myocytes prepared from AKAP5−/− mice (Fig. 6A). NMVCM were cultured for 5 days until confluence and then placed on a heated stage, and their contractions per min (bpm) were counted. Contractions of cardiomyocytes prepared from either mouse were similar, 125 ± 9 bpm in AKAP5+/+ myocytes versus 133 ± 9 bpm in AKAP5−/− myocytes (p > 0.05). Isoproterenol increased the contractions of cardiomyocytes prepared from AKAP5+/+ mice by 66 ± 6 bpm versus 112 ± 13 bpm in AKAP5−/− cardiomyocytes (p < 0.05, n = 18). We also compared the effect of subtype-selective β-adrenergic agonists on the contractility of cardiac myocytes derived from AKAP5+/+ and AKAP5−/− mice. Analysis of the concentration-response relationship between the nonselective β-agonist isoproterenol and the β1-selective agonist xamoterol on contractility indicated that maximal effects of isoproterenol and xamoterol on contractility occurred after 10 min and were attained at 0.5 and 2 μm, respectively (28). In cardiac myocytes derived from AKAP5+/+ mice, 0.5 μm isoproterenol increased contractility by 60 ± 5%, whereas 2 μm xamoterol increased contractility by 46 ± 5%, which is similar to their effects on heart rate in vivo in rodents (28, 29). A 2 μm concentration of the β2-selective agonist salmeterol caused a 22 ± 4% increase in contractility over basal in AKAP5+/+ cardiomyocytes, which was significantly less than the effect of isoproterenol or xamoterol on this parameter (p < 0.001 and p < 0.05 compared with isoproterenol and xamoterol, respectively). In AKAP5−/− cardiomyocytes, 0.5 μm isoproterenol or 2 μm xamoterol increased contractility by 102 ± 8 and 78 ± 7% over basal, respectively (p < 0.01 over their effect on AKAP5+/+ myocytes). However, salmeterol increased contractility by 29 ± 5% in AKAP5−/− NMVCM, which was not statistically different from its effect in AKAP5+/+ NMVCM. These results indicate that functional signaling by the β1-AR subtype on contractility was significantly enhanced in AKAP5−/− NMVCM.
FIGURE 6.
Effect of AKAP5 expression on cardiomyocyte function. A, contraction rates of cardiac myocytes prepared from AKAP5+/+ or AKAP5−/− mice were measured for 10 min before and for 30 min after the addition of 10 μm isoproterenol (ISO). B, increases in maximal contraction rates in A were compared between AKAP5+/+ myocytes and AKAP5−/− myocytes. C and E, cardiomyocytes from AKAP5+/+ mice or AKAP5−/− mice were stimulated with vehicle or 10 μm isoproterenol for 6 h and then fixed and stained for α-actinin (red) and β-myosin heavy chains (red). D and F, 80 cells per group per isolation were analyzed by morphometry in D and for fluorescence intensity in F. Each scale bar, 20 μm. Bars represent means ± S.E. A, analysis of variance was determined by Student's t test. **, p < 0.01, and ***, p < 0.001. B and D, **, p < 0.01, and ***, p < 0.001 for isoproterenol versus basal morphometry in WT and KO cells, respectively. **, p < 0.01, and ***, p < 0.001 for isoproterenol versus basal β-MHC in WT and KO respectively; one-way ANOVA with Newman-Keuls post tests.
Next, we sought to determine the effect of AKAP5 on surface areas of NMVCM because activation of the β1-AR subtype was associated with hypertrophy (30). Immunocytochemical and morphometric assays showed that surface areas of vehicle-treated AKAP5−/− NMVCM were significantly greater than AKAP5+/+ NMVCM (cell area, 829 ± 213 μm2 in AKAP5+/+ myocytes versus 1026 ± 210 μm2 in AKAP5−/− myocytes)(Fig. 6, C and D). As expected, isoproterenol increased the surface area of NMVCM prepared from AKAP5+/+ mice by 18 ± 2% (p < 0.01, n = 80). In AKAP5−/− NMVCM, the effect of isoproterenol on surface area was enhanced by an additional 26.9 ± 2.1% (p < 0.001, n = 90).
