Abstract
Recent studies suggest that the combination of caffeine-containing drinks together with alcohol might reduce the subjective feelings of alcohol intoxication—the so-called “masking effect”. In this study, we aimed to review the effects of alcohol in combination with caffeine or energy drink with special focus on the “masking effect”. Fifty-two healthy male volunteers were analysed concerning breath alcohol concentration and subjective sensations of intoxication using a 18 item Visual Analogue Scale in a randomised, double-blinded, controlled, four treatments cross-over trial after consumption of (A) placebo, (B) alcohol (vodka 37.5 % at a dose of 46.5 g ethanol), (C) alcohol in combination with caffeine at a dose of 80 mg (equivalent to one 250 ml can of energy drink) and (D) alcohol in combination with energy drink at a dose of 250 ml (one can). Primary variables were headache, weakness, salivation and motor coordination. Out of four primary variables, weakness and motor coordination showed a statistically significant difference between alcohol and non-alcohol group, out of 14 secondary variables, five more variables (dizziness, alterations in sight, alterations in walking, agitation and alterations in speech) also showed significant differences due mainly to contrasts with the non-alcohol group. In none of these end points, could a statistically significant effect be found for the additional ingestion of energy drink or caffeine on the subjective feelings of alcohol intoxication. This within-subjects study does not confirm the presence of a “masking effect” when combining caffeine or energy drink with alcohol.
Keywords: Energy drink, Red bull, Alcohol, Caffeine, Masking, Intoxication
Introduction
So-called “energy drinks”—usually based on caffeine, carbohydrates, vitamins and other ingredients such as taurine—have become popular and capture one percent of the total soft drink market. Since 2004, energy drinks have been the fastest growing sector of the beverage market, for example the market in Western Europe has grown by 12.9 % between 2007 and 2011 (Database 2012). One of the most popular energy drinks is Red Bull®, which has been available in Austria since 1987 and in the United States since 1997 (Reissig et al. 2009). It is now available in more than 160 countries.
The ingestion of caffeine-containing drinks together with alcohol is not a new phenomenon (e.g. rum coke/cola), and recently the combination of energy drinks with alcohol has become popular, with 20 % of students occasionally combining energy drinks with alcohol (de Haan et al. 2012). In the past, concerns have been raised suggesting that the combination of both substances might reduce the subjective feelings of alcohol intoxication—the so-called “masking effect” (Ferreira et al. 2006). Therefore the risk for dangerous activities, such as driving a car, would be increased. Thus possible effects of combined alcohol and caffeine consumption are an important research topic.
Several studies have already investigated whether caffeine counteracts the neuro-cognitive effects of alcohol consumption, with inconsistent results (Ferreira et al. 2006; Alford et al. 2012; Azcona et al. 1995; Marczinski et al. 2011). Some studies report a significant antagonising effect of caffeine on alcohol such as influencing weakness and impairment of motor coordination (Ferreira et al. 2006), or they suggest that the combination of caffeine and alcohol may lead to longer drinking and to an increase in stimulation compared to alcohol-only consumption (Attwood et al. 2012). Other investigations could not find any antagonising effects (Alford et al. 2012; Marczinski et al. 2012; Verster et al. 2012).
One of the most cited studies regarding the so-called “masking effect” was performed by (Ferreira et al. 2006). In this study, twenty-six young and healthy male volunteers were tested concerning breath alcohol concentration, subjective sensations of intoxication, motor coordination, and visual reaction time after consumption of energy drink (3.57 ml/kg bw), alcohol (0.6 or 1.0 g/kg bw) or both, using a mixed design. The additional ingestion of energy drinks did not modify breath alcohol concentration, motor coordination, and visual reaction time. Regarding subjective sensations of intoxication, more descriptors registered similar impairment with the energy drink and alcohol combination versus alcohol alone, than showed reduced impairment after co-administration of energy drink with alcohol. However, the authors interpreted these findings as a so-called “masking effect”, since they found significant differences in headache, weakness, salivation and motor coordination.
