Abstract
Males of all reed frog species (Anura: Hyperoliidae) have a prominent, often colourful, gular patch on their vocal sac, which is particularly conspicuous once the vocal sac is inflated. Although the presence, shape, and form of the gular patch are well-known diagnostic characters for these frogs, its function remains unknown. By integrating biochemical and histological methods, we found strong evidence that the gular patch is a gland producing volatile compounds, which might be emitted while calling. Volatile compounds were confirmed by gas chromatography–mass spectrometry in the gular glands in 11 species of the hyperoliid genera Afrixalus, Heterixalus, Hyperolius, and Phlyctimantis. Comparing the gular gland contents of 17 specimens of four sympatric Hyperolius species yielded a large variety of 65 compounds in species-specific combinations. We suggest that reed frogs might use a complex combination of at least acoustic and chemical signals in species recognition and mate choice.
Keywords: Afrixalus, Amphibia, chemical communication, Heterixalus, histology, Hyperolius, gular gland anatomy, pheromones, Phlyctimantis
Introduction
In the animal kingdom male signals usually offer information about the individual's condition, underpinning beneficial traits that might be passed on to the next generation. Females recognize these signals and use them in mate choice (Zahavi, 1975). In most anuran amphibians advertisement calls are the predominant signal in inter- and intrasexual communication (Ryan, 1985; Gerhardt & Huber, 2002; Dorcas et al., 2010). The male advertisement call attracts conspecific females and signals a readiness to defend territories against conspecific males (Duellman & Trueb, 1986). Hence calling behaviour plays a vital role in reproductive success, and is essential for sexual selection. The calling behaviour of frogs and toads has been the subject of a large number of studies, whereas other channels of communication possibly used in a reproductive context (e.g. seismic, visual, or chemical communication) in these organisms have been almost completely neglected (Taylor, Buchanan & Doherty, 2007; Coleman, 2009), but see the review by Hödl & Amézquita (2001) and two studies on seismic communication in frogs (Lewis et al., 2001; Caldwell et al., 2010).
The anuran vocal sac and its role in communication
In males of most anuran species the vocal sac is associated with producing advertisement calls. The main function of the vocal sac is to recycle the air coming from the lungs during calling. Furthermore, it also minimizes the loss of sound energy by decreasing the impedance mismatch between the frog's body cavity and its environment, increases the call rate, and distributes sound waves omnidirectionally (Bucher, Ryan & Bartholomew, 1982; Rand & Dudley, 1993; Pauly et al., 2006). Because the vocal sac inevitably moves while a male is calling, it might send a fixed composite signal (sensu Partan & Marler, 2005) consisting of the acoustic signal component and a visual trait, with increased detectability arising from the movement (Endler & Thery, 1996; Fleishman et al., 1998; Rosenthal, Rand & Ryan, 2004; Taylor et al., 2008). However, the way in which multimodal signals interact is species specific, and might differ immensely. Hirschmann & Hödl (2006) showed that in Phrynobatrachus krefftii Boulenger, 1909 the conspicuous yellow vocal sac functions as a signal in male–male agonistic interactions, even without calls within the human audible range being emitted. As chemosignals can usually be produced at low costs (Hedin, Maxwell & Jenkins, 1974), the use of pheromones might be a widespread phenomenon in anuran species recognition and mate choice also, but to date the possibility of chemical communication in anurans has not been considered by most studies (Waldman & Bishop, 2004; Belanger & Corkum, 2009), probably because of the more conspicuous signal modalities present to the human observer (e.g. acoustic and visual), or because of the overly costly and elaborate analyses necessary to investigate pheromones.