Increased myocardial size is associated with increased expression of several hypertrophy markers, such as β-MHC (Myh7) and others (31). The effect of isoproterenol on expression of β-MHC in AKAP5+/+ NMVCM versus AKAP5−/− NMVCM was determined by immunohistochemistry. For this purpose, we used a validated highly specific antibody to β-MHC (33). As shown in Fig. 6E, expression of β-MHC was not statistically different between untreated NMVCM (Fig. 6, E and F). Isoproterenol increased β-MHC expression in AKAP5+/+ cardiac myocytes by 1.3 ± 0.3-fold, and this effect was significant (p < 0.01, n = 80). The effect of isoproterenol on β-MHC expression in AKAP5−/− NMVCM was more pronounced (1.6 ± 0.3-fold), and this effect was highly significant (p < 0.001, n = 80). Therefore, the effect of isoproterenol on cardiomyocyte contractions, cell area, and β-MHC expression was enhanced in NMVCM prepared from AKAP5−/− mice as compared with NMVCM prepared from AKAP5+/+ mice.
DISCUSSION
The hallmark for the activation of the β1-AR is the generation of the second messenger cyclic AMP, which activates PKA by releasing the catalytic kinase subunits of PKA. The role of AKAP in this process is fundamental because they have PKA binding and networking domains to diversify spatiotemporal signaling of PKA (1–3). Our studies on the role of AKAPs in trafficking of the human β1-AR in HEK-293 cells identified a key role for AKAP79 (AKAP5) in this phenomenon (13). Trafficking of the GPCR is a result of chronic activation of the GPCR by relatively high concentrations of the specific agonist. The first step in GPCR trafficking is its internalization, which is a consequence of phosphorylating the agonist-occupied GPCR by G protein-coupled receptor kinase (GRK) (34, 35). GRK-mediated phosphorylation increases the binding affinity of the phosphorylated region of the GPCR to β-arrestins. The binding of β-arrestin to the GPCR facilitates its internalization via clathrin-coated pit-mediated endocytosis (36). Thus, the GRK-β-arrestin axis provides a major pathway for desensitization and regulated endocytosis of the GPCR (36). It should be noted, however, that activation of PKA occurred in earlier time frames and required lower concentrations of β-agonists than the activation of GRK (37).
After their internalization, trafficking of internal GPCR diverges into two distinct pathways. One pathway leads to recycling of GPCR back to the membrane and the other leads to retention of GPCR and their eventual proteolysis in late endosomes/lysosomes (34, 35). The mechanism for recycling of internal GPCR back to the cell membrane is still obscure. Studies concerning the trafficking of the β1-AR have determined that the intracellular trafficking itinerary was imprinted prior to its internalization (16). This process involved a pool of PKA that was targeted via AKAP5 to the β1-AR (13). Because activation of PKA occurred before that of GRK, the notion has arisen that the “PKA-recycling signal” was imprinted prior to GRK-mediated internalization of the GPCR (16). In the case of the β1-AR, the recycling signal appears to be phospho-Ser312 in the third intracellular loop of the β1-AR, which is phosphorylated by a pool of PKA bound to the β1-AR (13, 16). The mechanism for specific targeting of PKA to the β1-AR is centered at the type 1 PDZ in the extreme C terminus of the β1-AR because inactivation of the PDZ prevented the recycling of the β1-AR (Fig. 3). The type 1 PDZ of the β1-AR binds to the scaffolding protein SAP97 (16). SAP97 is a bipartite binding protein that binds to the β1-AR PDZ through the PDZ2-binding region of SAP97 and to the AKAP5-PKA complex via the i3 binding domain of SAP97 (25, 26). Thus, targeting of PKA to the β1-AR was mediated by the binding between the type 1 PDZ of the β1-AR and the SAP97-AKAP5-PKA complex.
