Background: MAP kinase phosphatase-1 (MKP-1) plays a critical role in regulating inflammation in innate and adaptive immunity.
Results: mTOR inhibition leads to induction of MKP-1 through the activation of AKT1 and MEK1/MEK2 pathways. Rapamycin pretreatment of macrophages inhibits LPS-mediated p38 activation and IL-6 and nitric oxide production.
Conclusion: Both AKT1 and MEK1/2 regulate rapamycin-mediated MKP-1 induction.
Significance: mTORC1 inhibition regulates immunity through MKP-1 induction.
Keywords: Akt PKB, Dual Specificity Phosphoprotein Phosphatase, MAP Kinases (MAPKs), mTOR Complex (mTORC), Signal Transduction
Abstract
Mitogen-activated protein kinase phosphatase-1 (MKP-1), also known as dual specificity phosphatase-1 (DUSP-1), plays a crucial role in the deactivation of MAPKs. Several drugs with immune-suppressive properties modulate MKP-1 expression as part of their mechanism of action. We investigated the effect of mTOR inhibition through rapamycin and a dual mTOR inhibitor (AZD2014) on MKP-1 expression. Low dose rapamycin led to a rapid activation of both AKT and ERK pathways with a subsequent increase in MKP-1 expression. Rapamycin treatment led to phosphorylation of CREB, transcription factor 1 (ATF1), and ATF2, three transcription factors that bind to the cyclic AMP-responsive elements on the Mkp-1 promoter. Inhibition of either the MEK/ERK or the AKT pathway attenuated rapamycin-mediated MKP-1 induction. AZD2014 did not activate AKT but activated the ERK pathway, leading to a moderate MKP-1 induction. Using bone marrow-derived macrophages (BMDMs) derived from wild-type (WT) mice or mice deficient in AKT1 and AKT2 isoforms or BMDM from targeted deficiency in MEK1 and MEK2, we show that rapamycin treatment led to an increased MKP1 expression in BMDM from WT but failed to do so in BMDMs lacking the AKT1 isoform or MEK1 and MEK2. Importantly, rapamycin pretreatment inhibited LPS-mediated p38 activation and decreased nitric oxide and IL-6 production. Our work provides a conceptual framework for the observed immune modulatory effect of mTOR inhibition.
Introduction
Mammalian target of rapamycin (mTOR)2 is a multifunctional kinase complex that plays a central role in cell growth and metabolism (1, 2). mTOR can interact with the regulatory-associated proteins of mTOR (raptor) to constitute the mTORC1 complex or, alternatively, interacts with rapamycin-insensitive companion (rictor) to form the mTORC2 complex (1, 2). mTORC1 and mTORC2 exert their actions by regulating several important kinases, such as S6K and AKT. Although AKT activates mTORC1, the TORC2 complex directly phosphorylates AKT at Ser-473 (3, 4). Although the role of mTORCs in cancer is well studied, the signaling input and output of mTORCs in the immune system are not well understood. Rapamycin, a natural product of the bacterium Streptomyces hygroscopicus and a macrolide antibiotic, has emerged as a potent anti-proliferative medication with immunosuppressive properties (5).
Activation of both AKT and mTOR influences signaling of various receptors such as toll-like receptors (TLRs) or cytokine receptors (6, 7). The immunosuppressive effect of rapamycin has been largely attributed to inhibition of the clonal expansion of lymphocytes, an altered cytokine production, or differential regulation of effector and regulatory T cell linage commitment (6, 8). However, mTOR plays an important role in terminally differentiated macrophages and in regulation of innate immune responses (6–8).
Three principal MAPK subfamilies, including the extracellular signal-regulated kinases 1 and 2 (ERK1 and -2), c-Jun amino-terminal kinases (JNKs), and p38, are essential for initiation of acute inflammation in response to mitogens or stress signals (9). Although activation of MAPKs is important in cellular processes, failure to terminate this activation may lead to detrimental systemic effects and chronic inflammatory diseases (9–11). MAP kinase phosphatases (MKPs) are a group of dual specificity phosphatases (DUSPs) that dephosphorylate both threonine and tyrosine in the TXY motif on MAPKs and thereby modulate inflammation (10, 12). MKP-1 preferentially dephosphorylates p38 and JNK, whereas MKP-3 (DUSP-6) and DUSP-5 prefer ERKs as substrates (10, 12). The MKP-1 (DUSP-1) gene is rapidly induced in response to growth factors, stress, and several cytokines (TNF-α and TGF-β) (13, 14). The exact transcriptional regulation of MKP-1 is not well understood, but it has been reported that all three kinases (ERK, p38, and JNK) are involved in its regulation (15, 16). For example, ERK positively regulates MKP-1 through both transcriptional and post-transcriptional mechanisms (16). The effects of several anti-inflammatory drugs, such as corticosteroids and cyclic AMP analogs, have been shown to depend, at least in part, on MKP-1 induction (17). For example, the anti-inflammatory effect of corticosteroids is impaired in MKP-1−/− macrophages and MKP-1 knock-out mice (18). Additionally, the induction of MKP-1 is required for the optimal anti-inflammatory effect of two potent anti-inflammatory cytokines, IL-10 and TGF-β (19, 20). Moreover, MKP-1 is necessary in T-cell activation and function (21). We hypothesized that the immunosuppressive effect of rapamycin is, at least in part, due to up-regulation of MKP-1 expression.
We show that rapamycin rapidly activates both AKT and ERK pathways (both in murine macrophages and bone marrow-derived macrophages (BMDMs)), enhances the activities of several transcription factors that directly bind to the Mkp-1 promoter, and up-regulate MKP-1 expression in macrophages. Moreover, blocking either the MEK/ERK pathway or the AKT pathway attenuates rapamycin-mediated MKP-1 induction in response to mTORC1 inhibition. Finally, we explore the role of AKT1 and -2 isoform and MEK1 and -2 in rapamycin-mediated MKP-1 induction using macrophages deficient in the corresponding genes.
EXPERIMENTAL PROCEDURES
Chemicals and Antibodies
Rapamycin and pharmacological inhibitors of kinases (LY294002, wortmannin, U0126, and PD98059) and c-RAF inhibitor were purchased from Calbiochem. AZD2014 was purchased from Selleckchem (Houston, TX). Phospho-specific antibodies against phospho-AKT, PI3K (pp85), ERK1/2, p38, JNK, CREB/ATF1, RAF-1, MSK1/2, ATF1, ATF2, and C/EBPβ as well as β-actin were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against total ATF2, phospho-p300, and MKP-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Specific antibodies against AKT1, AKT2, MEK1, and MEK2 and horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit IgG secondary antibodies were purchased from Cell Signaling Technology, and HRP-conjugated anti-goat antibody was purchased from Bio-Rad. All other chemicals were purchased from Sigma unless specified otherwise.
Cell Culture
Murine macrophage-like cell line (B6-MCL) was cultured as previously described (22). Briefly, B6-MCL were grown in Iscove's modified Dulbecco's medium supplemented with 10% heat-inactivated fetal calf serum (Invitrogen), antibiotics, sodium pyruvate, nonessential amino acids, and β-mercaptoethanol.
Mice and Isolation of BMDMs
Mek1(flox) f/f Mek2−/− and Mek1d/d Mek2+/+ Sox2Cre+ were generated onto the 129 cc background as previously described (23, 24). AKT1−/− and AKT2−/− mice were generated as previously described (25, 26). All mice were backcrossed onto the corresponding backgrounds at least 10 times. WT and MEK2−/−MEK1(f)/f and Mek1d/dMek2+/+Sox2Cre+ mice were maintained at animal facilities at the Centre de Recherche sur le Cancer de l'Université Laval (Québec, CA) and at the University of Pennsylvania (Philadelphia, PA). Animal studies were approved by the corresponding Universities Committee on Use and Care of Animals.