Given the variability of results regarding the perception of impairment, no valid evidence can be derived from this study. Therefore, the results and conclusion of this study should be re-assessed and discussed again, especially concerning the importance of the four mentioned subjective effects of intoxication (headache, weakness, salivation and motor coordination) which were reported to be significant. Since the publication of this study, several methodological flaws of this study have been discussed, in particular the statistical analysis, and the interpretation of the results are not undisputed (Alford et al. 2012; Verster et al. 2012). However, the data have neither been confirmed nor rebutted, and in a review published in 2012, Verster et al. conclude that the masking effect of energy drinks cannot be confirmed by currently available data (Verster et al. 2012).
In this study, we aimed to replicate Ferreira’s study with a higher number of healthy participants and a within-subjects design to re-examine the effects of alcohol in combination with caffeine or energy drink with special focus on the so-called “masking effect”.
Methods
Subjects
Fifty-two healthy male volunteers participated in the study. Their age was 20–26 years (24.4 ± 1.5), with body mass indices between 21 and 25 kg/m2 (23.2 ± 1.1), body weight between 68 and 85 kg (77.0 ± 3.8) and at least 12–14 years of formal education. All volunteers were in good general health as determined by medical history and screening investigations. All were taking no regular medication and had no history of psychiatric disorders.
Further inclusion criteria were: Moderate alcohol consumption (less than 190.4 g/week) according to the Daily Drink questionnaire (Collins et al. 1985), sporadic users of energy drinks (less than ten cans of 250 ml in the last 6 months), and confirmation from the participant’s general practitioner that they do not know of any reason that would advise against participation in the study. Participants were similar regarding social and demographic data, patterns of use of alcoholic beverages and energy drinks as well as quality of life (Martinez et al. 2000) and having a similar level of physical activity (Baecke et al. 1982). Exclusion criteria were a consumption of less than two or more than four caffeine-containing drinks per day within 3 months of screening; smoking of more than ten cigarettes per day or equivalent within 3 months of screening; consumption of more than 190.4 g alcohol per week; a history of alcohol or drug abuse or consumption of less than one alcoholic drink per week.
Volunteers were informed about the procedures of the study and signed an informed consent form. The Committee of Ethics of the Medical University of Vienna approved the study (ClinicalTrials.gov Identifier: NCT01350089).
Treatments
Four mixtures listed below were consumed orally within 10–20 min, on one occasion each in a randomised order. Volunteers wore a nose clip to optimise blinding. As far as possible all investigational products were identical in appearance and taste, differing only in the absence/presence of alcohol, caffeine and energy drink. The final volume of the mixtures was 500 ml for each treatment. The caffeine, alcohol and energy drink doses were chosen as used by Ferreira et al. because they were within the range of doses usually ingested on a single occasion (Ferreira et al. 2006).
The placebo (A) consisted of (carbonated) water (250 ml), artificial fruit juice [21 g/l prepared with (carbonated) water].
The comparator B was a mixture of 46.5 g ethanol (in form of vodka 37.5 vol %) (carbonated) water (250 ml), artificial fruit juice [21 g/l prepared with (carbonated) water].
The comparator C was a mixture of 46.5 g ethanol (in form of vodka 37.5 vol %), caffeine (80 mg, equivalent to one 250 ml can of a typical energy drink), (carbonated) water (250 ml), artificial fruit juice [21 g/l prepared with (carbonated) water].
The comparator D was a mixture of 46.5 g ethanol (in form of vodka 37.5 vol %), Red Bull Energy Drink (250 ml, equivalent to one can, without flavour to optimise blinding), artificial fruit juice [21 g/l prepared with (carbonated) water].
Subjective effects of intoxication
This was evaluated through a visual analogue scale (VAS) of somatic symptoms (Bond and Lader 1972; Greenwood et al. 1975) including all items used in Ferreira et al. 2006. This was assessed before and 30, 75 and 120 min after the treatments. Each 100 mm line represented the whole range of possible intensity of each listed symptom (e.g. salivation–dry mouth). The volunteers marked the location that corresponded to the intensity of their sensation with a vertical line. We tested the items: agitation, alterations in motor coordination, hearing, walking and speech, sensation of well-being, tiredness, headache, dizziness, tremor, weakness, muscular tension, nausea, salivation, perspiration, visual disturbances, tachycardia and difficulty in breathing.
