Abstract
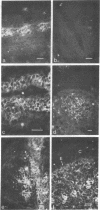
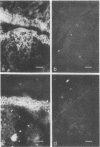
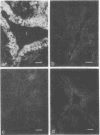
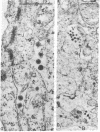
Oncornaviral antigen was detected in the bursal epithelium and in a subpopulation of bursal follicular cells of 15B X 72 chickens. This antigen is present in the bursal epithelium at 11 days of embryogenesis and persists there for at least 3 weeks after hatching. The absence of detectable antigen in the intestinal epithelium contiguous to the bursal epithelium indicates that the accumulation of viral antigen is a specific property of the bursal epithelium. The observation of C-type particles in the intraepithelial spaces suggests that the viral antigen in synthesized and assembled into virions by the bursal epithelial cells. In embryonic bursas, viral antigen-positve cells radiate from the surface epithelium toward the central region of the follicles. In bursas from post-hatch chickens, viral antigen-positive cells, including intrafollicular epithelial cells and cells resembling lymphocytes, are confined to the medullary region of the follicles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bockman D. E., Cooper M. D. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer's patches. An electron microscopic study. Am J Anat. 1973 Apr;136(4):455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Cooper M. D., Purchase H. G., Bockman D. E., Gathings W. E. Studies on the nature of the abnormality of B cell differentiation in avian lymphoid leukosis: production of heterogeneous IgM by tumor cells. J Immunol. 1974 Oct;113(4):1210–1222. [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V., Smith E. J. Genetic control of RAV-O production in chickens. Virology. 1977 Jan;76(1):90–97. doi: 10.1016/0042-6822(77)90285-9. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Friis R. R., Mason W. S. Expression of the Major Viral Glycoprotein of Avian Tumor Virus in Cells of chf(+) Chicken Embryos. J Virol. 1975 May;15(5):1131–1140. doi: 10.1128/jvi.15.5.1131-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Cooper M. D. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chains. J Immunol. 1971 Feb;106(2):371–382. [PubMed] [Google Scholar]
- Koppenheffer T. L., Phillips J. H., Jr, Vankin G. L. C-type virus-lymphocyte interactions in developing mouse thymus. Am J Anat. 1978 Sep;153(1):165–170. doi: 10.1002/aja.1001530111. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Halpern M. S. Subunit structure of the glycoprotein complex of avian tumor virus. J Virol. 1976 Jun;18(3):956–968. doi: 10.1128/jvi.18.3.956-968.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Wilson C. B., Villano B. C., McConahey P. J., Dixon F. J. Endogenous oncornaviral gene expression in adult and fetal mice: quantitative, histologic, and physiologic studies of the major viral glycorprotein, gp70. J Exp Med. 1976 Jan 1;143(1):151–166. doi: 10.1084/jem.143.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann D., Sachs L. Co-regulation of type C RNA virus production and cell differentiation in myeloid leukemic cells. Cell. 1978 Nov;15(3):823–835. doi: 10.1016/0092-8674(78)90267-2. [DOI] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., Purchase H. G. Studies of the interrelationship of chicken leukosis virus and host cell genomes by RNA-DNA hybridzation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):875–883. doi: 10.1101/sqb.1974.039.01.102. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Immunosuppressive activity of antibody directed against endogenous C-type virus interferes with early events of the immune response. J Immunol. 1978 Jun;120(6):1913–1916. [PubMed] [Google Scholar]
- Sorvari T., Sorvari R., Ruotsalainen P., Toivanen A., Toivanen P. Uptake of environmental antigens by the bursa of Fabricius. Nature. 1975 Jan 17;253(5488):217–219. doi: 10.1038/253217a0. [DOI] [PubMed] [Google Scholar]
- Staber F. G., Schläfli E., Moroni C. Expression of endogenous C-type viral antigen on normal mouse haemopoietic stem cells. Nature. 1978 Oct 19;275(5681):669–671. doi: 10.1038/275669a0. [DOI] [PubMed] [Google Scholar]
- Thorbecke G. J., Warner N. L., Hochwald G. M., Ohanian S. H. Immune globulin production by the bursa of Fabricius of young chickens. Immunology. 1968 Jul;15(1):123–134. [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Waksal S. D., Smolinsky S., Cohen I. R., Feldman M. Transformation of thymocytes by thymus epithelium derived from AKR mice. Nature. 1976 Oct 7;263(5577):512–514. doi: 10.1038/263512a0. [DOI] [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T. Expression of MuLV GP71-like antigen in normal mouse spleen cells induced by antigenic stimulation. Nature. 1977 Oct 13;269(5629):598–600. doi: 10.1038/269598a0. [DOI] [PubMed] [Google Scholar]