Background: LRP1 is a cell signaling receptor in neurons.
Results: LRP1, NMDA receptor, and Trk compose a single system, activating cell signaling in response to tPA and α2-macroglobulin. MAG binding to LRP1 recruits p75NTR but not Trk.
Conclusion: Ligand-specific co-receptor recruitment explains how LRP1 activates distinct cell signaling pathways in response to different ligands.
Significance: Distinct co-receptor assemblies allow LRP1 to regulate the cellular response to its microenvironment.
Keywords: Cell Signaling, ERK, Lipoprotein-like Receptor (LRP), Protease Inhibitor, Tissue-type Plasminogen Activator (tPA)
Abstract
In addition to functioning as an activator of fibrinolysis, tissue-type plasminogen activator (tPA) interacts with neurons and regulates multiple aspects of neuronal cell physiology. In this study, we examined the mechanism by which tPA initiates cell signaling in PC12 and N2a neuron-like cells. We demonstrate that enzymatically active and inactive tPA (EI-tPA) activate ERK1/2 in a biphasic manner. Rapid ERK1/2 activation is dependent on LDL receptor-related protein-1 (LRP1). In the second phase, ERK1/2 is activated by tPA independently of LRP1. The length of the LRP1-dependent phase varied inversely with the tPA concentration. Rapid ERK1/2 activation in response to EI-tPA and activated α2-macroglobulin (α2M*) required the NMDA receptor and Trk receptors, which assemble with LRP1 into a single pathway. Assembly of this signaling system may have been facilitated by the bifunctional adapter protein, PSD-95, which associated with LRP1 selectively in cells treated with EI-tPA or α2M*. Myelin-associated glycoprotein binds to LRP1 with high affinity but failed to induce phosphorylation of TrkA or ERK1/2. Instead, myelin-associated glycoprotein recruited p75 neurotrophin receptor (p75NTR) into a complex with LRP1 and activated RhoA. p75NTR was not recruited by other LRP1 ligands, including EI-tPA and α2M*. Lactoferrin functioned as an LRP1 signaling antagonist, inhibiting Trk receptor phosphorylation and ERK1/2 activation in response to EI-tPA. These results demonstrate that LRP1-initiated cell signaling is ligand-dependent. Proteins that activate cell signaling by binding to LRP1 assemble different co-receptor systems. Ligand-specific co-receptor recruitment provides a mechanism by which one receptor, LRP1, may trigger different signaling responses.
Introduction
Tissue-type plasminogen activator (tPA)2 is a serine protease and activator of fibrinolysis, used in recombinant form to treat ischemic stroke, myocardial infarction, and other disorders in which thrombosis is central to the pathogenesis (1). In neurons and neuron-like cell lines, membrane depolarization results in the release of tPA (2, 3). tPA also may be transported across the blood-brain barrier by LDL receptor-related protein-1 (LRP1) (4). It is thus important to understand the numerous effects of tPA on neuronal cell biology and function, including effects on long term potentiation (5–7), excitotoxic neurotoxicity (3, 8), axonal regrowth following injury (9), neuroprotection in hypoxia and ischemia (10, 11), and permeability of the blood-brain barrier (12).
Various mechanisms have been proposed to explain the activity of tPA in the CNS, including pathways that require plasminogen activation (9, 13, 14), direct proteolytic cleavage of the NR1 subunit of the NMDA receptor (NMDA-R) (3), binding to LRP1 and activation of LRP1-dependent cell signaling (7, 10, 12, 15), and binding to annexin A2 (16). In addition to tPA, other proteins that bind to LRP1, such as apolipoprotein E (apoE) and activated α2-macroglobulin (α2M*), trigger cell signaling in neurons and neuron-like cell lines, activating kinases such as ERK/MAPK (ERK1/2), promoting neuronal survival, and supporting neurite outgrowth (17–21). There is substantial evidence that the mechanism by which LRP1 signals in response to tPA, apoE, or α2M* in neurons requires NMDA-R as a co-receptor and that targeting either LRP1 or the NMDA-R may attenuate the signaling response (22–26). Other receptors in the LDL receptor family, such as apoER2/LRP8, also signal in association with NMDA-R (27, 28). The bifunctional adaptor protein, postsynaptic density protein 95 (PSD-95), plays an important role, bridging NMDA-R to LRP1 and other LDL receptor family members (27, 29, 30).
We recently demonstrated that the ability of α2M* to promote neurite outgrowth in PC12 cells, N2a cells, and cerebellar granule neurons requires binding of α2M to LRP1 and Trk receptor transactivation, which occurs downstream of activated Src family kinases (SFKs) (31). Antagonizing SFK activity or Trk receptor blocked ERK1/2 activation and inhibited neurite outgrowth in response to activated α2M* and tPA. Trk transactivation has been described as a potentially distinct pathway by which LRP1 may activate cell signaling independently of NMDA-R (32).
In this study, we examined cell signaling in PC12 and N2a neuron-like cell lines treated with enzymatically inactive tPA (EI-tPA). By using an inactive form of tPA, we focused on receptor-dependent signaling mechanisms, in isolation from pathways that require plasminogen activation or NR1 cleavage. We find that ERK1/2 activation in response to EI-tPA occurs in two phases: a rapid phase that is dependent on LRP1 and a slower response that is LRP1-independent. Similar results were obtained with enzymatically active tPA.
We then focused on the LRP1-dependent phase of tPA-initiated cell signaling and demonstrated that NMDA-R and Trk receptors function with LRP1 as part of a single signaling system to activate ERK1/2. p75 neurotrophin receptor (p75NTR), which serves as an essential LRP1 co-receptor for activation of RhoA and inhibition of neurite outgrowth when myelin-associated glycoprotein (MAG) binds to LRP1 (33), was not part of the LRP1 signaling receptor complex formed in response to EI-tPA or α2M*. Recruitment of PSD-95 to LRP1 occurred selectively in cells treated with tPA and α2M*. These results suggest a model in which the effects of LRP1 on cell signaling and cell physiology are regulated in a ligand-specific manner by selective co-receptor recruitment.
MATERIALS AND METHODS
Proteins and Reagents
Active tPA (85% single-chain, 15% two-chain) and EI-tPA, which is mutated at two amino acids (S478A and R275E) and thus inactive and non-cleavable were purchased from Molecular Innovations (Novi, MI). The enzymatic activity of both forms of tPA was determined by measuring the rate of hydrolysis of H-d-isoleucyl-l-prolyl-l-arginine p-nitroanilide 2HCl (S-2288, Chromogenix, Bedford, MA). We compared hydrolysis of 1.0 mm S-2288 by 12 nm active tPA and EI-tPA. The first order rate constant (v/Et) for active tPA was 16 s−1. Comparing this value with kinetic parameters published by the manufacturer (kcat and Km) suggested that the tPA preparation was nearly 100% active. S-2288 hydrolysis was completely absent with EI-tPA.