In this report, we verified many of the salient features of this mechanism and the critical role of AKAP5 in recycling of the β1-AR in mammalian cells (Figs. 1–4). For example, AKAP5 was bound to the WT β1-AR but not to the β1-ARΔPDZ (Fig. 1). Inactivation of the PDZ, Ser312, or PKA inhibited the recycling of the β1-AR in human HEK-293 cells and in rodent cardiomyocytes. However, mutating Ser312 in the third intracellular loop of the β1-AR to the phospho-Ser mimic Asp312 facilitated the recycling of the β1-AR in HEK-293 and in rodent cardiomyocytes, even when the β1-AR PDZ, PKA, or AKAP5 was inactivated (Figs. 1–4). These results support previous conclusions, which have indicated that phosphorylation of Ser312 was apparently the ultimate recycling signal for the β1-AR (16).
AKAP5 contains a membrane binding domain and sites to scaffold at least three signaling enzymes, namely PKA, PKC, and calcineurin (1, 7–9). In Fig. 4, we determined that membrane binding and anchorage of PKA or calcineurin to AKAP5 were required for the recycling of the β1-AR in HEK-293 cells. These results suggest that AKAP5 exerted its effect on recycling of the β1-AR by multiple molecular mechanisms. One of these mechanisms apparently involved the calcineurin-binding sequence between 321 and 360 of AKAP5. This finding was surprising because a role for calcineurin bound to AKAP5 in GPCR trafficking has not been described. Because the binding of AKAP5 to the β1-AR required SAP97, we affirmed that binding of SAP97 to the various AKAP5* deletion mutants was not affected (16). These experiments verified that the SAP97 binding domain in AKAP5 between amino acids 151 and 300 was not affected by these deletions (7). Therefore, these results highlighted a novel role for calcineurin bound to AKAP5 in trafficking of the β1-AR.
Many studies have shown that there was a delicate balance between the binding of calcineurin to an AKAP and the activity of the AKAP-bound PKA. For example, in the hippocampus, recruitment of AKAP5 to membrane-associated guanylate kinase-glutamate receptor complexes was dependent on the proper localization of AKAP5 to the membrane cytoskeleton (18, 19, 38, 39). Loss of the calcineurin-binding site from AKAP5 affected the optimal positioning of MAGUK-AKAP5-glutamate receptor complexes in the hippocampus and interfered with the phosphorylation and trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (38, 39). There is also anecdotal evidence for calcineurin and PKA that are bound to the same AKAP in regulating the trafficking of several proteins. In adult cardiac myocytes, calcineurin and PKA bound to muscle AKAP exerted opposite effects on trafficking of myopodin between Z-discs and the nucleus (23). Moreover, a calcineurin-binding AKAP was also involved in the translocation of aquaporin-2 from endosomes to the membrane of inner medullary collecting ducts (40). Trafficking of aquaporin-2 or myopodin was mediated by their phosphorylation by an AKAP-anchored PKA and was inhibited by the phosphatase activity of AKAP-anchored calcineurin (23, 40, 41). Furthermore, translocation of aquaporin-2 from endosomes to the membrane of inner medullary collecting ducts or myopodin from Z-discs to the nucleus was inhibited by manipulations that increased the activity or the amount of calcineurin (23, 40). Therefore, it is conceivable that loss of the calcineurin-binding site from AKAP5 might have affected optimal SAP97-AKAP5 geometry at the β1-AR or interfered with the targeting or efficiency of PKA in phosphorylating Ser312 or other trafficking proteins. Another possibility would be that delocalization of calcineurin from AKAP5 by the Δ321–360 deletion, which is known to hyperactivate the phosphatase activity of calcineurin, could have interfered with the recycling of β1-AR by enhancing the dephosphorylation of Ser312 or other trafficking proteins (42, 43).