BMDMs from mice were prepared as described previously (22). Macrophages were cultured with Iscove's modified Dulbecco's medium supplemented with 30% L929 supernatant containing macrophage-stimulating factor, glutamine, sodium pyruvate, 10% heat-inactivated FBS, and antibiotics for 5–7 days. BMDM were re-plated at a density of 2 × 106 cells/well the day before the experiment.
Protein Extraction and Immunoblotting
After the appropriate treatments, cells were washed with PBS and harvested in RIPA buffer (Sigma) containing protease inhibitor and antiphosphatase cocktails as previously described (22). Equal amounts of proteins (10–20 μg) were mixed with the same volume of 2× sample buffer, separated on 10% SDS-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad) at 20 V for 30 min using a SemiDry Transfer Cell (Bio-Rad) as previously described (22). The PVDF membrane was blocked with 5% dry milk in Tris-buffered saline with 0.1% Tween 20, rinsed, and incubated with primary antibody overnight. The blots were washed and incubated with HRP-conjugated secondary anti-IgG antibody. Membranes were washed, and immunoreactive bands were visualized using a chemiluminescent substrate (ECL-Plus, GE Healthcare). Images were captured on Hyblot CL film (Denville, Scientific Inc, Metuchen, NJ). Optical density analysis of signals was performed using ImageQuant software (version 5, Molecular Dynamics).
Cell Viability
Cell viability was measured using the MTT (3-(4,5)-dimethyl thiazol-2,5-diphenyl tetrazolium bromide) assays as described (22).
Nitrite Determination by Griess Reaction
NO levels were assessed by nitrite quantification as previously described (22).
Enzyme-linked Immunosorbent Assay (ELISA)
Murine TNF-α, IL-10, and IL-6 cytokine levels in cell culture supernatants were measured using ELISA DuoKits (R&D Systems) as previously described (27).
RNA Extraction and Quantitative Reverse Transcriptase/Real Time-PCR
Total RNA was extracted using Stat 60 (Iso-Tex Diagnostics) and reverse-transcribed using the Reverse Transcription System (Promega, Madison, WI). The primers (targeting MKP-1, MKP-3, and a reference gene, β-actin) were used to amplify the corresponding cDNA by using iQ SYBR Green Supermix (Bio-Rad). Quantitative analysis of mRNA expression was performed using the MX3000p instrument (Stratagene, La Jolla, CA). PCR amplification was performed in a total volume of 20 μl containing 2 μl of each cDNA preparation and 20 pg of primers (Invitrogen). The PCR amplification protocol was performed as described previously (27). Relative mRNA levels were calculated after normalizing to β-actin. Data were analyzed using the unpaired, two-tailed Student's t test, and the results were expressed as relative -fold of change. The following primers were used in the PCR reactions: β-actin, forward (GATTACTGCTCTGGCTCCTAGC) and reverse (GACTCATCGTACTCCTGCTTGC); MKP-1 forward (5′-ACAACAATGACTTGACCGCA-3′) and reverse (5′-GGAATGGTTAATACTGGTGG-3′); MKP-3 (DUSP6) forward (5′-TCGGGCTGCTGCTCAAGAAAC-3′) and reverse (5′-CGGTCAAGGTCAGACTCAATGTCC-3′).
Separation of Cytoplasmic and Nuclear Fractions
The cytoplasmic and nuclear fractions were separated as described previously (28). Briefly, after treatment the cells were resuspended in a hypotonic buffer (10 mm HEPES, pH 7.9, 0.5% Igepal, 2 mm MgCl2, 10 mm KCl, 0.1 mm EDTA, 0.5 mm phenylmethylsulfonyl fluoride, 1.0 μg/ml leupeptin, and 1.0 μg/ml aprotinin) and incubated on ice for 10 min. After centrifugation at 14,000 × g for 1 min at 4 ºC, the supernatant (cytoplasmic) and the pellets (nuclear fraction) were collected.
Biotin Pulldown Assay
Cells were harvested after treatment with rapamycin or dexamethasone, and nuclear proteins were extracted according to a protocol described previously (29). Nuclear extracts were incubated with 100 μl of streptavidin-agarose beads and 5–10 μg/ml concentrations of the following biotinylated double-stranded DNA probes (the binding site sequences are underlined): Mkp-1 ATF/CREB/AP-1 site 1, 5′-CCTAGGCCGATGACGTCTTCGGC-3′; site 2, 5′-Mkp-1 C/EBP site, 5′-CGGCACCCCTTCAGAGTTGTGCAACTCTG-3′. The final volume was adjusted to 250 μl with binding buffer. The mixture was placed on a rotating shaker with gentle mixing at room temperature, and the beads were then thoroughly washed and proteins analyzed by Western blotting to assess binding of transcription factors.
Statistical Analyses
Statistical analyses were performed using SPSS software, Version 19.0 (SPSS Inc; Chicago, IL). One way analysis of variance (ANOVA) test and post hoc repeated measure comparisons (least significant difference) were performed on all obtained data. Quantitative reverse transcriptase-PCR results were expressed as the means ± S.E. For all analyses, two-tailed p values of less than 0.05 were considered significant.
RESULTS
Rapamycin Potently Induces MKP-1 Expression
Rapamycin has been used as an anti-inflammatory and immune modulatory agent (5). We hypothesized that the anti-inflammatory and immune modulatory activities of rapamycin might rely on MKP-1 induction. The effect of rapamycin was investigated using a B6-MCL murine macrophage cell line. B6-MCL cells were treated with rapamycin (10 ng/ml) for different time periods (Fig. 1A). Cell lysates were assessed by immunoblotting using an MKP-1 antibody, and equal loading was verified with a β-actin antibody. Rapamycin stimulation led to an early decrease in basal MKP-1 expression (within 15 min) followed by a rapid induction of MKP-1 proteins starting at 30 min post-stimulation. By 60 min, the MKP-1 expression increased 20-fold above basal level (Fig. 1, A and B). An elevated MKP-1 expression was sustained for up to 12 h (data not shown). To determine whether the effect of rapamycin on MKP-1 induction is selective, we performed Western blotting to detect MKP-3 (DUSP6) and MKP-5 (DUSP10). Rapamycin did not have an appreciable effect on MKP-3 or MKP-5 protein levels in murine macrophages (data not shown).
FIGURE 1.
Time-dependent effect of rapamycin on MKP-1 expression. A, rapamycin increases MKP-1 protein levels. Murine macrophages (B6-MCL) were treated with rapamycin (Rapa, 10 ng/ml) for different time periods. Whole cell extracts were subjected to Western blot analysis using antibodies against MKP-1 and β-actin. B, densitometric quantification of three blots for MKP-1 plotted against time; error bars, means ± S.E. (ANOVA Mann-Whitney U test; * = p < 0.05; ** = p < 0.01). C and D, rapamycin increases MKP-1 but not MKP-3 mRNA expression. B6-MCL cells were treated with rapamycin (10 ng/ml) for 5, 15, 30, and 60 min. RNA was isolated from cells and subjected to RT-PCR assessing MKP-1 (C) and MKP-3 (D) expression. Values were normalized to β-actin and are shown as -fold change. The data represent the mean values ± S.E. of four independent experiments, each performed in triplicate. * = p < 0.05; ** = p < 0.01. E and F, induction and decay of MKP-1 in response to rapamycin and CHX. Cells were treated with rapamycin (10 ng/ml) for 60 min. Cells were then treated with 100 ng/ml CHX for 10, 30, 60, and 90 min. Cell lysates were subjected to Western blot analysis using antibodies against MKP-l and β-actin. F, densitometric quantification of three blots for MKP-1 plotted against time.