α2M was purified from human plasma by the method of Imber and Pizzo (34) and activated for binding to LRP1 by dialysis against 200 mm methylamine HCl (α2M*) (35). Modification of α2M by methylamine was confirmed by demonstrating the characteristic increase in α2M electrophoretic mobility by nondenaturing PAGE (36). The LRP1 ligand-binding antagonist, receptor-associated protein (RAP), was expressed as a GST fusion protein (GST-RAP) in bacteria and purified as previously described (37). As a control, we expressed GST in bacteria transformed with the empty vector, pGEX-2T. MAG was expressed as an Fc fusion protein and purified by affinity chromatography on Protein A-Sepharose (GE Healthcare) (33). Free Fc also was prepared as a control. Purified Fc fusion proteins, RAP and GST, were subjected to chromatography on Detoxi-Gel endotoxin-removing columns (Thermo Scientific, Rockford, IL). Lactoferrin was from Sigma-Aldrich. Murine NGF-β was from Invitrogen. The Rho assay reagent, in which residues 7–89 of the rhotekin Rho-binding domain is expressed as a GST fusion protein (TRBD-GST) was from Millipore (Billerica, MA).
For immunoblotting experiments, we used an antibody that detects the LRP1 85-kDa β-chain (Sigma). Rabbit polyclonal antibodies that detect the NR1 subunit of the NMDA-R, PSD-95, RhoA, phospho-Trk, phospho-ERK, and total ERK were from Cell Signaling Technologies (Danvers, MA). The polyclonal antibody that detects p75NTR was from Millipore. Monoclonal antibody specific for β-actin was from Sigma. Horseradish peroxidase-conjugated secondary antibodies were from Cell Signaling Technologies.
Cell Culture
Rat PC12 pheochromocytoma cells were obtained from the ATCC (catalog no. CRL-1721) and cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose; Invitrogen) containing 10% fetal bovine serum (FBS; Hyclone), 5% heat-inactivated horse serum (Omega Scientific Inc.), penicillin (100 units/ml), and streptomycin (1 mg/ml). Mouse N2a neuroblastoma cells were a generous gift from Dr. Katerina Akassoglou (Gladstone Institute of Neurological Disease, University of California, San Francisco, CA). N2a cells were cultured in DMEM containing 10% FBS, penicillin, and streptomycin.
Gene Silencing
Rat NR1-specific siRNA ON-TARGETplus SMARTpool, which targets the NR1 subunit of the NMDA-R, the previously described LRP1-specific siRNA, L2 (CGAGCGACCUCCUAUCUUUUU) (20), and pooled non-targeting control (NTC) siRNA were from Dharmacon. PC12 cells (2 × 106) were transfected with NR1-specific siRNA (50 nm), LRP1-specific siRNA (25 nm), or NTC siRNA (25–50 nm) by electroporation using the Cell Line Nucleofector Kit V (Amaxa), as described previously (38). The degree of gene silencing was determined at the mRNA level by real-time qPCR and at the protein level by immunoblot analysis. Experiments were performed 48 h after siRNA transfection.
Activation of TrkA and ERK1/2
PC12 and N2a cells were plated in 100-mm dishes at a density of 2 × 106 cells/well in serum-containing medium and cultured until ∼70% confluent. The cultures were then transferred into serum-free medium (SFM) for 4 h before adding candidate cell signaling activators, including α2M*, EI-tPA, MAG, lactoferrin, NGF-β, or vehicle. Where indicated, the NMDA-R antagonist, MK801 (1 μm), was added 2 h before adding stimulants. RAP (250 nm) or GST was added 30 min before other LRP1 ligands. Incubations were conducted for 10 min unless otherwise stated. The cells were then rinsed twice with ice-cold phosphate-buffered saline (PBS). Cell extracts were prepared in radioimmune precipitation assay buffer (PBS with 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor mixture, and sodium orthovanadate). The protein concentration in cell extracts was determined by a bicinchoninic acid assay. An equivalent amount of cellular protein (40 μg for analysis of ERK1/2 activation and 100 μg for analysis of TrkA activation) was subjected to 8 or 12% SDS-PAGE and electrotransferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk in 20 mm Tris-HCl, 150 mm NaCl, pH 7.4, with Tween 20 and incubated with primary antibodies. The membranes were then washed and treated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immunoblots were developed by enhanced chemiluminescence (PerkinElmer Life Sciences). Immunoblotting studies were performed at least three times.
Co-immunoprecipitation Experiments
N2a cells were cultured until 80% confluent in 10-cm tissue culture dishes and then treated with α2M* (10 nm), EI-tPA (12 nm), lactoferrin (20 nm), MAG-Fc (20 nm), or Fc (20 nm). The cultures were washed twice with ice-cold PBS and extracted in 1 ml of 50 mm Hepes, 150 mm NaCl, pH 7.4, 1% Triton X-100, 10% glycerol, protease inhibitor mixture, 2 mm EDTA, 1 mm sodium vanadate (cell extraction buffer). Extracts were cleared by centrifugation at 4 °C. The supernatants were collected, and protein content was determined by a bicinchoninic acid assay. Equal amounts of cellular protein were subjected to sequential immunoprecipitation with control IgG and then LRP1-specific antibody (7.5 μg), which were precoupled to Protein A-Sepharose beads by incubation for 2 h at 4 °C. Incubations with cell extracts were allowed to progress for 12 h at 4 °C. The beads were collected by centrifugation and washed three times with ice-cold extraction buffer. Immunoprecipitates were subjected to SDS-PAGE. Immunoblot analysis was then performed to detect LRP1 and co-immunoprecipitated proteins.
RhoA Activity Assays
Affinity precipitation of active RhoA was performed using TRBD-GST. N2a cells were plated in 100-mm dishes at a density of 2 × 106 cells/well in serum-containing medium and cultured until ∼85% confluent. The cultures were then transferred into SFM for 4 h before adding candidate LRP1 ligands, including MAG, lactoferrin, α2M*, and EI-tPA. Cell extracts were obtained and incubated with 30 μg of TRBD-GST coupled to glutathione-Sepharose for 45 min at 4 °C. The beads were washed and then treated with SDS sample buffer to dissociate TRBD-GST and active GTP-coupled Rho. Immunoblot analysis was performed to detect active RhoA. Samples of each cell extract were subjected to immunoblot analysis prior to incubation with TRBD-GST to determine total RhoA.
Statistical Analysis
Data processing and statistical analysis were performed using Microsoft Excel (Microsoft Corp.) and GraphPad Prism (GraphPad Software, Inc.). p values less than 0.05 were regarded as statistically significant and are indicated with an asterisk. p values of ≤0.005 are indicated with a double asterisk.