A major remaining question not addressed adequately in HEK-293 cells is whether there were additional AKAPs involved in trafficking of the β1-AR. Studies in HEK-293 cells have implicated AKAP5 as the AKAP involved in trafficking of the β1-AR (13). These studies, however, were hampered by the limited repertoire of AKAPs that are expressed in these cells, which include gravin (AKAP12), AKAP5, AKAP15/18, (AKAP7), and AKAP149 (AKAP1) (13). Heart cells, however, express these and many additional AKAPs, such as muscle AKAP (AKAP6), yotiao (AKAP9), AKAPlbc (AKAP13), and others (5, 6, 44). Thus, studies in heart cells would address more broadly whether an AKAP other than AKAP5 was involved in trafficking of the β1-AR. To determine where AKAP5 would fit in this model, we confirmed that IPs of the β1-AR co-immunoprecipitated the rodent homolog of AKAP5 from rat cardiac myocytes. Next, we determined that WT β1-AR were internalized in response to isoproterenol in neonatal mouse cardiac myocytes and internal β1-AR recycled efficiently back into the cell membrane (Fig. 4). These results in mouse cardiac myocytes are unlike those reported in another study, which have implied that the WT β1-AR did not internalize in mouse cardiac myocytes in response to isoproterenol (45). Our results clearly showed that the β1-AR was efficiently trafficked in mouse cardiac myocytes. Furthermore, a specific role for AKAP5 in this phenomenon was demonstrated by showing that deletion of AKAP5 did not affect the internalization of the WT β1-AR in mouse cardiac myocytes but rather prevented the recycling of internal β1-AR. Expression of AKAP5 and EGFP from the same RNA transcript rescued the recycling of the β1-AR in AKAP5−/− cardiac myocytes. Because the expression of other cardiac AKAPs was not affected by the AKAP5 deletion, we interpret these results as further proof that AKAP5 was the major and perhaps the only AKAP involved in trafficking of the agonist-internalized β1-AR in mammalian cells.
Despite great strides in delineating the molecular mechanisms that govern the trafficking of GPCR, the role of trafficking in GPCR-mediated effects in mammalian cells is still obscure. Initially, internalization mechanisms leading to GPCR sequestration away from the post-synaptic membrane were regarded as termination signals, but a large body of data exist in support of continued signaling from internalized GPCR (46). For example, signaling by the agonist-internalized receptors of thyroid-stimulating hormone, parathyroid hormone, and catecholamines to cyclic AMP seems to continue after these receptors have been internalized (46, 47). With regard to the role of recycling and re-insertion of the reactivated GPCR into the cell membrane, there are many reports that have shown that this process was involved in GPCR resensitization and subsequent signaling from the recycled GPCR (14, 26, 34, 35, 48, 49). In addition, recycling of the GPCR is intimately involved in maintaining receptor density in cells and tissues because prolonged incubation of cells with isoproterenol did not cause significant degradation of β2-AR even after several hours of continuous exposure to high levels of the β-agonist (50). However, mutations that interfered with the recycling of the β2-AR resulted in degradation of mutant β2-AR in cells chronically exposed to β-agonists (50). Therefore, recycling of the GPCR was associated with maintenance of GPCR density and signaling intensity. This property might be important in maintaining cellular levels of GPCR in conditions associated with elevated levels of hormones or neurotransmitters. For example, cardiac insufficiency is associated with elevated circulating and synaptic levels of catecholamines (51). Thus, recycling of the β-AR might play a fundamentally protective role in preventing acute down-regulation of myocardial β-AR in pathological conditions associated with sympathetic hyperactivation.
Another salient discovery reported here was the role of AKAP5 in regulating the signaling output of the β1-AR in cardiac myocytes. In cardiac myocytes derived from AKAP5−/− mice we did not detect an increase in cyclic AMP levels as compared with their levels in AKAP5+/+ cells, but we detected an increase in cyclic AMP elicited by activated β1-AR. Nichols et al. (11) also reported that production of cyclic AMP in response to isoproterenol was similar in adult mouse cardiomyocytes prepared from AKAP5+/+ or AKAP−/− mice, but the output from each β-AR subtype was not determined. In addition, Nichols et al. (11) reported that deletion of AKAP5 resulted in loss of β-adrenergic-stimulated Ca2+ transients and phosphorylation of substrates involved in Ca2+ handling. These findings indicate that deletion of AKAP5 impaired β-AR-mediated modulation of excitation-contraction coupling (11).