Procedures
The volunteers were instructed to drink no alcohol or high-energy products during each study period with the exception of study treatment. Sufficient sleep (at least 7 h) was required the night before testing and controlled via questionnaire on test days. No alcohol during a period of at least 72 h prior to each test dose was allowed. On the test days, the consumption of at least two and no more than four caffeine-containing drinks was controlled via questionnaire. On every treatment day, the volunteers were instructed to arrive fasting 15 min before the beginning of the treatment administration, which started around midday. A standard meal of 1,000 kcal (1 Big Mac, small French fries and water) was given 45 min before treatment. Sugar-free fluid was allowed until 1 h before treatment, no further fluids were allowed until 2.5 h after dosing. We used a double blind procedure throughout the experiment.
Safety
Supine and standing vital signs were evaluated before (in triplicate) and 60 and 150 min after the treatments.
Breath alcohol concentration
This was determined by using a breath analyzer (AlcoQuant 6,020, Envitec, Germany) before and 15, 30, 60, 90, 120 and 150 min after the treatments. The alcohol dose used aimed for a mean breath alcohol concentration of 0.05 % similar to that of Ferreira et al. (2006).
Statistical analyses
To check for differences between the four treatment groups mixed models were applied to consider the special data structure of the cross-over design. A restricted maximum likelihood (REML) method was used. The primary variables were the symptoms headache, weakness, salivation and motor coordination, as they yielded statistically significant differences in Ferreira’s study. To adjust for multiple comparisons a Bonferroni correction was applied for the four primary variables resulting in a local significance level of 0.05/4 = 0.0125 for each single primary variable.
In a first step the data of all four treatment groups were used. As the descriptive plots partially revealed considerable differences between the alcohol groups and the non-alcohol group, a further analysis was performed using only the three treatment groups that included alcohol.
In each of the models, the 20 min pre-treatment values of the respective treatment day were included as baseline values. The following measurements at 30, 90 and 120 min post-treatment were treated as autoregressive. We assumed, that due to the long wash-out period between the treatments no carry-over effect could occur, but in order to account for possible habituation effects the number of the visit was included in the model. Therefore, the fixed effects included in the model were baseline, treatment, number of the visit, time of measurement, and the interaction between treatment and time of measurement. A random influence of each patient was included in the model.