RESULTS
LRP1-dependent and -independent Phases of tPA-initiated Cell Signaling
In neurons and neuron-like cell lines, activation of ERK1/2 is essential for neurite outgrowth and as a prosurvival signal (39–41). α2M that is transformed into the receptor-recognized conformation with methylamine (α2M*) is a highly specific ligand for LRP1, with a binding affinity (KD) of about 1 nm (42–44). Fig. 1A shows that ERK1/2 was robustly phosphorylated in PC12 cells that were treated with 1.0, 10, or 50 nm α2M* for 10 min. GST-RAP completely blocked ERK1/2 phosphorylation induced by α2M*, suggesting that LRP1 is necessary for the signaling response.
FIGURE 1.
The role of LRP1 in ERK1/2 activation by EI-tPA and α2M*. PC12 cells were treated with vehicle (SFM) or with increasing concentrations of α2M* (1–50 nm) (A) or EI-tPA (1–50 nm) (B) for 10 min. GST-RAP (250 nm) was added to the cultures 30 min before α2M* or EI-tPA, as indicated (+RAP). C, PC12 cells were transfected with NTC or LRP1-specific siRNA. Relative LRP1 mRNA expression was determined by qPCR. D, transfected PC12 cells were treated with NGF-β (50 ng/ml) or EI-tPA (12 nm) for 10 min. E, PC12 cells were pretreated with GST-RAP (250 nm) for 30 min (+RAP) or with vehicle (−RAP) and then with EI-tPA (12 nm) for the indicated times, up to 2 h. Equal amounts of cellular protein (50 μg) were loaded into each lane and subjected to SDS-PAGE. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK (T-ERK). The blots shown are representative of at least three independent studies. **, p ≤ 0.005.
Next, we studied EI-tPA. By examining an enzymatically inactive form of tPA, we ruled out mechanisms of activating cell signaling that require plasminogen activation or cleavage of cell surface proteins (3, 9, 13, 14). tPA binds directly to LRP1 but with substantially lower affinity then α2M* (45–47). Nevertheless, ERK1/2 was robustly activated in PC12 cells that were treated with EI-tPA for 10 min at concentrations as low as 1.0 nm (Fig. 1B). RAP blocked ERK1/2 phosphorylation in response to 12 nm EI-tPA but consistently failed to block ERK1/2 phosphorylation when the concentration of EI-tPA was 50 nm or higher.
The effects of RAP on ERK1/2 phosphorylation by EI-tPA suggested a role for LRP1 but did not rule out involvement of other LDL receptor gene family members that also are antagonized by RAP (48). To confirm that LRP1 was involved, we silenced the LRP1 gene with siRNA. Fig. 1C shows that the extent of LRP1 gene silencing was about 85% at the mRNA level. Fig. 1D shows that LRP1 gene silencing blocked ERK1/2 phosphorylation in response to 12 nm EI-tPA in a 10-min incubation. As a specificity control, we treated PC12 cells in which the LRP1 gene was silenced with NGF-β. ERK1/2 phosphorylation was not blocked but, instead, increased. This result is discussed below.
To determine why ERK1/2 phosphorylation was not blocked by RAP when PC12 cells were treated with 50 nm EI-tPA (see Fig. 1B), first we conducted a time course experiment. Fig. 1E shows that ERK1/2 was robustly phosphorylated within 5 min in response to 12 nm EI-tPA, and the level of phospho-ERK1/2 remained essentially unchanged through 2 h. In the initial 15 min, ERK1/2 phosphorylation was inhibited by RAP, indicating an essential role for LRP1. Subsequently, the signaling response became RAP-resistant.
The results presented in Fig. 1, B and E, suggested a model in which rapid cell signaling in response to EI-tPA requires LRP1. When LRP1 is neutralized, EI-tPA still activates ERK1/2; however, the response is slower. To test whether ERK1/2 activation may be accelerated when LRP1 is neutralized by increasing the EI-tPA concentration, PC12 cells were treated with various concentrations of EI-tPA for 10 min. In the absence of RAP, ERK1/2 activation was similar throughout the EI-tPA concentration range (Fig. 2A). When RAP was added, the extent of ERK1/2 phosphorylation at 10 min increased as a function of the EI-tPA concentration.
FIGURE 2.
Enzymatically active tPA and EI tPA demonstrate similar activity. A, PC12 cells were pretreated with 250 nm GST-RAP (+RAP) or with vehicle (−RAP) for 30 min and then with increasing concentrations of EI-tPA (2–60 nm) for 10 min. B, PC12 cells were pretreated with 250 nm GST-RAP (+RAP) or with vehicle (−RAP) for 30 min and then treated with EI-tPA (12 nm) for 10 min or with enzymatically active tPA (12 nm) for different periods of times up to 1 h. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK).
Next, we examined ERK1/2 activation in PC12 cells treated with enzymatically active tPA (12 nm). The results were equivalent to those obtained with EI-tPA. ERK1/2 phosphorylation was observed at 10–60 min (Fig. 2B). At 10 min, the response to active tPA was blocked by RAP. By 30 min, comparable levels of ERK1/2 phosphorylation were observed in the presence and absence of RAP.
As a second model system, we studied N2a cells, which express high levels of LRP1 (33). EI-tPA (12 nm) and α2M* (10 nm) induced ERK1/2 phosphorylation in these cells at 10 min, and the response was blocked by RAP (Fig. 3A). In Fig. 3B, we treated N2a cells with 12 nm EI-tPA for increasing periods of time. As was the case with PC12 cells, robust ERK1/2 phosphorylation was observed by 5 min, and the response was sustained for 2 h. In N2a cells, phosphorylated ERK1 (p44) appeared more stable than phosphorylated ERK2 (p42). When RAP was added, the rapid phase of ERK1/2 phosphorylation was inhibited; however, by 30 min, ERK1/2 was equally phosphorylated in the presence and absence of RAP.
FIGURE 3.
EI-tPA-initiated cell signaling is biphasic in relation to the role of LRP1 in N2a cells. A, N2a cells were pretreated with RAP (250 nm) for 30 min as indicated (+RAP) and then treated with vehicle (SFM), α2M* (10 nm), or EI-tPA (12 nm) for 10 min. B, N2a cells were pretreated with GST-RAP (250 nm) for 30 min, as indicated (+RAP), and then treated with EI-tPA (12 nm) for different times up to 2 h. C, N2a cells were pretreated with GST-RAP (250 nm) for 30 min and then with increasing concentrations of EI-tPA (2–60 nm). Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK).
Fig. 3C shows that in N2a cells, as in PC12 cells, the requirement for LRP1 in order to observe rapid ERK1/2 phosphorylation in response to EI-tPA (at 10 min) was overcome by increasing the EI-tPA concentration. When cells were treated with 60 nm EI-tPA, equivalent ERK1/2 phosphorylation was observed at 10 min in the presence and absence of RAP.