Loss of β-AR-mediated modulation of excitation-contraction coupling in adult AKAP5−/− cardiomyocytes might be due to delocalization of cyclic AMP (such as delocalization of adenylyl cyclase 5/6) or to delocalization of PKA. Stimulation of β-AR in knock-in AKAP5Δ36 cardiomyocytes (derived from D36 mice that express AKAP5 without the PKA-binding site) elicited similar [Ca2+]i transients as those recorded in cardiomyocytes derived from AKAP5+/+ mice. Thus, AKAP5-anchored PKA was not essential for β-AR-mediated stimulation of [Ca2+]i transients (11). These results are not at odds with our findings because distinct populations of AKAP5 might be involved in recycling of the β1-AR and in excitation-contraction coupling. As we described at length throughout this report, the pool of PKA involved in phosphorylating Ser312 was PKA-anchored to the β1-AR PDZ by the SAP97-AKAP5 complex, which accounts for a small percentage of AKAP5-PKA complex. However, the defect in β-AR stimulated [Ca2+]i transients was more related to altered AKAP5 association with adenylyl cyclase V/VI or caveolin 3 (11). Moreover, cardiomyocytes from AKAP7−/− (AKAP13/18) responded normally to β-AR stimulation, even though AKAP7 was implicated in β-AR-mediated stimulation of [Ca2+]i transients in neonatal and adult rodent cardiac myocytes (6, 44, 52). Therefore, in vivo outcomes cannot be easily gleaned from the extrapolation of in vitro results.
To probe with more precision whether signaling by β1-AR in AKAP5−/− cardiomyocytes was selectively enhanced, myocardial β1-ARs were selectively activated by xamoterol, and these experiments verified that second messenger production by the β1-AR subtype was increased. In addition, we selected three sets of parameters to assess signaling properties of β1-AR in cardiac myocytes derived from neonatal transgenic AKAP5 mice. The first parameter was to compare the effect of isoproterenol on heart rate in AKAP5+/+ versus AKAP5−/− myocytes because the β1-AR subtype mediated the chronotropic effects of isoproterenol in mice (30). These analyses indicated that isoproterenol increased the contraction rate of heart cells derived from AKAP5−/− mice more than it increased the contraction rate of heart cells derived from AKAP5+/+ mice. Another parameter affected by the activation of the β1-AR subtype was the size of cardiomyocytes (30). An extensive series of morphometric studies determined that the size of AKAP5−/− cardiomyocytes was greater than AKAP5+/+ myocytes. In addition, isoproterenol caused a greater increase in the surface area of cardiomyocytes derived from AKAP5−/− as compared with its effects in AKAP5+/+ cardiomyocytes. These results were intriguing and were verified by determining the effects of AKAP5 knockdown and isoproterenol on hypertrophic markers. Cardiac hypertrophy induced multiple changes, including genetic reprogramming manifested by re-expression of fetal genes and down-regulation of adult genes (53, 54). For example, β-MHC in rodent cardiac myocytes is expressed normally during fetal development and replaced by α-MHC after birth (54). β-MHC is re-expressed in adult hearts in response to stress, injury, or excessive α1- or β1-adrenergic receptor stimulation (53, 55). Once more, isoproterenol induced a larger increase in β-MHC expression in cardiac myocytes prepared from AKAP5−/− as compared with those prepared from AKAP5+/+ mice. Thus, three independent methods implied that signaling via β1-AR was enhanced in cardiac myocytes prepared from AKAP5−/− mice. These results led us to hypothesize that AKAP5 is cardioprotective because its knockdown resulted in myocardial cell hypertrophy and enhanced β-MHC expression, which are more associated with cardiac remodeling induced by cardiac insufficiency, stress, and/or injury (56).
Acknowledgment
We thank G. Stanley McKnight (University of Washington, Seattle) for providing heterozygous AKAP5 mice and help with genotyping.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-085848 (to S. W. B.).
- AKAP
- A-kinase anchoring protein
- β-AR
- β-adrenergic receptor
- GPCR
- G-protein coupled receptors
- HBSS
- Hanks' balanced salt solution
- ANOVA
- analysis of variance
- IP
- immunoprecipitation
- NRVCM
- neonatal rat ventricular cardiac myocyte
- NMVCM
- neonatal mouse ventricular cardiac myocyte
- EGFP
- enhanced GFP
- ICI
- ICI-118,551
- bpm
- beats/min
- CFP
- cyan fluorescent protein
- C/Y
- ratio of CFP/YFP
- CGP
- CGP-20712A
- GRK
- G protein-coupled receptor kinase.