To further explore whether enhanced MKP-1 and lack of MKP-3 protein expression in response to rapamycin is regulated through a transcriptional mechanism, we evaluated mRNA levels of MKP-1 and MKP-3 using quantitative reverse transcriptase-PCR. Rapamycin treatment led to a rapid induction of MKP-1 mRNA (Fig. 1C), whereas the mRNA levels of MKP-3 did not significantly change (Fig. 1D).
To assess de novo MKP-1 protein synthesis in response to rapamycin, we next asked whether cycloheximide (CHX) inhibits rapamycin-mediated MKP-1 induction. As there was a robust induction of MKP-1 protein about 1 h after rapamycin treatment, this time point was chosen to assess the effect of CHX on rapamycin-mediated MKP-1 protein induction. Cells were exposed to CHX (100 ng/ml) after 60 min of rapamycin treatment and harvested after various time periods. Cell lysates were examined by immunoblotting using a MKP-1 antibody and β-actin antibody. As shown in Fig. 1E, rapamycin led to a robust MKP-1 induction by 1 h. A 30-min CHX treatment after 1 h of treatment with rapamycin led to a substantial decrease in MKP-1 protein levels (Fig. 1, E and F). These data collectively indicate that translation is required to maintain rapamycin-mediated MKP-1 protein induction.
Rapamycin Selectively Activates the ERK Pathway
All three MAP kinase subfamilies, ERK, JNK, and p38, have been implicated in MKP-1 induction in response to diverse stimuli (13, 15). Murine macrophages were treated with rapamycin for different time periods. Cell lysates were subjected to immunoblotting using antibodies against the active forms of ERK1/2, p38, and JNK1/2. As shown in Fig. 2A, rapamycin treatment led to a rapid ERK phosphorylation that was substantially increased within 5 min and reached a peak level at 30 min (Fig. 2, A and D). In contrast, we did not detect any activation of p38 in response to rapamycin (Fig. 2, B and D) but a weak JNK activation was detected at 60 min after rapamycin treatment (Fig. 2, C and D).
FIGURE 2.
Rapamycin treatment activates ERK but not JNK and p38. Murine macrophages (B6-MCL) were treated with rapamycin (Rapa, A–C) for different time periods. Whole cell lysates were made and subjected to Western blot analysis using a phosphoepitope-specific antibody against pERK (Thr-202/Tyr-204), pp38 (Thr-180/Tyr-182), or pJNK (Thr-183/Tyr-185). Equal loading was verified by using antibodies against total ERK, JNK, or p38, respectively. D, densitometric quantification of three blots for pERK, pJNK, and pp38 plotted against time shows time-dependent response of MAPKs to rapamycin treatment.
Rapamycin Activates the AKT and cRAF/MEK/ERK Pathways
It has been shown that inhibition of mTORC1 through rapamycin activates the PI3K/AKT and ERK pathways through a negative feedback that increases mTORC2/PI3K output (30–32). These observations were made mostly in cancer cells or in response to growth factors or insulin. We examined whether rapamycin treatment in our cell system leads to AKT activation. Macrophages were treated with rapamycin for different time periods, and total cell lysates were subjected to Western blot analysis using an antibody against the phosphorylated form of the regulatory subunit of PI3K (pp85) and antibodies against the Ser-473- and Thr-308-phosphorylated forms of AKT. As shown in Fig. 3A, rapamycin treatment led to a rapid and robust phosphorylation of AKT on Ser-473, whereas phosphorylation of the regulatory subunit of PI3K occurred later than AKT phosphorylation (Fig. 3A). These data are consistent with the model that mTORC2 phosphorylates AKT on Ser-473. Even a lower dose of rapamycin (1 ng/ml) led to phosphorylation of AKT on Ser-473 within 5 min (data not shown). c-RAF (RAF-1) is a serine/threonine protein kinase and one of the main effectors recruited by GTP-bound RAS to activate the MEK/ERK pathway (33). Previously, it has been shown that expression of MKP-1 in response to macrophage-colony-stimulating factor and LPS is dependent on RAF-1 activation (34). Therefore, we investigated whether rapamycin-induced ERK activation and MKP-1 induction is mediated through the c-RAF/MEK1/2 pathway. Macrophages were treated with rapamycin for the indicated time periods, and activation of c-RAF, MEK1/2, and ERK were assessed by Western blot analysis using phospho-specific antibodies against these kinases. We found that a 5-min rapamycin treatment led to a rapid c-RAF phosphorylation at Ser-338 (Fig. 3B). Moreover, rapamycin-induced c-RAF phosphorylation was associated with a marked activation of MEK1/2, as indicated by MEK1/2 phosphorylation at Ser-217/221 (Fig. 3B). Accordingly, ERK exhibited a 10-fold increase in its phosphorylation after rapamycin treatment (Fig. 3B). Similarly, a lower dose of rapamycin (1 ng/ml) led to rapid phosphorylation of Raf-1 and MEK1/2 as well as ERK1/2 (data not shown).
FIGURE 3.

Rapamycin treatment leads to activation the PI3K/AKT and RAF/MEK1/2/ERK pathways. Murine macrophages (B6-MCL) were treated with rapamycin (Rapa, 10 ng/ml) for different time periods as indicated. A, rapamycin treatment activates the PI3K/AKT pathway. Whole cell lysates were subjected to Western blot analysis using phosphoepitope-specific antibodies against phospho p85 (Tyr-458) and phospho AKT (Ser-473 and Thr-308). As equal loading controls total p85 or total AKT were also detected. B, the effects of rapamycin on c-RAF and MEK1/2 and ERK1/2. Cell lysates were subjected to Western blot analysis using phosphoepitope-specific antibodies against RAF-1 (c-RAF, Ser-338), MEK1/2 (Ser-217/221), and ERK (Thr-202/Tyr-204). Loading controls were confirmed using total RAF, ERK, or β actin antibodies.
Inhibition of ERK and AKT Pathways Attenuates Rapamycin-mediated MKP-1 Induction
ERK activation has been shown to play an important role in MKP-1 expression (16). Because rapamycin treatment led to rapid ERK and AKT activation, we assessed whether inhibition of ERK by using U0126 or inhibition of the PI3K/AKT pathway by using wortmannin before rapamycin treatment abrogates MKP-1 expression. Macrophages were pretreated with U0126 or wortmannin and then stimulated with rapamycin for 90 min. MKP-1 levels in the cell lysates were assessed by Western blotting. Both inhibitors attenuated rapamycin-induced MKP-1 expression (Fig. 4A). Thus, we examined whether rapamycin-induced ERK and AKT activation are linked. Macrophages were pretreated with MEK inhibitor (U0126) or PI3K inhibitor (wortmannin) and then treated with rapamycin. Pretreatment with either the MEK inhibitor or PI3K/AKT inhibitor markedly attenuated rapamycin-induced ERK phosphorylation (Fig. 4B). Similar effects on rapamycin-mediated MKP-1 induction were observed using other ERK inhibitors (PD98059) or PI3K inhibitors (LY204002) before rapamycin treatment (data not shown). These data suggest that both the ERK and the AKT pathways are important in mediating MKP-1 induction after rapamycin treatment.
FIGURE 4.

Inhibition of AKT and ERK blocks the rapamycin-mediated induction of MKP-1. Murine macrophages (B6-MCL) were pretreated with U0126 (10 μm) or wortmannin (Wort, 50 ng/ml) for 30 min and then treated with rapamycin (Rapa) for 90 min (to detect MKP-1). A, total cell lysates were subjected to Western blot analysis using MKP-1 antibody, and equal loading of proteins was confirmed using β actin. The upper panel shows the densitometric quantification of MKP-1 blots (n = 3) after rapamycin treatment in the absence and presence of inhibitors. B, cells were treated with U0126 or wortmannin 30 min before rapamycin stimulation for 30 min, and cell lysates were subjected Western blot using phospho-ERK antibody and total ERK antibodies.