NMDA-R and Trk Receptors Collaborate with LRP1 to Form a Single tPA Signaling Receptor System
We previously demonstrated that in PC12 cells, ERK1/2 activation in response to EI-tPA and α2M* requires Trk receptors (31). In this study, we examined the role of NMDA-R in relation to Trk receptors. All of our incubations were conducted for 10 min to focus on the time period when LRP1 is required. First, we treated PC12 cells with MK-801, a noncompetitive antagonist of the NMDA-R. Fig. 4A shows that MK-801 blocked ERK1/2 phosphorylation in response to EI-tPA but not in response to the control protein, NGF-β. Similar results were obtained when we examined N2a cells. In this second model system, MK-801 blocked ERK1/2 phosphorylation in response to EI-tPA and α2M* (Fig. 4B). These results suggest that in PC12 and N2a cells, the NMDA-R is a necessary co-receptor for ERK1/2 activation in response to LRP1 ligands.
FIGURE 4.
NMDA-R is required for ERK1/2 activation by EI-tPA and α2M*. A, PC12 cells were pretreated with MK-801 (1 μm) or vehicle for 2 h and then treated with vehicle (SFM), NGF-β (50 ng/ml), or EI-tPA (12 nm) for 10 min. B, N2a cells were pretreated with MK-801 (1 μm) for 2 h and then treated with vehicle (SFM), EI-tPA (12 nm), or α2M* (10 nm) for 10 min. C, PC12 cells were transfected with NTC siRNA or NR1-specific siRNA (siNR1). NR1 mRNA was determined by qPCR. NR1 protein was determined by immunoblot analysis 48 h after transfection. D, PC12 cells in which NR1 was silenced and control cells transfected with NTC siRNA were treated with NGF-β (50 ng/ml) or EI-tPA (12 nm) for 10 min. E, PC12 cells in which NR1 was silenced and control cells transfected with NTC siRNA were treated with EI-tPA (12 nm) for different times up to 2 h. Immunoblot analysis was performed to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK). *, p < 0.05.
The amount of intact heterodimeric NMDA-R is low in some PC12 cell variants; however, the NR1 subunit is typically expressed in higher amounts (49). Fig. 4C shows that in our cells, the NR1 subunit was readily detected at the mRNA and protein levels. In order to prevent assembly of intact NMDA-R, we silenced the gene for the NR1 subunit in PC12 cells. NR1 gene silencing was effective, decreasing the level of NR1 mRNA by 80% and NR1 protein so that it was barely detectable. Fig. 4D shows that in PC12 cells in which the NR1 subunit was silenced, ERK1/2 phosphorylation in response to EI-tPA was blocked. The response to NGF-β was slightly increased.
Next, we treated PC12 cells, in which the NR1 subunit of the NMDA-R was silenced, with 12 nm EI-tPA for up to 2 h, well beyond the time period when LRP1 is required for ERK1/2 activation. Control cells, which were treated with NTC siRNA, also were treated with 12 nm EI-tPA for 2 h. ERK1/2 activation was not observed in cells in which the NR1 subunit was silenced throughout the time course (Fig. 4E).
When EI-tPA or α2M* binds to LRP1, SFKs are activated, and the SFKs transactivate Trk receptors (31). Fig. 5A shows that TrkA was phosphorylated in PC12 cells treated with 12 nm EI-tPA for 10 min and in cells treated with NGF-β, which binds directly to TrkA. Antagonizing the NMDA-R with MK-801 had no effect on TrkA phosphorylation in response to NGF-β, as anticipated. By contrast, MK-801 blocked TrkA phosphorylation in response to EI-tPA.
FIGURE 5.
NMDA-R is required for Trk receptor transactivation. A, PC12 cells were pretreated with MK-801 (1 μm) for 2 h and then with NGF-β (50 ng/ml) or EI-tPA (12 nm) for 10 min. B, PC12 cells were pretreated with MK-801 (1 μm) for 2 h and then with EI-tPA (12 or 60 nm) for 10 min or 1 h. C, PC12 cells in which the NR1 subunit of the NMDA-R was silenced and cells transfected with NTC siRNA were treated with NGF-β (50 ng/ml), EI-tPA (12 nm), or vehicle for 10 min. Equal amounts of cellular protein (100 μg) were subjected to SDS-PAGE and immunoblot analysis to detect phosphorylated Trk (p-Trk). Blots were reprobed to detect β-actin as a loading control. Each blot is representative of at least three independent studies.
Next, we tested whether MK-801 blocks TrkA phosphorylation in response to higher concentrations of EI-tPA (60 nm) or when the EI-tPA is incubated with cells for up to 60 min. Fig. 5B shows that MK-801 blocked TrkA phosphorylation under all of our experimental conditions.
To confirm that NMDA-R is required for TrkA phosphorylation in response to EI-tPA, we conducted studies using PC12 cells in which the NR1 subunit of the NMDA-R was silenced. NR1 gene silencing had no effect on TrkA phosphorylation in response to NGF-β; however, TrkA phosphorylation in response to EI-tPA was entirely blocked (Fig. 5C). Taken together with previously published work (10), these results suggest that LRP1 and the NMDA-R function as co-receptors upstream of TrkA in a single pathway by which EI-tPA activates ERK1/2.