Results
Breath alcohol concentration
Mean breath alcohol concentrations at 15, 30, 60, 90, 120 and 150 min after the treatments were 0.059, 0.059, 0.053, 0.047, 0.041 and 0.035 %,respectively; there was no difference within the alcohol groups.
Primary variables
The statistical significance of the regression coefficients of the mixed models for all four primary variables is presented in Table 1.
Table 1.
p values of the coefficients of the primary variables in the mixed model
| Parameter | Treatment | Baseline value | Treatment day | Time of measurement | Interaction treatment/time of measurement |
|---|---|---|---|---|---|
| Headache | 0.32295 | <0.0001a | 0.99001 | 0.11912 | 0.70058 |
| Weakness | 0.00038a | 0.00109a | 0.49429 | 0.05087 | 0.05634 |
| Salivation | 0.07444 | <0.0001a | 0.20777 | 0.00012a | 0.54592 |
| Motor coordination | <0.0001a | 0.03532 | 0.14744 | 0.00002a | 0.38169 |
asignificant at the significance-level 0.0125
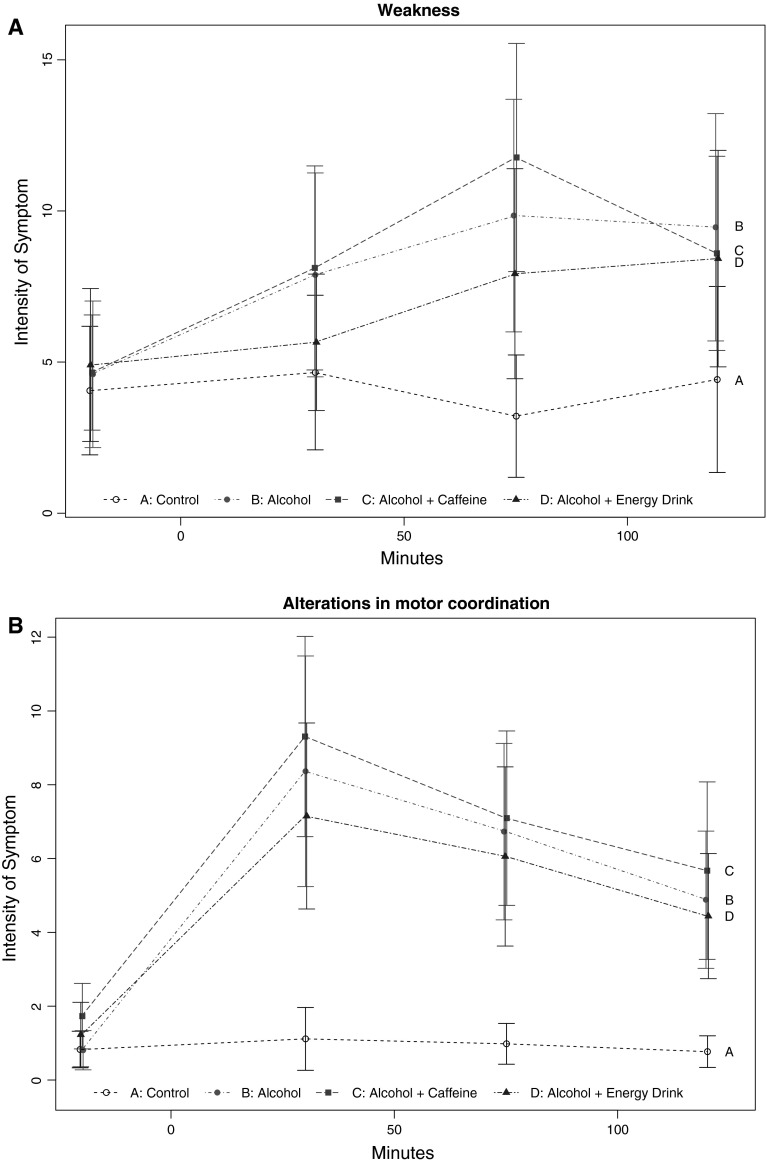
For the variables, weakness and motor coordination, a statistically significant difference between all four treatment groups was observed; in neither of these variables a statistically significant influence of the treatment could be revealed in the sub-analysis of the three groups with alcohol. This indicates that differences between the four treatment groups in weakness and motor coordination were mainly driven due to the linear values recorded for the non-alcohol group (see Fig. 1a, b).
Fig. 1.
Primary parameter. a Weakness shows the intensity of weakness at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts oft the total 0–100 scales. There was no difference between the alcohol groups, but a statistically significant difference was observed between the alcohol groups and the non-alcohol group when corrected for multiple testing. b Alterations in motor coordination shows the intensity of alterations in motor coordination at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts oft the total 0–100 scales. There was no difference between the alcohol groups, but a statistically significant difference was observed between the alcohol groups and the non-alcohol group when corrected for multiple testing (color figure online)
The time of measurement was significant for salivation, motor coordination and the three-group analysis of weakness, but no treatment–time interaction could be shown for any of the four primary variables. The number of the visit reflecting treatment order had no significant influence on any of the models.
Secondary variables
Eleven of the 14 secondary variables showed an uncorrected significance level of less than 0.05 %. Out of these, five revealed significant differences between the four treatment groups when corrected for multiple testing (see Table 2), i.e. dizziness, alterations in sight, alterations in walking, agitation and alterations in speech.