REFERENCES
- 1. Klauck T. M., Faux M. C., Labudda K., Langeberg L. K., Jaken S., Scott J. D. (1996) Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science 271, 1589–1592 [DOI] [PubMed] [Google Scholar]
- 2. Logue J. S., Scott J. D. (2010) Organizing signal transduction through A-kinase anchoring proteins (AKAPs). FEBS J. 277, 4370–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong W., Scott J. D. (2004) AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959–970 [DOI] [PubMed] [Google Scholar]
- 4. Bauman A. L., Soughayer J., Nguyen B. T., Willoughby D., Carnegie G. K., Wong W., Hoshi N., Langeberg L. K., Cooper D. M., Dessauer C. W., Scott J. D. (2006) Dynamic regulation of cyclic AMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 23, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pare G. C., Bauman A. L., McHenry M., Michel J. J., Dodge-Kafka K. L., Kapiloff M. S. (2005) The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J. Cell Sci. 118, 5637–5646 [DOI] [PubMed] [Google Scholar]
- 6. Hulme J. T., Lin T. W., Westenbroek R. E., Scheuer T., Catterall W. A. (2003) β-Adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc. Natl. Acad. Sci. U.S.A. 100, 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colledge M., Dean R. A., Scott G. K., Langeberg L. K., Huganir R. L, Scott J. D. (2000) Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron 27, 107–119 [DOI] [PubMed] [Google Scholar]
- 8. Dell'Acqua M. L., Faux M. C., Thorburn J., Thorburn A., Scott J. D. (1998) Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4,5-bisphosphate. EMBO J. 17, 2246–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dell'Acqua M. L., Dodge K. L., Tavalin S. J., Scott J. D. (2002) Mapping the protein phosphatase-2B anchoring site on AKAP79. J. Biol. Chem. 277, 48796–48802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horvat S. J., Deshpande D. A., Yan H., Panettieri R. A., Codina J., DuBose T. D., Jr., Xin W., Rich T. C., Penn R. B. (2012) A-kinase anchoring proteins regulate compartmentalized cyclic AMP signaling in airway smooth muscle. FASEB J. 26, 3670–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols C. B., Rossow C. F., Navedo M. F., Westenbroek R. E., Catterall W. A., Santana L. F., McKnight G. S. (2010) Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 107, 747–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Valentine C. D., Haggie P. M. (2011) Confinement of β1- and β2-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol. Biol. Cell 22, 2970–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gardner L. A., Tavalin S. J., Goehring A. S., Scott J. D., Bahouth S. W. (2006) AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the β1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J. Biol. Chem. 281, 33537–33553 [DOI] [PubMed] [Google Scholar]
- 14. Gardner L. A., Delos Santos N. M., Matta S. G., Whitt M. A., Bahouth S. W. (2004) Role of the cyclic AMP-dependent protein kinase in homologous resensitization of the β1-adrenergic receptor. J. Biol. Chem. 279, 21135–21143 [DOI] [PubMed] [Google Scholar]
- 15. Snyder E. M., Colledge M., Crozier R. A., Chen W. S., Scott J. D., Bear M. F. (2005) Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J. Biol. Chem. 280, 16962–16968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardner L. A., Naren A. P., Bahouth S. W. (2007) Assembly of an SAP97-AKAP79-cyclic AMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human β1-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J. Biol. Chem. 282, 5085–5099 [DOI] [PubMed] [Google Scholar]
- 17. Bahouth S. W., Cui X., Beauchamp M. J., Park E. A. (1997) Thyroid hormone induces β1-adrenergic receptor gene transcription through a direct repeat separated by five nucleotides. J. Mol. Cell. Cardiol. 129, 3223–3237 [DOI] [PubMed] [Google Scholar]
- 18. Ehlers M. D. (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 [DOI] [PubMed] [Google Scholar]