The LRP1 Signaling Response Is Ligand-specific
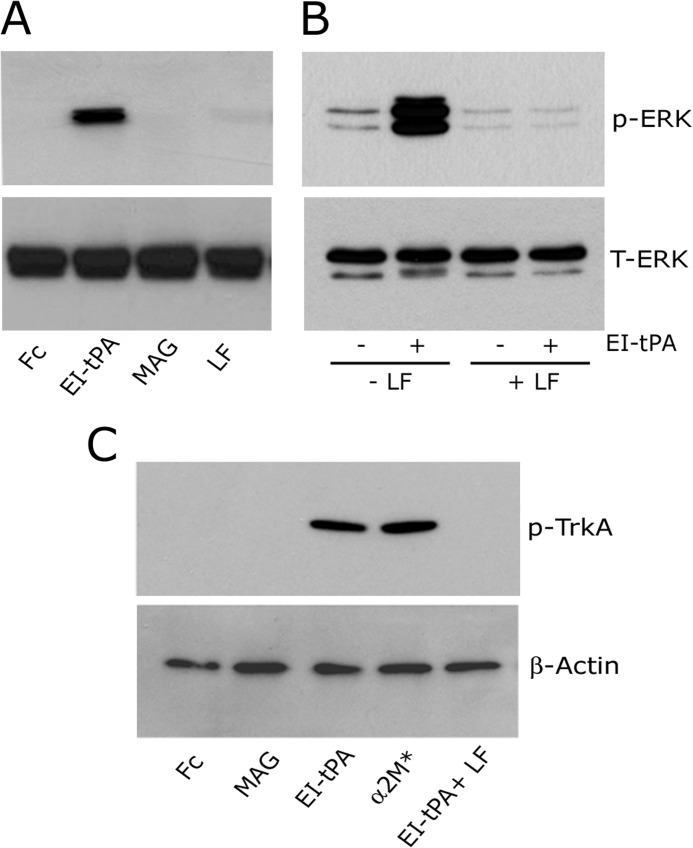
When the CNS is injured, proteins in myelin, including MAG, inhibit axonal regeneration (50, 51). We demonstrated that binding of MAG to LRP1 is necessary for inhibition of neurite outgrowth in PC12 cells, N2a cells, and cerebellar granule neurons (33), in contrast with tPA and α2M*, which promote neurite outgrowth in the same cell types (20, 31). MAG binding to LRP1 recruits p75NTR into a complex with LRP1 to activate RhoA (33). p75NTR recruitment has not been described with other LRP1 ligands. Like MAG, RAP has effects on cell signaling that are different from those induced by tPA and α2M*. RAP induces no discernible, independent signaling response. Instead, RAP inhibits activation of cell signaling and effects on cell physiology induced by other LRP1 ligands (7, 12, 17, 19, 20, 22, 26, 31). This prior work raised the hypothesis that LRP1-initiated cell signaling may be ligand-specific. To test this hypothesis, first we examined ERK1/2 phosphorylation in response to LRP1 ligands other than EI-tPA and α2M*. Fig. 6A shows that MAG (20 nm) failed to activate ERK1/2 in PC12 cells. Lactoferrin (20 nm) induced very weak ERK1/2 phosphorylation in some experiments and had no discernible effect in others. The response to EI-tPA is shown as a positive control. Because MAG is expressed as an Fc fusion protein, we treated PC12 cells with free Fc as a negative control. No ERK1/2 phosphorylation was observed.
FIGURE 6.
LRP1-initiated cell signaling is ligand-specific. A, PC12 cells were treated with free Fc (20 nm), EI-tPA (12 nm), MAG (20 nm), or lactoferrin (20 nm) for 10 min. Cell extracts were subjected to immunoblot analysis to detect phosphorylated ERK1/2 (p-ERK) and total ERK1/2 (T-ERK). B, PC12 cells were pretreated with lactoferrin (250 nm) or vehicle for 1 h and then treated with vehicle or EI-tPA (12 nm) for 10 min. C, PC12 cells were treated with free Fc (20 nm), MAG (20 nm), EI-tPA (12 nm), α2M* (10 nm), or EI-tPA in the presence of 250 nm lactoferrin for 10 min. Cell extracts were subjected to immunoblot analysis to detect phosphorylated TrkA (p-TrkA) and β-actin as a loading control.
Because lactoferrin failed to trigger a significant cell signaling response when it bound to LRP1, we tested its effects on cell signaling initiated by another LRP1 ligand. In Fig. 6B, PC12 cells were treated with EI-tPA (12 nm) in the presence of 250 nm lactoferrin. ERK1/2 activation in response to EI-tPA was blocked. These results suggest that lactoferrin may function similarly to RAP, as an LRP1 signaling antagonist in the presence of other LRP1 ligands.
We also examined TrkA transactivation in PC12 cells treated with different LRP1 ligands. TrkA was not phosphorylated in response to MAG (Fig. 6C). Robust TrkA phosphorylation was demonstrated with EI-tPA and α2M*. TrkA phosphorylation was not observed in lactoferrin-treated cells (results not shown). When PC12 cells were treated with 12 nm EI-tPA in the presence of 250 nm lactoferrin, TrkA phosphorylation in response to EI-tPA was blocked, confirming the ability of lactoferrin to function as an LRP1 signaling antagonist in PC12 cells.
We previously reported that MAG binding to LRP1 activates RhoA (33). This result is confirmed in Fig. 7A. Lactoferrin, EI-tPA, and α2M* all failed to activate RhoA. Next, we performed co-immunoprecipitation experiments to test whether p75NTR functions as an LRP1 co-receptor in response to ligands other than MAG. N2a cells were treated with EI-tPA, α2M*, lactoferrin, or MAG. Vehicle and Fc were studied as controls. As demonstrated previously (33), p75NTR co-immunoprecipitated with LRP1 from N2a cells treated with MAG (Fig. 7B). By contrast, neither EI-tPA nor α2M* caused p75NTR to associate with LRP1. p75NTR also was not observed in LRP1 immunoprecipitates obtained from cells treated with lactoferrin or Fc. These results suggest that p75NTR, like Trk receptor, functions as a ligand-specific LRP1 co-receptor.
FIGURE 7.
p75NTR associates with LRP1 selectively in MAG-treated cells. A, N2a cells were incubated with free Fc (20 nm), MAG (20 nm), lactoferrin (20 nm), EI-tPA (12 nm), or α2M* (10 nm) for 10 min. GTP-bound RhoA was determined by affinity precipitation. The original cell extracts were studied by immunoblot analysis using the same antibody to determine total RhoA. B, N2a cells were incubated with vehicle (SFM), EI-tPA (12 nm), α2M* (10 nm), lactoferrin (20 nm), free Fc (20 nm), or MAG (20 nm) for 10 min. Extracts were prepared and adsorbed sequentially with nonspecific IgG coupled to Protein A-Sepharose and LRP1-specific antibody coupled to Protein A-Sepharose. Proteins that precipitated (IP) with LRP1-specific antibody were subjected to immunoblot analysis (IB) to detect p75NTR, PSD-95, and LRP1. The cell extracts from which the immunoprecipitates were prepared also were subjected to immunoblot analysis to detect PSD-95 and LRP1. The blots are representative of five independent experiments.
PSD-95 is a bifunctional intracellular adapter protein that has been implicated in the bridging of LRP1 to NMDA-R (27, 29, 30). Fig. 7B shows that PSD-95 co-immunoprecipitated with LRP1 selectively from cells treated with EI-tPA or α2M*. Trace levels of PSD-95 were observed in immunoprecipitates isolated from MAG-treated cells. PSD-95 was not detected in LRP1 immunoprecipitates isolated from lactoferrin-treated cells or cells treated with Fc or vehicle.
DISCUSSION
In addition to its primary function as an activator of fibrinolysis, there is abundant evidence demonstrating a role for tPA in cell signaling, especially in the central nervous system, where tPA regulates neuronal cell physiology (5–12). Different mechanisms have been described to explain tPA-initiated cell signaling. Even when the described mechanisms are limited to those that do not require enzymatic activity, the role of LRP1 versus other receptors remains unsettled. The results presented here demonstrate that experimental design parameters, including the tPA concentration and the time of incubation with cells, may influence whether a role for LRP1 is detected. In N2a and PC12 cells, rapid activation of ERK1/2 was LRP1-dependent and, thus, inhibited by LRP1 gene silencing or by RAP. This was followed by a second LRP1-independent phase that was not inhibited by RAP. Thus, in these two cell culture model systems, standardizing the incubation time could lead to the conclusion that tPA-induced ERK1/2 activation either requires LRP1 or does not. Similar results were obtained with EI-tPA and with enzymatically active tPA. As might be predicted by kinetic models, the length of the first LRP1-dependent phase is shortened by increasing the tPA concentration.