Rapamycin Treatment Activates MSK1 and Induces Phosphorylation of Downstream Transcription Factors
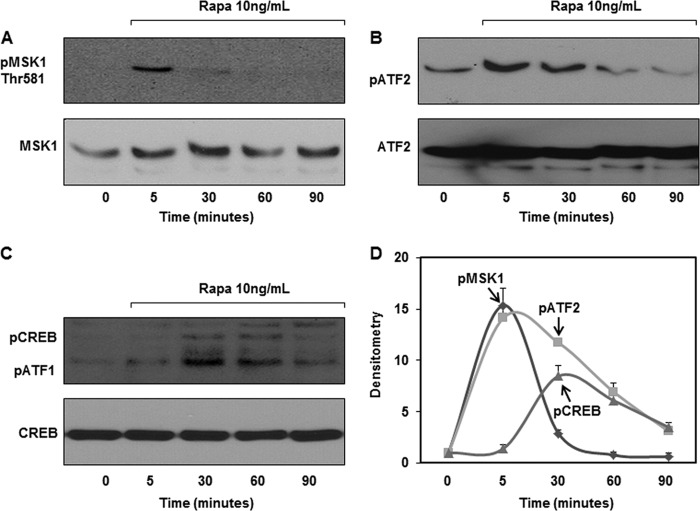
Mitogen- and stress-activated protein kinase (MSK)1 and MSK2 are two related kinases that are downstream of both p38 and ERK1/2 (35, 36). MSK1 and MSK2 activation acts as a feedback control mechanism to dampen the inflammatory response by up-regulating MKP-1 expression through phosphorylation of several transcription factors, including cAMP-response element-binding protein (CREB) and activating transcription factor 1 (ATF1) (35–37). Therefore, we assessed whether rapamycin also activates MSKs and their downstream transcription factors. Rapamycin treatment resulted in a rapid phosphorylation of MSK1 (Fig. 5, A and D). In contrast, MSK2 was not activated by rapamycin treatment even at higher concentrations (data not shown). After rapamycin treatment, ATF2 phosphorylation occurred promptly and reached its maximum levels at ∼5 min and then declined (Fig. 5, B and D). We found that rapamycin treatment resulted in a marked increase in CREB/ATF1 phosphorylation (Fig. 5, C and D), supporting the notion that MSKs mediate the phosphorylation of CREB/ATF1.
FIGURE 5.
Rapamycin activates MSK1, CREB/ATF1, and ATF2. A, cells were treated with rapamycin (Rapa, 10 ng/ml) for the times indicated, and whole cell lysates were subjected to Western blot analysis using phosphoepitope-specific antibody against pMSK1 (Thr-581). Equal loading was confirmed using MSK antibody. B and C, whole cell lysates were subjected to Western blot analysis using phosphoepitope-specific antibody against pATF2 (Thr-69/71) or pCREB/ATF1 (Ser-133). Equal loading was confirmed using total ATF2 or CREB antibodies. D, densitometric quantification of three blots for pMSK1, pCREB/ATF1, and pATF2.
Rapamycin Treatment Increases Nuclear Translocation of Activated CREB/ATF1/ATF2
To understand the regulation of the transcription factors relevant in Mkp-1 transcription, we treated macrophages with rapamycin and prepared nuclear and cytoplasmic protein fractions. These fractionated proteins were subjected to Western blot analysis. As expected, phospho-MSK1 was only detected in the nuclear fraction, and rapamycin treatment increased the levels of phospho-MSK1 (Thr-581) within 5 min (Fig. 6A). The fact that β-actin, a highly abundant cytosolic protein, was not detected in the nuclear fraction indicates the purity of the nuclear fraction. Although ATF2 was predominantly present in the cytoplasmic fraction, the phosphorylated form of ATF2 was detected exclusively in the nuclear fraction, supporting the notion that phosphorylation and nuclear localization are linked events (38). Rapamycin caused a time-dependent increase in phospho-ATF2 in the nuclear fraction. In contrast, phospho-CREB/ATF1 was detected in both the nuclear and cytoplasmic fractions. Our results confirmed previous findings that MKP-1 is exclusively located in the nucleus. Rapamycin treatment rapidly increased MKP-1 in the nucleus (Fig. 6B).
FIGURE 6.
Rapamycin mediates the activation and nuclear accumulation of CREB/ATF1 and ATF2 and their binding with the CRE sites in the Mkp-1 promoter. A, murine macrophages were treated with rapamycin (Rapa, 10 ng/ml). The nuclear and cytoplasmic proteins were fractionated and subjected to Western blot analysis using phosphoepitope-specific antibodies against phospho-MSK1 (Thr-581), phospho-ATF2 (Thr-69/71), and phospho-CREB/ATF1 (Ser-133). B, subcellular localization of MKP-1 in rapamycin-treated macrophages. Nuclear and cytoplasmic proteins were subjected to SDS-PAGE followed by Western blot analysis using the MKP-1 and β-actin antibodies. Densitometric data for MKP-1 expression represent the mean values ± S.E. of four independent experiments. C, upper panel, Mkp-1 (DUSP1) promoter structure and the CRE binding site in relation to the E Box and TATA Box and different cis elements. Lower panel, DNA pulldown assay for the consensus CREs on the murine Mkp-1 promoter. Nuclear extracts (250 μg) from unstimulated cells (lane 1) or cells treated with either rapamycin (10 ng/ml) (lane 2) or dexamethasone (100 ng/ml) (positive control, lane 3) were incubated with biotinylated double-stranded nucleotides containing the consensus CRE site on the Mkp-1 promoter (−124 to −117). The nucleotide-protein complexes were purified using streptavidin-agarose beads. Pulldown complexes were subjected to Western blot analysis using phosphorylated antibodies against CREB, ATF2, C/EBPβ, and p300.
Rapamycin Stimulates the Binding of Phospho-CREB/ATF1/ATF2 to the CRE and Enhances the Recruitment of p300 and C/EBPβ to the CRE in the Mkp-1 Promoter
To understand the transcriptional regulation of Mkp-1, we searched three different software programs (TRANSFAC, JASPAR, and GENOMATIX) to identify high confidence, evolutionarily conserved transcription binding sites on the murine Mkp-1 promoter. We identified three highly conserved putative cAMP response elements (CREs) on the Mkp-1 promoter. We asked the question whether phosphorylated CREB/ATF1 could bind to the putative CREs of the Mkp-1 promoter after rapamycin treatment. We focused on two conserved CREs on the Mkp-1 promoter, one located at position −172 to −165 in relation to the transcriptional start site and the other located at position −124 to −117 (Fig. 6C, upper panel). We designed 5′-biotinylated DNA probes corresponding to these CREs on the Mkp-1 promoter. Nuclear extracts were prepared from unstimulated cells (negative control) or after stimulation with rapamycin or dexamethasone (positive control). The nuclear cell extracts were incubated with the two biotinylated nucleotides containing the consensus binding sites for CRE on the Mkp-1 promoter and streptavidin-agarose beads. The complexes were pulled down, and transcription factors in the complexes were assessed by Western blot analysis. Fig. 6C (lower panel) shows that rapamycin treatment led to increased binding of phospho-CREB and ATF2 on the proximal consensus CRE (−124 to −117) in the Mkp-1 promoter. Compared with dexamethasone treatment, there was overall less binding of phospho-CREB to the consensus CRE site after rapamycin treatment (compare lane 2 to lane 3). C/EBPβ, a member of the basic region-leucine zipper class of transcription factors, has been implicated as an important regulator in MKP-1 transcription (39). The E1 A-binding protein p300 has been shown to interact with both CREB and C/EBPβ on the promoter of targeted genes (39, 40) to facilitate the recruitment of the transcription machinery. Interestingly, rapamycin treatment substantially increased the recruitment of p300 and C/EBPβ to the proximal putative CRE site as compared with the dexamethasone treatment (Fig. 6C). In contrast, recruitment of phospho-ATF2 was comparable in both treatments (Fig. 6C). Similar results were obtained using the putative consensus CRE between −172 to −165 (data not shown).