Table 2.
p values of the coefficients of the secondary variables in the mixed model
| Symptom | Treatment | Baseline value | Treatment day | Time of measurement | Interaction treatment/time of measurement |
|---|---|---|---|---|---|
| Tiredness | 0.01911 | <0.0001 | 0.22595 | <0.0001 | 0.06376 |
| Dizziness | <0.0001 | 0.53792 | 0.41346 | <0.0001 | 0.00034 |
| Tremor | 0.01814 | <0.0001 | 0.76371 | 0.01015 | 0.51661 |
| Tension | 0.50898 | <0.0001 | 0.17191 | 0.39731 | 0.56081 |
| Nausea | 0.09716 | 0.00002 | 0.00566 | 0.68368 | 0.35981 |
| Perspiration | 0.00523 | <0.0001 | 0.30909 | <0.0001 | 0.54631 |
| Alterations in sight | <0.0001 | 0.00030 | 0.00701 | <0.0001 | 0.00312 |
| Tachycardia | 0.04811 | 0.00002 | 0.08747 | 0.30345 | 0.05890 |
| Breathing difficulty | 0.01281 | <0.0001 | 0.45282 | 0.19071 | 0.77901 |
| Alterations in walking | <0.0001 | 0.00222 | 0.82269 | <0.0001 | 0.00706 |
| Agitation | 0.00069 | <0.0001 | 0.12238 | 0.00012 | 0.27364 |
| Alterations in hearing | 0.00614 | 0.00138 | 0.17064 | 0.26217 | 0.09431 |
| Alterations in speech | <0.0001 | 0.00986 | 0.09885 | <0.0001 | 0.22069 |
| Well-being | 0.09532 | <0.0001 | 0.91220 | 0.04818 | 0.72580 |
Bold remain significant when corrected for multiple testing
In the sub-analysis of the three groups with alcohol none of these variables revealed a significant difference between treatments. This indicates that differences between the four treatment groups were mainly driven due to differences with the non-alcohol control group (see Fig. 2a–e).
Fig. 2.
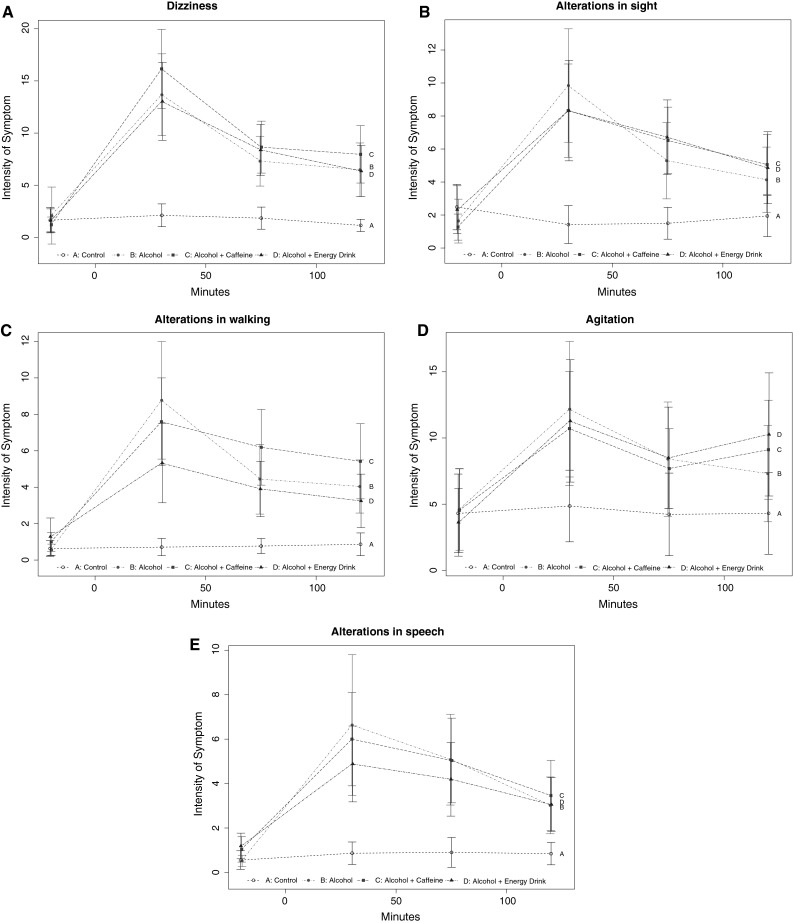
Secondary parameter. a Dizziness shows the intensity of dizziness at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts oft the total 0–100 scales. There was a significant difference between the four treatment groups when corrected for multiple testing, but no difference was observed in the sub-analysis of the three alcohol groups. b Alterations in sight shows the intensity of alterations in sight at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts of the total 0–100 scales. There was a significant difference between the four treatment groups when corrected for multiple testing, but no difference was observed in the sub-analysis of the three alcohol groups. c Alterations in walking shows the intensity of alterations in walking at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts of the total 0–100 scales. There was a significant difference between the four treatment groups when corrected for multiple testing, but no difference was observed in the sub-analysis of the three alcohol groups. d Agitation shows the intensity of agitation at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts of the total 0–100 scales. There was a significant difference between the four treatment groups when corrected for multiple testing, but no difference was observed in the sub-analysis of the three alcohol groups. e Alteration in speech shows the intensity of alteration in speech at four different time points (20 min pre-treatment, 30, 90 and 120 min post-treatment). The black A line shows the control group. The red B line shows the alcohol-only group. The green C line shows the alcohol and caffeine group. The blue D line shows the alcohol and energy drink group. The y-axis only shows the relevant parts oft the total 0–100 scales. There was a significant difference between the four treatment groups when corrected for multiple testing, but no difference was observed in the sub-analysis of the three alcohol groups (color figure online)