The biphasic nature of ERK1/2 activation in response to EI-tPA may be explained by different models. Biphasic ERK1/2 activation may be explained if EI-tPA interacts with two distinct and non-interacting cell surface receptors. It would be necessary that both receptors induce ERK1/2 phosphorylation to a saturated level independently of the other, as determined by immunoblotting, because we observed no evidence of an additive effect from engaging two receptors simultaneously. According to the “two-independent receptor model,” when LRP1 is neutralized, the second receptor, which engages tPA more slowly than LRP1, would eventually phosphorylate ERK1/2 to the level observed in the presence of LRP1.
We favor a second model in which LRP1 and an interacting receptor, which is most likely the NMDA-R, function as a unit to bind EI-tPA and trigger cell signaling. In this model, the second receptor still binds EI-tPA and activates ERK1/2 in the absence of LRP1 but with slower kinetics. Evidence that the second receptor is the NMDA-R includes our results showing that MK-801 and NR1 gene silencing block Trk receptor phosphorylation (Fig. 5B) and ERK1/2 activation (Fig. 4E) in response to high concentrations of EI-tPA for up to 2 h. Under these conditions, EI-tPA activates cell signaling independently of LRP1. Thus, the NMDA-R is either functioning as the primary EI-tPA binding site or in conjunction with a second receptor other than LRP1. If the NMDA-R binds EI-tPA and activates cell signaling in the absence of another receptor, our results suggest that tPA binding to the NMDA-R triggers cell signaling without cleaving NR1 because EI-tPA lacks enzymatic activity.
Although it has been suggested that the NMDA-R and Trk receptors may constitute alternative pathways for activation of cell signaling downstream of LRP1 (32), our results demonstrate that LRP1, the NMDA-R, and Trk receptors function as a single signaling system to activate ERK1/2 in response to EI-tPA and α2M*. A pivotal event required for Trk receptor transactivation downstream of diverse receptors is SFK activation (52). In PC12 cells, v-Src mimics the activity of NGF-β, probably by transactivating TrkA (53). SFKs may be activated as a result of NMDA-R channel activation (54), providing a possible link between EI-tPA, the NMDA-R, and Trk receptors. Fig. 8 presents a model in which LRP1 and the NMDA-R cooperate to activate SFKs in response to permissive ligands, such as EI-tPA or α2M*. The SFKs then transactivate Trk, which is necessary for ERK1/2 phosphorylation. Once activated, SFKs also may target sites on the NMDA-R, modulating the activity of this neurotransmitter receptor (55).
FIGURE 8.
Model showing LRP1, NMDA-R, and Trk receptor, functioning as a single system to activate cell signaling in response to LRP1 ligands. tPA and α2M* are shown as permissive ligands that activate cell signaling. RAP and lactoferrin (LF) bind to LRP1 but may antagonize cell signaling in response to permissive ligands. MAG binds with high affinity to LRP1 but apparently assembles an entirely different co-receptor network (not shown).
Cells in culture produce LRP1 ligands (48, 56). These ligands may activate LRP1 signaling to some degree in the absence of added proteins, such as EI-tPA or α2M*. Trk receptor transactivation downstream of LRP1 would be expected to down-regulate cell surface Trk receptor, possibly explaining why ERK1/2 activation in response to NGF-β was more robust when the LRP1-Trk signaling system was inactivated by silencing the LRP1 gene (Fig. 1D) or the gene for the NR1 subunit of the NMDA-R (Fig. 4D). Interestingly, TrkA phosphorylation in response to NGF-β was not increased in NR1 gene-silenced cells (Fig. 5C). Thus, other mechanisms may explain why the response to NGF-β appears more robust when LRP1 or the NMDA-R is neutralized. Understanding the relationship between Trk transactivation and direct Trk receptor activation by neurotrophins is an important problem.
The abundance of LRP1 ligands introduces unique questions regarding the function of this receptor in cell signaling. In neurons and neuron-like cell lines, neurite outgrowth has been studied as a cellular response linked to activation of LRP1-initiated cell signaling. Many LRP1 ligands, including tPA, α2M*, and apoE, promote neurite outgrowth (18–20, 31). By contrast, MAG binds with high affinity to LRP1 and inhibits neurite outgrowth (33). The results presented here provide a possible explanation for the apparently opposite effects of different LRP1 ligands by demonstrating ligand-specific co-receptor recruitment. In response to EI-tPA and α2M*, TrkA was recruited as a functional LRP1 co-receptor, allowing for activation of ERK1/2. TrkA phosphorylation was not observed with MAG. Conversely, p75NTR was recruited into a physically associated complex with LRP1 in response to MAG and not when cells were treated with EI-tPA or α2M*. Ligand-specific co-receptor recruitment may allow LRP1 to function as a true sensor of the cellular microenvironment, inducing different changes in cell physiology that depend on the spectrum of available ligands.
Finally, we demonstrated that lactoferrin is an LRP1 ligand that has little or no independent ability to activate cell signaling. Instead, lactoferrin inhibited cell signaling induced by another LRP1 ligand, EI-tPA. It may be appropriate to cluster lactoferrin and RAP into a category of ligands considered “LRP1 signaling antagonists.” tPA and α2M* may be clustered into a second category of ligands that transactivate Trk receptors. MAG would be placed in a third category that includes LRP1 ligands that recruit p75NTR. Determining how other LRP1 ligands sort into these three categories is an important goal.
This work was supported, in whole or in part, by National Institutes of Health R01 Grants HL-060551 and NS-054671.
- tPA
- tissue-type plasminogen activator
- α2M
- α2-macroglobulin
- α2M*
- α2M that has been treated with methylamine
- apoE
- apolipoprotein E
- EI-tPA
- enzymatically inactive tissue-type plasminogen activator
- MAG
- myelin-associated glycoprotein
- NMDA-R
- N-methyl-d-aspartate receptor
- p75NTR
- p75 neurotrophin receptor
- RAP
- receptor-associated protein
- SFK
- Src family kinase
- SFM
- serum-free medium
- NTC
- non-targeting control.