Chemical communication in amphibians
A large number of amphibians use chemical cues for navigation (Sinsch, 1990; Schulte et al., 2011) and predator detection (Flowers & Graves, 1997), both in aquatic and terrestrial environments. This leads to the assumption that many species may also have the physiological and anatomical ability to produce and detect hetero- and conspecific chemical signals (Byrne & Keogh, 2007; Woodley, 2010; Hamer, Lemckert & Banks, 2011). In aquatic and terrestrial urodeles there are many well-known cases of chemical communication in a sexual context. For instance, male newts of the genus Lissotriton release a bouquet, use their tail to fan it towards a female, and thus elicit responses necessary to coordinate spermatophore transfer (e.g. Malacarne & Giacoma, 1986; and see Treer et al., 2013). There are also a few reports of aquatic sex pheromones (i.e. molecules used for communication between conspecific animals in an aquatic environment) in anurans. In African clawed-frogs (Hymenochirus sp.), females tested in Y-maze experiments showed a clear preference for water containing homogenized male post-axillary breeding glands, or for water previously containing live males (Pearl et al., 2000). Wabnitz et al. (1999) found that female Litoria splendida Tyler, Davies & Martin, 1977 are attracted towards the male by splendipherin, an aquatic pheromone produced only by males in glands found on the head. The chemicals identified in L. splendida, Hymenochirus spp., as well as in newts and salamanders, are peptides, and can therefore only be spread in water or through direct contact (Rajchard, 2005; Houck, 2009). As a result of their direct contact with the female during amplexus, there are a considerable number of publications speculating on the possible use of skin glands, present in terrestrial male anurans, in chemical communication in a sexual context (e.g. Thomas, Tsang & Licht, 1993; Rödel et al., 2003; Lenzi-Mattos et al., 2005). But to the best of our knowledge, there are only four reported cases of volatile pheromone communication in terrestrial amphibians. Male American toads [Anaxyrus americanus (Holbrook, 1836)] show orientation towards female chemical cues in a Y-maze set-up (Forester & Thompson, 1998). Korbeck & (McRobert 2005) report, that poison frogs [Dendrobates auratus (Girard, 1855)] are attracted towards conspecifics of the opposite sex by olfactory cues. In the Australian toadlet Pseudophryne bibronii Günther, 1859, males call hidden in the leaf litter at night and secrete an odorous mucus produced by dorsal, axillary, and postfemoral skin glands, which is likely to help females in close-range mate localization (Byrne & Keogh, 2007). In a recent study on mantellid frogs, Poth et al. (2012) provided the first direct evidence for the occurrence of volatile compounds acting as pheromones in anurans. These compounds are emitted in species-specific cocktails from the prominent femoral glands that characterize the males of these endemic Malagasy frogs (Vences et al., 2007; Poth et al., 2012; Poth et al., 2013).
An extraordinary vocal sac structure in hyperoliids
The anuran family Hyperoliidae (reed frogs) is common in sub-Saharan Africa, Madagascar, and the Seychelles, and comprises over 200 species in 18 genera, of which Hyperolius is the most species rich (Frost, 2012). Within this clade there is substantial variation in coloration, morphology, and reproductive modes, but males of all reed frog species share a common feature: a prominent gular patch on the vocal sac (Fig. 1). Schiøtz (1967) described the gular patches as ‘protective flaps’, suggesting a function as a robust shield for the thin skin of the vocal sac. (Drewes1984) conducted a broad survey, during which he found different variations concerning the position and the shape of the gular patches in reed frogs, and described them as glands of which the ‘actual function remains obscure’. Some genera have gular patches but lack a vocal sac, and (Perret1961) suggested chemical communication in Acanthixalus spinosus (Buchholz & Peters, 1875). Rödel et al. (2003) provided further evidence for possible chemical communication in two apparently mute hyperoliid species from West and Central Africa [Acanthixalus sonjae Rödel, Kosuch, Veith & Ernst, 2003 and Acanthixalus spinosus (Buchholz & Peters, 1875), but see Amiet, 1972], in which males have two gland-like structures on the throat.
Figure 1.
From top left to bottom right: sympatric male individuals of Hyperolius cinnamomeoventris, Hyperolius kivuensis, Hyperolius viridiflavus, and Hyperolius lateralis with inflated vocal sacs. The prominent gular patch is visible in all pictures. (Photos by I. Starnberger and W. Hödl, taken at Kibale Forest National Park, Uganda).
The present study aims to shed light onto the structure and function of the conspicuous hyperoliid gular patch and its potential to bear a function in species recognition, mate choice, and also as a consequence in speciation. We first performed histological examinations to reveal the internal structure of the gular patch. Furthermore, we searched for volatile substances in the gular patch tissue of 11 members of the family Hyperoliidae that would enable airborne chemical communication. Finally, we tested the gular patches of four syntopic species of Hyperolius for the possible presence of species-specific cocktails of volatile substances, which would offer these frogs the opportunity of chemical species recognition. This study aims to lay the foundation for future experimental studies to empirically test the function of the gular patch.