Discussion
In our study, findings of a so-called “masking effect” could not be reproduced. First, the parameters Salivation and Headache showed no differences between the alcohol-containing treatment conditions and the placebo condition despite our larger population sample. With regards to the two other primary parameters perception of Weakness and Impairment of Motor Coordination, our data confirmed a significant effect of alcohol—that also remained robust following correction for multiple comparisons. However, when the subset of alcohol-containing treatment conditions were analysed in a three-group model—that excluded the overriding effects of alcohol—we were not able to detect a masking effect of the combination of energy drink or caffeine and alcohol compared to the alcohol-only treatment condition, even without correcting for multiple comparisons.
The use of VAS as a method to evaluate subjective feelings has a long tradition as “comparisons can be achieved with greater sensitivity than with semantic phrases or numeric rating scales” (Aitken 1969). Starting with the measurement of feelings and moods (Bond and Lader 1972; Aitken 1969; Folstein and Luria 1973), VAS were also used for rating physiological signs of emotions and adapted increasingly for clinical use (Greenwood et al. 1975). For their study, Ferreira had added five scales to the original 13 items of the Bond and Lader VAS without prior validation as a measure of intoxication (Ferreira et al. 2006; Bond and Lader 1972). This somatic symptom scale, which was also applied in the current study, may therefore not adequately assess the ‘mental’ experience of intoxication—but was chosen to partially replicate the Ferreira study (Ferreira et al. 2006). In a recent review of the literature (Hjermstad et al. 2011), VAS scales were compared to other methods of rating and confirmed as valid and the most frequently used option. The conditions of its use rather than the type of scale determined the quality of VAS-based methods (Hjermstad et al. 2011). Accordingly, the current study used well-established anchor descriptors (analogous to Ferreira et al. 2006) and a written instruction that was orally checked for comprehension.
When the statistical analysis of the data obtained by the VAS did not include corrections for multiple comparisons, 13 out of the investigated 18 parameters in the present study differed significantly between alcohol and non-alcohol containing drinks. Ferreira had detected effects of alcohol in fewer parameters, failing to detect differences in parameters such as Tremor, Perspiration, Tachycardia, Breathing difficulty, Agitation and Alteration in hearing. In contrast, Ferreira found significant differences in Headache and Salivation, whereas our data did not demonstrate differences between the alcohol and the non-alcohol group in these parameters. When corrected for multiple comparisons—as is imperative in this methodology—five of our parameters still remained significant with regards to alcohol effects. Unfortunately, this comparison cannot be performed with Ferreira’s paper, as this information is not given in his data. However, the overall broad overlap of results in response to alcohol between our study and that of Ferreira again supports the validity of the VAS as an assessment tool, and confirms the sensitivity of this tool in our population sample.