REFERENCES
- 1. Collen D. (1987) Molecular mechanisms of fibrinolysis and their application to fibrin-specific thrombolytic therapy. J. Cell Biochem. 33, 77–86 [DOI] [PubMed] [Google Scholar]
- 2. Gualandris A., Jones T. E., Strickland S., Tsirka S. E. (1996) Membrane depolarization induces calcium-dependent secretion of tissue plasminogen activator. J. Neurosci. 16, 2220–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicole O., Docagne F., Ali C., Margaill I., Carmeliet P., MacKenzie E. T., Vivien D., Buisson A. (2001) The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 7, 59–64 [DOI] [PubMed] [Google Scholar]
- 4. Benchenane K., Berezowski V., Ali C., Fernández-Monreal M., López-Atalaya J. P., Brillault J., Chuquet J., Nouvelot A., MacKenzie E. T., Bu G., Cecchelli R., Touzani O., Vivien D. (2005) Tissue-type plasminogen activator crosses the intact blood-brain barrier by low density lipoprotein receptor-related protein-mediated transcytosis. Circulation 111, 2241–2249 [DOI] [PubMed] [Google Scholar]
- 5. Baranes D., Lederfein D., Huang Y. Y., Chen M., Bailey C. H., Kandel E. R. (1998) Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron 21, 813–825 [DOI] [PubMed] [Google Scholar]
- 6. Madani R., Hulo S., Toni N., Madani H., Steimer T., Muller D., Vassalli J. D. (1999) Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 18, 3007–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhuo M., Holtzman D. M., Li Y., Osaka H., DeMaro J., Jacquin M., Bu G. (2000) Role of tissue plasminogen activator receptor LRP in hippocampal long-term potentiation. J. Neurosci. 20, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsirka S. E., Gualandris A., Amaral D. G., Strickland S. (1995) Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature 377, 340–344 [DOI] [PubMed] [Google Scholar]
- 9. Bukhari N., Torres L., Robinson J. K., Tsirka S. E. (2011) Axonal regrowth after spinal cord injury via chondroitinase and the tissue plasminogen activator (tPA)/plasmin system. J. Neurosci. 31, 14931–14943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Echeverry R., Wu J., Haile W. B., Guzman J., Yepes M. (2010) Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J. Clin. Invest. 120, 2194–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu F., Echeverry R., Wu J., An J., Haile W. B., Cooper D. S., Catano M., Yepes M. (2013) Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Mol. Cell Neurosci. 52, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yepes M., Sandkvist M., Moore E. G., Bugge T. H., Strickland D. K., Lawrence D. A. (2003) Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niego B., Freeman R., Puschmann T. B., Turnley A. M., Medcalf R. L. (2012) t-PA-specific modulation of a human blood-brain barrier model involves plasmin-mediated activation of the Rho kinase pathway in astrocytes. Blood 119, 4752–4761 [DOI] [PubMed] [Google Scholar]
- 14. Baron A., Hommet Y., Cassé F., Vivien D. (2010) Tissue-type plasminogen activator induces plasmin-dependent proteolysis of intracellular neuronal nitric oxide synthase. Biol. Cell 102, 539–547 [DOI] [PubMed] [Google Scholar]
- 15. Samson A. L., Nevin S. T., Croucher D., Niego B., Daniel P. B., Weiss T. W., Moreno E., Monard D., Lawrence D. A., Medcalf R. L. (2008) Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J. Neurochem. 107, 1091–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee H. Y., Hwang I. Y., Im H., Koh J. Y., Kim Y. H. (2007) Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J. Neurochem. 101, 1236–1247 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi H., Campenot R. B., Vance D. E., Vance J. E. (2007) Apolipoprotein E-containing lipoproteins protect neurons from apoptosis via a signaling pathway involving low-density lipoprotein receptor-related protein-1. J. Neurosci. 27, 1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holtzman D. M., Pitas R. E., Kilbridge J., Nathan B., Mahley R. W., Bu G., Schwartz A. L. (1995) Low density lipoprotein receptor-related protein mediates apolipoprotein E-dependent neurite outgrowth in a central nervous system-derived neuronal cell line. Proc. Natl. Acad. Sci. U.S.A. 92, 9480–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu Z., Hyman B. T., Rebeck G. W. (2004) Apolipoprotein E receptors mediate neurite outgrowth through activation of p44/42 mitogen-activated protein kinase in primary neurons. J. Biol. Chem. 279, 34948–34956 [DOI] [PubMed] [Google Scholar]
- 20. Mantuano E., Mukandala G., Li X., Campana W. M., Gonias S. L. (2008) Molecular dissection of the human α2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J. Biol. Chem. 283, 19904–19911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fuentealba R. A., Liu Q., Kanekiyo T., Zhang J., Bu G. (2009) Low density lipoprotein receptor-related protein 1 promotes anti-apoptotic signaling in neurons by activating Akt survival pathway. J. Biol. Chem. 284, 34045–34053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bacskai B. J., Xia M. Q., Strickland D. K., Rebeck G. W., Hyman B. T. (2000) The endocytic receptor protein LRP also mediates neuronal calcium signaling via N-methyl-d-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 11551–11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qiu Z., Strickland D. K., Hyman B. T., Rebeck G. W. (2002) α2-Macroglobulin exposure reduces calcium responses to N-methyl-d-aspartate via low density lipoprotein receptor-related protein in cultured hippocampal neurons. J. Biol. Chem. 277, 14458–14466 [DOI] [PubMed] [Google Scholar]
- 24. Qiu Z., Crutcher K. A., Hyman B. T., Rebeck G. W. (2003) ApoE isoforms affect neuronal N-methyl-d-aspartate calcium responses and toxicity via receptor-mediated processes. Neuroscience 122, 291–303 [DOI] [PubMed] [Google Scholar]
- 25. Martin A. M., Kuhlmann C., Trossbach S., Jaeger S., Waldron E., Roebroek A., Luhmann H. J., Laatsch A., Weggen S., Lessmann V., Pietrzik C. U. (2008) The functional role of the second NPXY motif of the LRP1 β-chain in tissue-type plasminogen activator-mediated activation of N-methyl-d-aspartate receptors. J. Biol. Chem. 283, 12004–12013 [DOI] [PubMed] [Google Scholar]
- 26. Sheng Z., Prorok M., Brown B. E., Castellino F. J. (2008) N-methyl-d-aspartate receptor inhibition by an apolipoprotein E-derived peptide relies on low density lipoprotein receptor-associated protein. Neuropharmacology 55, 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoe H. S., Pocivavsek A., Chakraborty G., Fu Z., Vicini S., Ehlers M. D., Rebeck G. W. (2006) Apolipoprotein E receptor 2 interactions with the N-methyl-d-aspartate receptor. J. Biol. Chem. 281, 3425–3431 [DOI] [PubMed] [Google Scholar]