- 19. Snyder E. M., Philpot B. D., Huber K. M., Dong X., Fallon J. R., Bear M. F. (2001) Internalization of ionotropic glutamate receptors in response to mGluR1 activation. Nat. Neurosci. 4, 1079–1085 [DOI] [PubMed] [Google Scholar]
- 20. Delos Santos N. M., Gardner L. A., White S. W., Bahouth S. W. (2006) Characterization of the residues in helix 8 of the human β1-adrenergic receptor that are involved in coupling the receptor to G proteins. J. Biol. Chem. 281, 12896–12907 [DOI] [PubMed] [Google Scholar]
- 21. Ponsioen B., Zhao J., Riedl J., Zwartkruis F., van der Krogt G., Zaccolo M., Moolenaar W. H., Bos J. L., Jalink K. (2004) Detecting cyclic AMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cyclic AMP indicator. EMBO Rep. 5, 1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faul C., Hüttelmaier S., Oh J., Hachet V., Singer R. H., Mundel P. (2005) Promotion of importin α-mediated nuclear import by the phosphorylation-dependent binding of cargo protein to 14-3-3. J. Cell Biol. 169, 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faul C., Dhume A., Schecter A. D., Mundel P. (2007) Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol. Cell. Biol. 27, 8215–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devic E., Xiang Y., Gould D., Kobilka B. (2001) β-Adrenergic receptor subtype-specific signaling in cardiac myocytes from β1- and β2-adrenoceptor knockout mice. Mol. Pharmacol. 60, 577–583 [PubMed] [Google Scholar]
- 25. Nikandrova Y. A., Jiao Y., Baucum A. J., Tavalin S. J., Colbran R. J. (2010) Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J. Biol. Chem. 285, 923–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nooh M. M., Naren A. P., Kim S.-J., Xiang Y. K., Bahouth S. W. (2013) SAP97 controls the trafficking and resensitization of the β1-adrenergic receptor through its PDZ2 and I3 domains. PLoS One 8, e63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 266, 15771–15781 [PubMed] [Google Scholar]
- 28. Kowalski M. T., Haworth D., Lu X., Thomson D. S., Barnett D. B. (1990) Comparison of the effects of xamoterol and isoprenaline on cardiac β-adrenoceptors: Studies of function and regulation. Br. J. Pharmacol. 99, 27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baker J. G., Kemp P., March J., Fretwell L., Hill S. J., Gardiner S. M. (2011) Predicting in vivo cardiovascular properties of β-blockers from cellular assays: a quantitative comparison of cellular and cardiovascular pharmacological responses. FASEB J. 25, 4486–4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schäfer M., Frischkopf K., Taimor G., Piper H. M., Schlüter K. D. (2000) Hypertrophic effect of selective β1-adrenoceptor stimulation on ventricular cardiomyocytes from adult rat. Am. J. Physiol. Cell Physiol. 279, C495–C503 [DOI] [PubMed] [Google Scholar]
- 31. Yoo B., Lemaire A., Mangmool S., Wolf M. J., Curcio A., Mao L., Rockman H. A. (2009) β1-Adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 297, H1377–H1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bisognano J. D., Weinberger H. D., Bohlmeyer T. J., Pende A., Raynolds M. V., Sastravaha A., Roden R., Asano K., Blaxall B. C., Wu S. C., Communal C., Singh K., Colucci W., Bristow M. R., Port D. J. (2000) Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. J. Mol. Cell. Cardiol. 32, 817–830 [DOI] [PubMed] [Google Scholar]
- 33. López J. E., Myagmar B. E., Swigart P. M., Montgomery M. D., Haynam S., Bigos M., Rodrigo M. C., Simpson P. C. (2011) β-Myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ. Res. 109, 629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Magalhaes A. C., Dunn H., Ferguson S. S. (2012) Regulation of GPCR activity, trafficking, and localization by GPCR-interacting proteins. Br. J. Pharmacol. 165, 1717–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 36. Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996) Role of β-arrestin in mediating agonist-promoted G-protein-coupled receptor internalization. Science 271, 363–366 [DOI] [PubMed] [Google Scholar]