In the Ferreira analysis, the differences in perception of salivation, headache, weakness and impairment of motor coordination were statistically significant between the non-alcohol and the alcohol-only group, but were not significantly different between the non-alcohol and the alcohol combined with energy drink group. As there were no significant effects detected in objective measures of visual reaction time or motor coordination, the authors interpreted these findings as a “masking effect”.
The differentiation between primary and secondary outcome parameters in our study was based on the assumption that the four parameters that described so-called “masking effects” in the Ferreira paper were a suitable subset for primary outcome parameters. As these primary outcome parameters failed to confirm a “masking effect” of combined ingestion of energy drink or caffeine with alcohol, and as this differentiation in primary and secondary outcome parameters was somewhat arbitrary, we also performed a statistical analysis of the other 14 symptoms measured by the VAS.
For half of these parameters, the two studies were in agreement: regarding tension and nausea neither Ferreira et al. nor we could find any significant differences (even without correction for multiple testing). Ferreira et al. showed a significant difference in the parameters Tiredness, Dizziness*, Alterations in Sight*, Alterations in Walking*, Alterations in Speech* between alcoholic and non-alcoholic drinks. We also found a similar difference between alcoholic and non-alcoholic drinks for these parameters, in four out of the five, even after correction for multiple testing (only Tiredness lost significance after correction for multiple testing).
For the other seven parameters, the two studies were not in agreement: regarding the parameters Tremor, Perspiration, Tachycardia, Breathing Difficulty, Agitation*, and Alterations in Hearing Ferreira et al. could not find a significant difference between all groups. In contrast, in our study we found a significant difference between the alcoholic and non-alcoholic drinks, which, however, was lost after correction for multiple testing for all parameters except Agitation.
As also demonstrated in Table 3, the agreement of any given parameter between the two studies strongly depended on the results of the correction for multiple testing. These data underline the importance of this statistical correction to reduce the risk of false positive findings. Out of the seven parameters (two primary and five secondary) that remained statistically significant after correction for multiplicity, six had also been described by Ferreira, resulting in an agreement of 86 %! In contrast, out of the six parameters that lost their statistical significance by correction for multiple testing, only one had also been described by Ferreira, resulting in an agreement of only 17 %! In other words, correction of multiplicity increased the likelihood for agreement between the two studies fivefold. None of these parameters demonstrated a “masking effect” of combined ingestion of energy drink or caffeine with alcohol. This lack of a “masking effect” was particularly well presented in the graphical display of the original data (Figs. 1, 2) that showed a clear separation between the alcohol and the non-alcohol containing drinks with no discernible effect of the addition of energy drink or caffeine across a range of subjective parameters.
Table 3.
Agreement between the Ulbrich and the Ferreira Studies´ variable parameters
| Ulbrich YES | Ulbrich NO | |
|---|---|---|
| 4 Primary parameters | ||
| Ferreira YES | Weakness, impairment of motor coordination | Dry mouth headache |
| 14 Secondary parameters | ||
| Ferreira YES | Tiredness, Dizziness, Alterations in sight, Alterations in walking, Alterations in speech | Well-being |
| Ferreira NO | Tremor, Perspiration, Tachycardia, Breathing difficulty, Agitation, Alterations in hearing | Tension, nausea |
Bold parameters remained statistically significant after correction for multiplicity
In conclusion, although testing twice the number of participants at the lower dose of alcohol, rendering our design more sensitive to the detection of such effects, this within-subjects study failed to reproduce results from Ferreira’s publication in 2006 with regards to a so-called “masking effect” when combining caffeine or energy drink with alcohol compared to alcohol-only consumption. As we did not perform objective measures in this study, our results do not allow conclusions regarding other parameters such as motor coordination and visual reaction time. These results thus add to other evidence reviewed by the UK Committee on Toxicity (2012) (http://cot.food.gov.uk/pdfs/tox201210.pdf) that the masking effect of energy drinks cannot be confirmed by currently available data.
Acknowledgments
We are grateful for the technical support by Axana Hellmann in preparing the manuscript.