Dual TORC1 and TORC2 Inhibition Is Less Potent in Induction of MKP-1
TORC1 inhibition increases the activities of receptor-tyrosine kinase and TORC2, two key proximal events leading to subsequent AKT Ser-473 phosphorylation and MEK/ERK activation (30, 41). AZD2014 is a newer second generation mTOR inhibitor that inhibits both TORC1 and TORC2. We tested whether AZD2014 similarly activates the ERK and the AKT pathways and enhances MKP-1 expression. Cells were treated with AZD2014 in different concentrations (up to 10 nm) for time periods as indicated, and cell lysates were subjected to Western blot analysis. Fig. 7 shows that AZD2014 treatment did not increase AKT phosphorylation, in contrast to rapamycin treatment. In fact, the base line of AKT phosphorylation was decreased after treatment with AZD2014 (Fig. 7A). AZD2014 treatment evoked a delayed ERK phosphorylation (Fig. 7B) and resulted in a delayed MKP-1induction (Fig. 7C). These data support the notion that rapamycin-mediated AKT activation depends on mTORC2 activity (4), as inhibition of both mTORCs attenuates such activation. Second, ERK phosphorylation depends on inhibition of at least TORC1 as both rapamycin and dual mTOR inhibitor enhanced ERK phosphorylation.
FIGURE 7.

Dual TORC inhibitor is less potent in the induction of MKP-1. Murine macrophages were treated with AZD2014 (10 nm) or rapamycin (10 ng/ml) for the time periods indicated. A, immunoblot analysis of whole cell lysate performed using an antibody detecting total AKT and phospho-AKT (Ser-473). B, immunoblot analysis of whole cell lysate performed using an antibody detecting total ERK and phospho-ERK (Thr202/Tyr204). C, detection of MKP-1. Immunoblot analysis of whole cell lysate performed using an antibody against MKP-1 and β-actin.
To characterize the role of RAF1 in rapamycin-induced MKP-1 expression, an ATP-competitive RAF kinase inhibitor was used before rapamycin treatment. The RAF1 inhibitor failed to inhibit rapamycin-mediated phosphorylation of ERK and AKT and subsequent MKP-1 induction (data not shown). Our observation confirms previous work done in human tumors indicating that although RAF inhibitors inhibit MAPK in BRAF and RAS mutants, in WT cells these inhibitors activate the MEK-ERK pathway (42). We concluded that the RAF activation might be redundant in rapamycin-mediated MKP-1 induction.
Intact Signaling through AKT1 and MEK1/MEK2 Is Required for Rapamycin-mediated MKP-1 Induction
There are three different AKT isoforms with distinct biological functions and differential tissue expression (25, 26). Because AKT activation in response to rapamycin coincided with MKP-1 induction, we asked whether deletion of the AKT isoforms affects rapamycin-mediated MKP-1 induction. To answer this question and to extend our observation in primary cells, we took advantage of a genetic approach using BMDMs from WT, AKT1−/−, and AKT2−/−mice. BMDMs from mice deficient in AKT1 and AKT2 or WT mice were cultured side by side under identical conditions and treated with rapamycin. Fig. 8A shows the absence of AKT1 and AKT2 in the corresponding AKT-deficient BMDM as determined by immunoblotting. There were no major differences between BMDM of the WT, AKT1−/−, and AKT2−/− mice in terms of MEK1 and MEK2 expression. Fig. 8, B and C, show that WT BMDMs responded to rapamycin with an increased MKP-1 induction (similar to our previous results in B6-MCL). In contrast, BMDMs from AKT1−/− mice exhibited an attenuated and delayed MKP-1 induction in response to rapamycin treatment, although the basal level of MKP-1 expression was similar. BMDMs from AKT2−/− mice show a lack of MKP-1 at base line; they responded to rapamycin with an enhanced MKP-1 expression. We concluded that AKT1 is critical for rapamycin-mediated MKP-1 induction.
FIGURE 8.
AKT1 and -2 and MEK1 and -2 are required for rapamycin-mediated MKP-1 induction. BMDM from WT, AKT1−/−, and AKT2−/− mice were cultured in the presence and absence of rapamycin (Rapa, 10 ng/ml) for the indicated periods. A, immunoblots performed on cell extracts using AKT1, AKT2, total AKT, MEK1, and MEK2 antibodies. B, immunoblot analysis of whole cell lysates performed using an antibody against MKP-1. Equal loading was confirmed using β-actin antibody. C, densitometric quantification of MKP-1 immunoblot in WT and AKT-deficient isoforms. The data represent the mean values ± S.E. of 4 independent experiments performed in triplicate (ANOVA Mann-Whitney U test; ** = p < 0.01). D, the levels of phospho AKT Ser-473, AKT-1, AKT-2, and total AKT in BMDM lysates from WT, MEK2−/− MEK1f/f, and Mek1d/d Mek2+/+ Sox2:Cre+ mice. BMDMs derived from WT, MEK2−/− MEK1f/f, and Mek1d/d Mek2+/+ Sox2:Cre+ mice were cultured in the absence and presence of rapamycin (10 ng/ml). E, immunoblot analysis of whole cell lysates performed using antibodies detecting pMEK1/2, MEK1, MEK2, and β-actin. F, immunoblot analysis of whole cell lysates performed using antibodies detecting MKP-1 and β-actin. G, densitometric quantification of MKP-1 immunoblots (n = 3) in WT and MEK2- and MEK1-deficient macrophages in response to rapamycin. The data represent the mean values ± S.E. of three independent experiments each performed in triplicates (ANOVA Mann-Whitney U test; ** = p < 0.01).
Because U0126 inhibited the rapamycin-mediated MKP-1 induction, we further investigated the role of MEK1 and MEK2 in this process. BMDMs from wild-type, MEK2−/− MEK1f/f, and MEK1d/dMEK2+/+Sox2Cre+ mice were cultured under the same conditions and treated with rapamycin for different time periods. There were no major differences between the expression of AKT1 and AKT2 in all three strains of mice. We observed a variation in phosphorylation of AKT between the WT, MEK2−/−MEK1f/f, and MEK1d/dMEK2+/+Sox2Cre+ at the base line and in response to rapamycin. WT BMDMs responded to rapamycin with an increased AKT phosphorylation. In contrast, we observed a higher basal phosphorylated form of AKT but only a minimal increase in AKT phosphorylation in response to rapamycin in both MEK1 and MEK2 deficient BMDM (Fig. 8D). BMDMs derived from MEK2−/−MEK1f/f mice lacked MEK2 protein expression, whereas MEK1d/dMEK2+/+Sox2Cre+ BMDMs were deficient in MEK1 protein expression (Fig. 8E). Although WT BMDMs responded to rapamycin with MEK1/2 phosphorylation, MEK1/2 phosphorylation was aberrant in both MEK1- and MEK2-deficient BMDMs. MEK2-deficient BMDMs show pMEK1/2 at the base line but no further response to rapamycin. MEK1-deficient BMDMs lacked phosphorylated MEK1/2 both at the base line and in response to rapamycin. Consistent with our previous results, BMDM of WT mice responded to rapamycin with an increased MKP-1 expression. In contrast, the MEK2−/− MEK1f/f BMDMs demonstrated a higher basal level of MKP-1 expression but failed to respond to rapamycin treatment with further enhanced MKP-1 expression. MEK1d/dMEK2+/+Sox2Cre+ BMDMs failed to express MKP-1 both at the base line and after rapamycin treatment. We conclude that MEK1 is critical for both basal and rapamycin-mediated MKP-1 expression, and MEK2 is important for rapamycin-mediated MKP-1 induction (Fig. 8, E and F).