- 28. Chen Y., Beffert U., Ertunc M., Tang T. S., Kavalali E. T., Bezprozvanny I., Herz J. (2005) Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 25, 8209–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. May P., Rohlmann A., Bock H. H., Zurhove K., Marth J. D., Schomburg E. D., Noebels J. L., Beffert U., Sweatt J. D., Weeber E. J., Herz J. (2004) Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24, 8872–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakajima C., Kulik A., Frotscher M., Herz J., Schäfer M., Bock H. H., May P. (2013) Low density lipoprotein receptor-related protein 1 (LRP1) modulates N-methyl-d-aspartate (NMDA) receptor-dependent intracellular signaling and NMDA-induced regulation of postsynaptic protein complexes. J. Biol. Chem. 288, 21909–21923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shi Y., Mantuano E., Inoue G., Campana W. M., Gonias S. L. (2009) Ligand binding to LRP1 transactivates Trk receptors by a Src family kinase-dependent pathway. Sci. Signal. 2, ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rebeck G. (2009) Nontraditional signaling mechanisms of lipoprotein receptors. Sci. Signal. 2, pe28. [DOI] [PubMed] [Google Scholar]
- 33. Stiles T. L., Dickendesher T. L., Gaultier A., Fernandez-Castaneda A., Mantuano E., Giger R. J., Gonias S. L. (2013) LDL receptor-related protein-1 is a sialic-acid-independent receptor for myelin-associated glycoprotein that functions in neurite outgrowth inhibition by MAG and CNS myelin. J. Cell Sci. 126, 209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imber M. J., Pizzo S. V. (1981) Clearance and binding of two electrophoretic “fast” forms of human α2-macroglobulin. J. Biol. Chem. 256, 8134–8139 [PubMed] [Google Scholar]
- 35. Gonias S. L., Reynolds J. A., Pizzo S. V. (1982) Physical properties of human α2-macroglobulin following reaction with methylamine and trypsin. Biochim. Biophys. Acta 705, 306–314 [DOI] [PubMed] [Google Scholar]
- 36. Barrett A. J., Brown M. A., Sayers C. A. (1979) The electrophoretically “slow” and “fast” forms of the α2-macroglobulin molecule. Biochem. J. 181, 401–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Webb D. J., Hussaini I. M., Weaver A. M., Atkins T. L., Chu C. T., Pizzo S. V., Owens G. K., Gonias S. L. (1995) Activated α2-macroglobulin promotes mitogenesis in rat vascular smooth muscle cells by a mechanism that is independent of growth-factor-carrier activity. Eur. J. Biochem. 234, 714–722 [DOI] [PubMed] [Google Scholar]
- 38. Campana W. M., Li X., Dragojlovic N., Janes J., Gaultier A., Gonias S. L. (2006) The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells. Possible implications in peripheral nerve injury. J. Neurosci. 26, 11197–11207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sano M., Kohno M., Iwanaga M. (1995) The activation and nuclear translocation of extracellular signal-regulated kinases (ERK-1 and -2) appear not to be required for elongation of neurites in PC12D cells. Brain Res. 688, 213–218 [DOI] [PubMed] [Google Scholar]
- 40. Creedon D. J., Johnson E. M., Lawrence J. C. (1996) Mitogen-activated protein kinase-independent pathways mediate the effects of nerve growth factor and cAMP on neuronal survival. J. Biol. Chem. 271, 20713–20718 [DOI] [PubMed] [Google Scholar]
- 41. Perron J. C., Bixby J. L. (1999) Distinct neurite outgrowth signaling pathways converge on ERK activation. Mol. Cell. Neurosci. 13, 362–378 [DOI] [PubMed] [Google Scholar]
- 42. Ney K. A., Gidwitz S., Pizzo S. V. (1985) Binding and endocytosis of α2-macroglobulin-plasmin complexes. Biochemistry 24, 4586–4592 [DOI] [PubMed] [Google Scholar]
- 43. Hussaini I. M., Srikumar K., Quesenberry P. J., Gonias S. L. (1990) Colony-stimulating factor-1 modulates α2-macroglobulin receptor expression in murine bone marrow macrophages. J. Biol. Chem. 265, 19441–19446 [PubMed] [Google Scholar]
- 44. Strickland D. K., Ashcom J. D., Williams S., Burgess W. H., Migliorini M., Argraves W. S. (1990) Sequence identity between the α2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J. Biol. Chem. 265, 17401–17404 [PubMed] [Google Scholar]
- 45. Bu G., Warshawsky I., Schwartz A. L. (1994) Cellular receptors for the plasminogen activators. Blood 83, 3427–3436 [PubMed] [Google Scholar]
- 46. Bu G., Williams S., Strickland D. K., Schwartz A. L. (1992) Low density lipoprotein receptor-related protein/α 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc. Natl. Acad. Sci. U.S.A. 89, 7427–7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Willnow T. E., Orth K., Herz J. (1994) Molecular dissection of ligand binding sites on the low density lipoprotein receptor-related protein. J. Biol. Chem. 269, 15827–15832 [PubMed] [Google Scholar]
- 48. Strickland D. K., Gonias S. L., Argraves W. S. (2002) Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 13, 66–74 [DOI] [PubMed] [Google Scholar]
- 49. Vazhappilly R., Wee K. S., Sucher N. J., Low C. M. (2010) A non-muscle myosin II motor links NR1 to retrograde trafficking and proteasomal degradation in PC12 cells. Neurochem. Int. 56, 569–576 [DOI] [PubMed] [Google Scholar]
- 50. Filbin M. T. (2003) Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat. Rev. Neurosci. 4, 703–713 [DOI] [PubMed] [Google Scholar]
- 51. Yiu G., He Z. (2006) Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Domeniconi M., Chao M. V. (2010) Transactivation of Trk receptors in spinal motor neurons. Histol. Histopathol. 25, 1207–1213 [DOI] [PubMed] [Google Scholar]
- 53. Thomas S. M., Hayes M., D'Arcangelo G., Armstrong R. C., Meyer B. E., Zilberstein A., Brugge J. S., Halegoua S. (1991) Induction of neurite outgrowth by v-src mimics critical aspects of nerve growth factor-induced differentiation. Mol. Cell. Biol. 11, 4739–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Head B. P., Patel H. H., Tsutsumi Y. M., Hu Y., Mejia T., Mora R. C., Insel P. A., Roth D. M., Drummond J. C., Patel P. M. (2008) Caveolin-1 expression is essential for N-methyl-d-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 22, 828–840 [DOI] [PubMed] [Google Scholar]
- 55. Salter M. W., Kalia L. V. (2004) Src kinases. A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 [DOI] [PubMed] [Google Scholar]
- 56. Fernandez-Castaneda A., Arandjelovic S., Stiles T. L., Schlobach R. K., Mowen K. A., Gonias S. L., Gaultier A. (2013) Identification of the low density lipoprotein (LDL) receptor-related protein-1 interactome in central nervous system myelin suggests a role in the clearance of necrotic cell debris. J. Biol. Chem. 288, 4538–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]