Conflict of interest
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and all authors, excluded Christoph Aufricht (CA) declare, that they got no support from any organisation for the submitted work [AU, SH, AL, SD, EP, SF, MH] and all authors [AU; SH; AL; SD; EP; SF; MH], excluded CA, have no financial relationships with any organisation that might have an interest in the submitted work in the previous 3 years. All authors declare [AU; SH; AL; SD; EP; SF; MH, CA] that no other relationships or activities appeared which influenced the submitted work. Christoph Aufricht (CA) got support from Red Bull GmbH for the submitted work and CA had specified relationship with Red Bull GmbH in the previous 3 years. The Medical University of Vienna (Christoph Aufricht) has received an unrestricted research grant from Red Bull GmbH. Christoph Aufricht is cofounder of Zytoprotec GmbH, a spin-off of the Medical University Vienna focusing on cytoprotective therapies.
References
- Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62(10):989–993. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford C, Hamilton-Morris J, Verster JC. The effects of energy drink in combination with alcohol on performance and subjective awareness. Psychopharmacology. 2012;222(3):519–532. doi: 10.1007/s00213-012-2677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood AS, Rogers PJ, Ataya AF, Adams S, Munafo MR. Effects of caffeine on alcohol-related changes in behavioural control and perceived intoxication in light caffeine consumers. Psychopharmacology. 2012;221(4):551–560. doi: 10.1007/s00213-011-2601-0. [DOI] [PubMed] [Google Scholar]
- Azcona O, Barbanoj MJ, Torrent J, Jane F. Evaluation of the central effects of alcohol and caffeine interaction. Br J Clin Pharmacol. 1995;40(4):393–400. doi: 10.1111/j.1365-2125.1995.tb04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- Bond AJ, Lader MH. Residual effects of hypnotics. Psychopharmacologia. 1972;25(2):117–132. doi: 10.1007/BF00423189. [DOI] [PubMed] [Google Scholar]
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: the effects of social interaction and model status on the self-administration of alcohol. J Consult Clin Psychol. 1985;53(2):189–200. doi: 10.1037/0022-006X.53.2.189. [DOI] [PubMed] [Google Scholar]
- Committee on toxicity of chemicals in food CpatEC (2012) Statement on the interaction of caffeine and alcohol and their combined effects on health and behaviour: lay summary
- Database CW (2012) Creating a buzz. Engery drinks are the star performer on the European soft drinks market. Inside drinks magazine
- de Haan L, de Haan HA, van der Palen J, Olivier B, Verster JC. Effects of consuming alcohol mixed with energy drinks versus consuming alcohol only on overall alcohol consumption and negative alcohol-related consequences. Int J Gen Med. 2012;5:953–960. doi: 10.2147/IJGM.S38020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Pompeia S, de Souza-Formigoni ML. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30(4):598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychol Med. 1973;3(4):479–486. doi: 10.1017/S0033291700054283. [DOI] [PubMed] [Google Scholar]
- Greenwood MH, Lader MH, Kantameneni BD, Curzon G. The acute effects of oral (−)-tryptophan in human subjects. Br J Clin Pharmacol. 1975;2(2):165–172. doi: 10.1111/j.1365-2125.1975.tb01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, et al. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi: 10.1016/j.jpainsymman.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Bardgett ME, Howard MA. Effects of energy drinks mixed with alcohol on behavioral control: risks for college students consuming trendy cocktails. Alcohol Clin Exp Res. 2011;35(7):1282–1292. doi: 10.1111/j.1530-0277.2011.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA, Fillmore MT, Henges AL, Ramsey MA, Young CR. Effects of energy drinks mixed with alcohol on information processing, motor coordination and subjective reports of intoxication. Exp Clin Psychopharmacol. 2012;20(2):129–138. doi: 10.1037/a0026136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez TY, Pereira CA, dos Santos ML, Ciconelli RM, Guimaraes SM, Martinez JA. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117(6):1627–1632. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. Caffeinated energy drinks—a growing problem. Drug Alcohol Depend. 2009;99(1–3):1–10. doi: 10.1016/j.drugalcdep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verster JC, Aufricht C, Alford C. Energy drinks mixed with alcohol: misconceptions, myths, and facts. Int J Gen Med. 2012;5:187–198. doi: 10.2147/IJGM.S29313. [DOI] [PMC free article] [PubMed] [Google Scholar]