Rapamycin Pretreatment Inhibits the LPS-mediated p38 Phosphorylation and Nitric Oxide and IL-6 Production
We further investigated whether induction of MKP-1 by rapamycin affects the biological processes regulated by p38. BMDMs from WT mice were either stimulated with LPS or pretreated with rapamycin for 30 min and then stimulated with LPS, and p38 phosphorylation as well as IL-6 and nitric acid production were analyzed. LPS treatment led to a rapid p38 phosphorylation and rapamycin pretreatment significantly attenuated LPS-mediated p38 activation (Fig. 9, A and B). p38 activation regulates several proinflammatory cytokines, including IL-6, and mediates the production of nitric oxide via enhancing inducible nitric-oxide synthase expression (42). Pretreatment of BMDMs with rapamycin before LPS stimulation led to a significant inhibition of both IL-6 and NO production at 24 h (Fig. 9, C and D).
FIGURE 9.
Rapamycin pretreatment attenuates LPS-induced p38 phosphorylation and IL-6 and nitric oxide production. Cultured BMDM from WT mice were treated with LPS (100 ng/ml) directly or pretreated with rapamycin (Rapa) for 30 min and then treated with LPS for different time periods. A, phosphorylated p38 determined in cell extracts by Western blot analysis. B, -fold changes in the densitometric readings of pp38 normalized to p38. The data represent the mean values ± S.E. of three independent experiments. C, cells were pretreated with rapamycin and then challenged with and without LPS for 24 h. IL-6 levels in condition medium were measured via ELISA. D, NO levels in condition medium were detected using the Griess reaction. The data represent mean values ± S.E. of three independent experiments each performed in triplicates (ANOVA Mann-Whitney U test, ** = p < 0.01).
DISCUSSION
Cellular immune responses rely on signal transduction cascades regulated by phosphorylation and dephosphorylation of specific kinases. MAPKs are central regulators of inflammatory processes to exogenous (such as infection) or endogenous (tissue injury or repair) stimuli (9). During the immune response MAPK activation is under tight control, with MKPs controlling both the magnitude and duration of MAPK activation, thereby impacting cellular homeostasis (10, 12). Recent studies indicate that MKP-1, a key phosphatase, not only negatively regulates innate immunity predominantly through p38 modulation (13, 43, 44) but also plays a critical role in adaptive immunity such as T cell function (21) through modulation of both T helper 1 (Th1) and Th17 differentiation (43). The Mkp-1 gene is an immediate-early gene and is rapidly induced in response to growth factors, some cytokines, most TLR agonists, and several anti-inflammatory drugs including glucocorticoids (13, 17). MKP-1 expression is positively or negatively regulated by ERK, p38, and JNK and some of their downstream targets (MSK1, MSK2, ATF1, ATF2, c-Jun, and CREB) (12, 15, 35, 39). Here we show that AKT and MEK/ERK activation in response to rapamycin mediates MKP-1 induction. Both ERK and AKT can activate the CREB family (45). The Mkp-1 promoter contains three consensus CRE sites for the CREB family, including ATF1, ATF2, ATF3, CREB1, and Jun (34). Based on in silico analysis, neither promoter of MKP-3 nor MKP-5 contains consensus binding sites for the CREB family; consistent with our experimental data, rapamycin induces neither MKP-3 nor MKP-5. Our study shows that rapamycin-activated CREB/ATF1 and ATF2 could directly bind to the two consensus CREs on the Mkp-1 promoter, and their binding recruits C/EBPβ and p300 to the CRE on the Mkp-1 promoter. C/EBPβ, a downstream target of ERK (46), directly binds to the Mkp-1 promoter, and it has been shown that glucocorticoid-mediated induction of MKP-1 occurs through a mechanism involving C/EBPβ (39). The effect of rapamycin was slightly different from dexamethasone, as rapamycin treatment led to a higher C/EBPβ and p300 recruitment on the Mkp-1 promoter. Both p300 and the CBP are important in localized chromatin structure remodeling to permit the transcriptional machinery access to the DNA (40).
In addition to its anti-neoplastic and anti-proliferative properties, rapamycin has immune-modulatory properties (47, 48). This effect of rapamycin has been attributed to inhibition of clonal expansion of lymphocytes and/or through a mechanism involving regulatory T (TReg) cells and IL-10. However, rapamycin also modulates the response of innate immune cells such as macrophages and dendritic cells (6, 7), but the upstream signaling event associated with such modulation remains poorly understood. Most other studies have used rapamycin as an inhibitor before treatment with diverse stimuli such as TLR ligands or growth factors (EGF, serum, or insulin) to activate mTOR. In contrast, we first applied rapamycin as a single agent to study its effects on several pivotal cell signaling pathways. Rapamycin alone did not induce any cytokines (IL-1β, IL-6, TNF-α, and IL-10) or NO production and did not activate NF-κB in murine macrophages or change cell viability (data not shown). However, rapamycin alone had a significant effect on both the ERK and the AKT pathways and enhanced the expression of MKP-1. Moreover, rapamycin-mediated MKP-1 induction attenuated p38 phosphorylation in response to TLR ligands such as LPS and led to a significant decrease in IL-6 and nitric oxide production. We have observed a similar inhibitory effect on JNK phosphorylation in rapamycin-pretreated cells in response to LPS, but an inhibition was not observed in terms of ERK phosphorylation (data not shown).
Inhibition of TORC1 through rapamycin is associated with increased receptor-tyrosine kinase receptor/PI3K and TORC2 activity leading to AKT and ERK activation (31, 41, 49). Although mTORC2 is the primary kinase involved in phosphorylation of AKT on Ser-473, PI3K regulates PDK1 and phosphorylates AKT on Thr-308 (3, 4). TORC2 and PI3K are structurally related kinases. It has been shown that both wortmannin and LY294002 block in vitro phosphorylation of AKT by rictor-TORC2 kinase in addition to an inhibitory effect on PI3K (4, 50). AKT activation appears to be important in immune tolerance through negative regulation of TLR pathways (51–53). This is further supported by the findings that MKP-1 induction in response to heat-killed Acinetobacter baumannii is augmented by PTEN deficiency but attenuated by PI3K deficiency (52). Despite the important role of AKT signaling and its regulation via mTORC1, mTORC2, and PI3K in various cellular processes including immune regulation, the differential roles of the AKT isoforms have not been well studied. Several recent studies indicate that AKT1 and AKT2 have differential roles in macrophages and T cell polarization (54, 55). Although all three AKT isoforms share common upstream activators and some downstream substrates, it appears that they play distinct and non-redundant roles in biological processes. AKT1 KO BMDMs exhibit a more proinflammatory phenotype (M1), whereas AKT2 KO BMDMs display a reduced inflammation and a M2 phenotype in response to TLR4 ligand (54). Our study indicates that AKT1 but not AKT2 is important in rapamycin-mediated MKP-1 induction. Previously, it has been shown that inhibition of TORC1 using rapamycin activates ERK (41); we also observed that treatment with the dual TORC inhibitor similarly led to ERK activation, but this treatment provoked an attenuated AKT phosphorylation below its basal level and had a delayed and less pronounced effect on MKP-1 induction relative to rapamycin treatment. We reasoned that mTORC2 activity is required for AKT activation, whereas mTORC1 inhibition is responsible for ERK activation.