- 37. Freedman N. J., Liggett S. B., Drachman D. E., Pei G., Caron M. G., Lefkowitz R. J. (1995) Phosphorylation and desensitization of the human β1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J. Biol. Chem. 270, 17953–17961 [DOI] [PubMed] [Google Scholar]
- 38. Gorski J. A., Gomez L. L., Scott J. D., Dell'Acqua M. L. (2005) Association of an A-kinase-anchoring protein signaling scaffold with cadherin adhesion molecules in neurons and epithelial cells. Mol. Biol. Cell 16, 3574–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dell'Acqua M. L., Smith K. E., Gorski J. A., Horne E. A., Gibson E. S., Gomez L. L. (2006) Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur. J. Cell Biol. 85, 627–633 [DOI] [PubMed] [Google Scholar]
- 40. Gooch J. L., Guler R. L., Barnes J. L., Toro J. J. (2006) Loss of calcineurin Aα results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J. Cell Sci. 119, 2468–2476 [DOI] [PubMed] [Google Scholar]
- 41. Jo I., Ward D. T., Baum M. A., Scott J. D., Coghlan V. M., Hammond T. G., Harris H. W. (2001) AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex. Am. J. Physiol. Renal. Physiol. 281, F958–F965 [DOI] [PubMed] [Google Scholar]
- 42. Li H., Pink M. D., Murphy J. G., Stein A., Dell'Acqua M. L., Hogan P. G. (2012) Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat. Struct. Mol. Biol. 19, 337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coghlan V. M., Perrino B. A., Howard M., Langeberg L. K., Hicks J. B., Gallatin W. M., Scott J. D. (1995) Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science 267, 108–111 [DOI] [PubMed] [Google Scholar]
- 44. Kapiloff M. S. (2002) Contributions of protein kinase A anchoring proteins to compartmentation of cAMP signaling in the heart. Mol. Pharmacol. 62, 193–199 [DOI] [PubMed] [Google Scholar]
- 45. Xiang Y., Devic E., Kobilka B. (2002) The PDZ binding motif of the β1-adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J. Biol. Chem. 277, 33783–33790 [DOI] [PubMed] [Google Scholar]
- 46. Calebiro D., Nikolaev V. O., Persani L., Lohse M. J. (2010) Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci. 31, 221–228 [DOI] [PubMed] [Google Scholar]
- 47. Irannejad R., Tomshine J. C., Tomshine J. R., Chevalier M., Mahoney J. P., Steyaert J., Rasmussen S. G., Sunahara R. K., El-Samad H., Huang B., von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu S. S., Lefkowitz R. J., Hausdorff W. P. (1993) β-Adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J. Biol. Chem. 268, 337–341 [PubMed] [Google Scholar]
- 49. Pippig S., Andexinger S., Lohse M. J. (1995) Sequestration and recycling of β2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 47, 666–676 [PubMed] [Google Scholar]
- 50. Gage R. M., Kim K. A., Cao T. T., von Zastrow M. (2001) A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J. Biol. Chem. 276, 44712–44720 [DOI] [PubMed] [Google Scholar]
- 51. Cohn J. N. (1989) Sympathetic nervous system activity and the heart. Am. J. Hypertens. 2, 353S–356S [PubMed] [Google Scholar]
- 52. Jones B. W., Brunet S., Gilbert M. L., Nichols C. B., Su T., Westenbroek R. E., Scott J. D., Catterall W. A., McKnight G. S. (2012) Cardiomyocytes from AKAP7 knockout mice respond normally to adrenergic stimulation. Proc. Natl. Acad. Sci. U.S.A. 109, 17099–17104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. (1979). Myosin isoenzyme redistribution in chronic heart overload. Nature 282, 105–107 [DOI] [PubMed] [Google Scholar]
- 54. Izumo S., Nadal-Ginard B., Mahdavi V. (1988) Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc. Natl. Acad. Sci. U.S.A. 85, 339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O'Connell T. D., Swigart P. M., Rodrigo M. C., Ishizaka S., Joho S., Turnbull L., Tecott L. H., Baker A. J., Foster E., Grossman W., Simpson P. C. (2006) α1-Adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J. Clin. Invest. 116, 1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Swynghedauw B. (1999) Molecular mechanisms of myocardial remodeling. Physiol. Rev. 79, 215–262 [DOI] [PubMed] [Google Scholar]