Our current data are in line with previous studies showing that rapamycin treatment activates receptor-tyrosine kinase including EGF receptor through attenuating mTOR-mediated feedback inhibition and triggers activation of the survival kinases ERK1/2 and p90RSK in cancer cells (41). We extended these observations and confirmed that rapamycin activates the MEK-ERK pathway in both macrophages cell line and BMDM. One important finding of our study is that the rapamycin effect on MKP-1 induction depends on the presence of both MEK1 and MEK2. MEK2-deficient macrophages exhibited a higher expression of MKP-1 at the base line with no further induction after rapamycin treatment. In contrast, MEK1-deficient cells exhibited a lack of MKP-1 expression both at base line and in response to rapamycin. We observed an aberrant response to rapamycin in both MEK1- and MEK2-deficient cells in terms of phosphorylation of MEK1/2 and AKT. Because MEK1 regulates MEK2 activity by building a MEK1-MEK2 complex deactivating the regulatory subunit (56), it is not entirely surprising that MEK1 deficiency caused a more pronounced attenuation in MKP-1 induction. Our investigation is the first study to dissect the role of MEK1 and -2 in response to rapamycin and show their downstream effect on MKP-1. Enhanced MKP-1 expression was found in various cancers; at the same time MEK/ERK and AKT activation has been considered as an escape pathway promoting cancer cell survival (57, 58). Perhaps the enhanced AKT and ERK activities explain why MKP-1 overexpression is observed in a variety of human cancers. Because MKP-1 negatively regulates p38 and JNK, elevated MKP-1 potentially gives cancer cells a survival advantage against the stress in the cancer environment. Thus, our current findings are not only relevant in the inflammatory field but may prove of importance in the field of cancer biology as well.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HL113508 (to L. S.) and R01 DK56886 (to M. J. B.). This work was also supported by the Department of Medicine and the Center for Molecular Medicine and Genetics, Wayne State University School of Medicine (to L. S.).
- mTOR
- mammalian target of rapamycin
- TLR
- toll-like receptor
- MAP
- mitogen-activated protein
- MKP
- MAP kinase phosphatase
- DUSP
- dual specificity phosphatase
- BMDM
- bone marrow-derived macrophage
- ANOVA
- analysis of variance
- CHX
- cycloheximide
- CREB
- cAMP-response element (CRE)-binding protein
- ATF1
- transcription factor 1.
REFERENCES
- 1. Wullschleger S., Loewith R., Hall M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 2. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR. From growth signal integration to cancer, diabetes, and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guertin D. A., Stevens D. M., Thoreen C. C., Burds A. A., Kalaany N. Y., Moffat J., Brown M., Fitzgerald K. J., Sabatini D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev cell 11, 859–871 [DOI] [PubMed] [Google Scholar]
- 4. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 5. Janes M. R., Fruman D. A. (2009) Immune regulation by rapamycin. Moving beyond T cells. Sci Signal 2, pe25. [DOI] [PubMed] [Google Scholar]
- 6. Cao W., Manicassamy S., Tang H., Kasturi S. P., Pirani A., Murthy N., Pulendran B. (2008) Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI3K-mTOR-p70S6K pathway. Nat. Immunol. 9, 1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weichhart T., Costantino G., Poglitsch M., Rosner M., Zeyda M., Stuhlmeier K. M., Kolbe T., Stulnig T. M., Hörl W. H., Hengstschläger M., Müller M., Säemann M. D. (2008) The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29, 565–577 [DOI] [PubMed] [Google Scholar]
- 8. Delgoffe G. M., Pollizzi K. N., Waickman A. T., Heikamp E., Meyers D. J., Horton M. R., Xiao B., Worley P. F., Powell J. D. (2011) The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol 12, 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dong C., Davis R. J., Flavell R. A. (2002) MAP kinases in the immune response. Annu Rev Immunol 20, 55–72 [DOI] [PubMed] [Google Scholar]
- 10. Jeffrey K. L., Camps M., Rommel C., Mackay C. R. (2007) Targeting dual-specificity phosphatases. Manipulating MAP kinase signalling and immune responses. Nat. Rev. Drug Discov. 6, 391–403 [DOI] [PubMed] [Google Scholar]
- 11. Rastogi R., Du W., Ju D., Pirockinaite G., Liu Y., Nunez G., Samavati L. (2011) Dysregulation of p38 and MKP-1 in response to NOD1/TLR4 stimulation in sarcoid bronchoalveolar cells. Am. J. Resp. Crit. Care Med. 183, 500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y., Shepherd E. G., Nelin L. D. (2007) MAPK phosphatases. Regulating the immune response. Nat. Rev. Immunol. 7, 202–212 [DOI] [PubMed] [Google Scholar]
- 13. Chi H., Barry S. P., Roth R. J., Wu J. J., Jones E. A., Bennett A. M., Flavell R. A. (2006) Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 2274–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang X., Meng X., Kuhlman J. R., Nelin L. D., Nicol K. K., English B. K., Liu Y. (2007) Knockout of Mkp-1 enhances the host inflammatory responses to gram-positive bacteria. J. Immunol. 178, 5312–5320 [DOI] [PubMed] [Google Scholar]
- 15. Kristiansen M., Hughes R., Patel P., Jacques T. S., Clark A. R., Ham J. (2010) Mkp1 is a c-Jun target gene that antagonizes JNK-dependent apoptosis in sympathetic neurons. J Neurosci 30, 10820–10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J., Zhou J. Y., Wu G. S. (2007) ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 67, 11933–11941 [DOI] [PubMed] [Google Scholar]
- 17. Wang X., Nelin L. D., Kuhlman J. R., Meng X., Welty S. E., Liu Y. (2008) The role of MAP kinase phosphatase-1 in the protective mechanism of dexamethasone against endotoxemia. Life Sci. 83, 671–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abraham S. M., Lawrence T., Kleiman A., Warden P., Medghalchi M., Tuckermann J., Saklatvala J., Clark A. R. (2006) Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 203, 1883–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hammer M., Mages J., Dietrich H., Schmitz F., Striebel F., Murray P. J., Wagner H., Lang R. (2005) Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur. J. Immunol. 35, 2991–3001 [DOI] [PubMed] [Google Scholar]
- 20. Xiao Y. Q., Malcolm K., Worthen G. S., Gardai S., Schiemann W. P., Fadok V. A., Bratton D. L., Henson P. M. (2002) Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-β. J. Biol. Chem. 277, 14884–14893 [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y., Reynolds J. M., Chang S. H., Martin-Orozco N., Chung Y., Nurieva R. I., Dong C. (2009) MAP kinase phosphatase 1 is necessary for T cell activation and function. J. Biol. Chem. 284, 30815–30824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bauerfeld C. P., Rastogi R., Pirockinaite G., Lee I., Hüttemann M., Monks B., Birnbaum M. J., Franchi L., Nuñez G., Samavati L. (2012) TLR4-mediated AKT activation is MyD88/TRIF-dependent and critical for induction of oxidative phosphorylation and mitochondrial transcription factor A in murine macrophages. J. Immunol. 188, 2847–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bélanger L. F., Roy S., Tremblay M., Brott B., Steff A. M., Mourad W., Hugo P., Erikson R., Charron J. (2003) Mek2 is dispensable for mouse growth and development. Mol. Cell. Biol. 23, 4778–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bissonauth V., Roy S., Gravel M., Guillemette S., Charron J. (2006) Requirement for Map2k1 (Mek1) in extra-embryonic ectoderm during placentogenesis. Development 133, 3429–3440 [DOI] [PubMed] [Google Scholar]
- 25. Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- 26. Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. (2001) Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 [DOI] [PubMed] [Google Scholar]
- 27. Samavati L., Rastogi R., Du W., Hüttemann M., Fite A., Franchi L. (2009) STAT3 tyrosine phosphorylation is critical for interleukin 1β and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 46, 1867–1877 [DOI] [PubMed] [Google Scholar]
- 28. Liu R. M., Choi J., Wu J. H., Gaston Pravia K. A., Lewis K. M., Brand J. D., Mochel N. S., Krzywanski D. M., Lambeth J. D., Hagood J. S., Forman H. J., Thannickal V. J., Postlethwait E. M. (2010) Oxidative modification of nuclear mitogen-activated protein kinase phosphatase 1 is involved in transforming growth factor β1-induced expression of plasminogen activator inhibitor 1 in fibroblasts. J. Biol. Chem. 285, 16239–16247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuwano Y., Kim H. H., Abdelmohsen K., Pullmann R., Jr., Martindale J. L., Yang X., Gorospe M. (2008) MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol. Cell. Biol. 28, 4562–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X. G., Liu F., Song X. F., Wang Z. H., Dong Z. Q., Hu Z. Q., Lan R. Z., Guan W., Zhou T. G., Xu X. M., Lei H., Ye Z. Q., Peng E. J., Du L. H., Zhuang Q. Y. (2010) Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol. Carcinog. 49, 603–610 [DOI] [PubMed] [Google Scholar]
- 31. Carracedo A., Ma L., Teruya-Feldstein J., Rojo F., Salmena L., Alimonti A., Egia A., Sasaki A. T., Thomas G., Kozma S. C., Papa A., Nardella C., Cantley L. C., Baselga J., Pandolfi P. P. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 118, 3065–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun S. Y., Rosenberg L. M., Wang X., Zhou Z., Yue P., Fu H., Khuri F. R. (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 65, 7052–7058 [DOI] [PubMed] [Google Scholar]
- 33. Chang F., Steelman L. S., Lee J. T., Shelton J. G., Navolanic P. M., Blalock W. L., Franklin R. A., McCubrey J. A. (2003) Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors. Potential targeting for therapeutic intervention. Leukemia 17, 1263–1293 [DOI] [PubMed] [Google Scholar]
- 34. Sánchez-Tilló E., Comalada M., Farrera C., Valledor A. F., Lloberas J., Celada A. (2006) Macrophage-colony-stimulating factor-induced proliferation and lipopolysaccharide-dependent activation of macrophages requires Raf-1 phosphorylation to induce mitogen kinase phosphatase-1 expression. J. Immunol. 176, 6594–6602 [DOI] [PubMed] [Google Scholar]
- 35. Ananieva O., Darragh J., Johansen C., Carr J. M., McIlrath J., Park J. M., Wingate A., Monk C. E., Toth R., Santos S. G., Iversen L., Arthur J. S. (2008) The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat. Immunol. 9, 1028–1036 [DOI] [PubMed] [Google Scholar]
- 36. Deak M., Clifton A. D., Lucocq L. M., Alessi D. R. (1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shimada M., Nakadai T., Fukuda A., Hisatake K. (2010) cAMP-response element-binding protein (CREB) controls MSK1-mediated phosphorylation of histone H3 at the c-fos promoter in vitro. J. Biol. Chem. 285, 9390–9401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lau E., Ronai Z. A. (2012) ATF2. At the crossroad of nuclear and cytosolic functions. J. Cell Sci. 125, 2815–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johansson-Haque K., Palanichamy E., Okret S. (2008) Stimulation of MAPK-phosphatase 1 gene expression by glucocorticoids occurs through a tethering mechanism involving C/EBP. J. Mol. Endocrinol. 41, 239–249 [DOI] [PubMed] [Google Scholar]
- 40. Schwartz C., Beck K., Mink S., Schmolke M., Budde B., Wenning D., Klempnauer K. H. (2003) Recruitment of p300 by C/EBPβ triggers phosphorylation of p300 and modulates coactivator activity. EMBO J. 22, 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaturvedi D., Gao X., Cohen M. S., Taunton J., Patel T. B. (2009) Rapamycin induces transactivation of the EGFR and increases cell survival. Oncogene 28, 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen C. C., Wang J. K. (1999) p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol. Pharmacol. 55, 481–488 [PubMed] [Google Scholar]
- 43. Arthur J. S., Fong A. L., Dwyer J. M., Davare M., Reese E., Obrietan K., Impey S. (2004) Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J. Neurosci. 24, 4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao Q., Shepherd E. G., Manson M. E., Nelin L. D., Sorokin A., Liu Y. (2005) The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide. Attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J. Biol. Chem. 280, 8101–8108 [DOI] [PubMed] [Google Scholar]
- 45. Carloni S., Girelli S., Scopa C., Buonocore G., Longini M., Balduini W. (2010) Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 6, 366–377 [DOI] [PubMed] [Google Scholar]
- 46. Buck M., Chojkier M. (2007) C/EBPβ phosphorylation rescues macrophage dysfunction and apoptosis induced by anthrax lethal toxin. Am. J. Physiol. Cell Physiol. 293, C1788–C1796 [DOI] [PubMed] [Google Scholar]
- 47. Moua T., Olson E. J., Jean H. C., Ryu J. H. (2012) Resolution of chylous pulmonary congestion and respiratory failure in LAM with sirolimus therapy. Am. J. Resp. Crit. Care Med. 186, 389–390 [DOI] [PubMed] [Google Scholar]
- 48. Mushaben E. M., Kramer E. L., Brandt E. B., Khurana Hershey G. K., Le Cras T. D. (2011) Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J. Immunol. 187, 5756–5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Reilly K. E., Rojo F., She Q. B., Solit D., Mills G. B., Smith D., Lane H., Hofmann F., Hicklin D. J., Ludwig D. L., Baselga J., Rosen N. (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 66, 1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brunn G. J., Williams J., Sabers C., Wiederrecht G., Lawrence J. C., Jr., Abraham R. T. (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin, and LY294002. EMBO J. 15, 5256–5267 [PMC free article] [PubMed] [Google Scholar]
- 51. Brown J., Wang H., Suttles J., Graves D. T., Martin M. (2011) Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J. Biol. Chem. 286, 44295–44305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Günzl P., Bauer K., Hainzl E., Matt U., Dillinger B., Mahr B., Knapp S., Binder B. R., Schabbauer G. (2010) Anti-inflammatory properties of the PI3K pathway are mediated by IL-10/DUSP regulation. J. Leukoc. Biol. 88, 1259–1269 [DOI] [PubMed] [Google Scholar]
- 53. Medina E. A., Morris I. R., Berton M. T. (2010) Phosphatidylinositol 3-kinase activation attenuates the TLR2-mediated macrophage proinflammatory cytokine response to Francisella tularensis live vaccine strain. J. Immunol. 185, 7562–7572 [DOI] [PubMed] [Google Scholar]
- 54. Arranz A., Doxaki C., Vergadi E., Martinez de la Torre Y., Vaporidi K., Lagoudaki E. D., Ieronymaki E., Androulidaki A., Venihaki M., Margioris A. N., Stathopoulos E. N., Tsichlis P. N., Tsatsanis C. (2012) Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc. Natl. Acad. Sci. U.S.A. 109, 9517–9522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim J. S., Sklarz T., Banks L. B., Gohil M., Waickman A. T., Skuli N., Krock B. L., Luo C. T., Hu W., Pollizzi K. N., Li M. O., Rathmell J. C., Birnbaum M. J., Powell J. D., Jordan M. S., Koretzky G. A. (2013) Natural and inducible TH17 cells are regulated differently by Akt and mTOR pathways. Nat Immunol 14, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Catalanotti F., Reyes G., Jesenberger V., Galabova-Kovacs G., de Matos Simoes R., Carugo O., Baccarini M. (2009) A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat. Struct. Mol. Biol. 16, 294–303 [DOI] [PubMed] [Google Scholar]
- 57. Huang J., Wang H., Song Z., Lin X., Zhang C. (2011) Involvement of MAPK phosphatase-1 in dexamethasone-induced chemoresistance in lung cancer. J. Chemother. 23, 221–226 [DOI] [PubMed] [Google Scholar]
- 58. Staples C. J., Owens D. M., Maier J. V., Cato A. C., Keyse S. M. (2010) Cross-talk between the p38α and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to UV radiation. J. Biol. Chem. 285, 25928–25940 [DOI] [PMC free article] [PubMed] [Google Scholar]