Material and Methods
Collection and molecular identification of specimens
To test the possiblity of species recognition via chemical cues, we collected syntopic specimens in Kibale Forest National Park, Uganda, near the Makerere University Biological Field Station (0°33′41.4786″N, E 30°21′23.6838″). Free-living adult specimens of Hyperolius cinnamomeoventris Bocage, 1866 (n = 3), Hyperolius kivuensis Ahl, 1931 (n = 3), Hyperolius lateralis Laurent, 1940 (n = 3), Hyperolius viridiflavus Dumeril & Bibron, 1841 (n = 8), and Phlyctimantis verrucosus (Boulenger, 1912) (n = 1) were caught in the field at night (when the males were calling). The frogs were sedated by the application of a small quantity of benzocaine on the back, which is absorbed through the frog's skin, and then killed by an overdose of the same substance in the field laboratory. Tissue from the vocal sac was removed and immediately fixed, either in formalin for histology (see below) or in methanol for chemical analysis. The latter samples were stored in 1 mL gas chromatography vials sealed with Teflon-coated caps to prevent modification or evaporation of chemical compounds. In addition, small quantities of muscle tissue for DNA analysis were preserved in absolute ethanol. To allow for a broader biochemical survey, specimens of additional hyperoliid species were collected in Cameroon (genera Afrixalus and Hyperolius) and Madagascar (Heterixalus) in the framework of other research projects, and fixed using the same protocols as in Kibale Forest. The species collected in Cameroon were identified based on morphological traits (Amiet, 2012). The Heterixalus specimens collected in Madagascar could be clearly distinguished by their species-specific calls, as only calling males were sampled. In three specimens of Heterixalus spp. we also examined samples from the skin of the belly and the skin of the vocal sac after excision of the gular gland. A list of voucher specimens is provided in Table S3.
Because many East African Hyperolius species are morphologically similar to each other, the identification of all specimens collected in Kibale Forest was confirmed by DNA sequences. DNA extraction from ethanol-preserved tissues, polymerase chain reaction (PCR), and direct sequencing of a DNA fragment of the mitochondrial 16S rRNA gene (∼500 nucleotides) followed standard methods (e.g. Vences et al., 2003). We used the primers 16S-AL and 16S-BH for PCR, and primer 16S-AL for sequencing (see Vences et al., 2003 for primer sequences and thermocycling protocols). All newly determined sequences were deposited in GenBank (accession numbers GenBank KF447778–KF447836). Haplotypes determined from specimens of each of the four species (H. cinnamomeoventris, H. kivuensis, H. lateralis, and H. viridiflavus) were almost invariable within species and highly divergent between species, thus confirming that the specimens indeed belong to four distinct species. BLAST searches against sequences in GenBank confirmed the species identification of each of the four clusters.
Compound extraction and analysis
The gular patch tissue samples stored in methanol were concentrated and directly analysed by gas chromatography–mass spectrometry (GC-MS) methods. GC-MS analysis was used to investigate the gland constituents, as they are presumably released during calling and should therefore be volatile. The analyses were performed on an Agilent 7890A GC system fitted with an HP-5MS-fused silica capillary column (30 m, 0.25 mm i.d., 0.25 μm film; J&W Scientific), connected to an Agilent 5975C inert mass detector using the following method: 5 min at 50 °C, then increasing with 10 °C min–1 to 320 °C, operated in splitless mode (60-s valve time). The detected compounds were characterized by their molecular mass, their base peak, and their retention index (RI). Most of the compounds were assigned to one of the four substance classes most abundant in the samples (sesquiterpenes, fatty acid esters, macrolides, alcohols), based on their characteristic fragmentation patterns. The structures of some volatile compounds were identified by the comparison of their mass spectra and retention indices with data from the literature.
Histology
For histological analyses specimens of H. viridiflavus (n = 2, both green morphs) were killed as described above and fixed in 10% neutral buffered formalin, as described by (Carson1997), in the field lab. The samples were submerged in Bouin's fixative and after 24 h transferred to ethanol (70%). The samples were dehydrated in a graded series of ethanol (70, 80, and 90%) and embedded in paraffin. The tissue was cut at 5 μm using a rotary microtome (Leica RM 2265). The 5 μm series was stained using Heidenhain's AZAN stain for general histology. We used only one species for detailed histological analysis, because a pilot study showed that the gland tissue anatomy does not vary considerably between closely related Hyperolius species (I. Starnberger, unpubl. data).
Statistical analyses
To graphically visualize variation in individual compound profiles within and among species of H. cinnamomeoventris, H. kivuensis, H. lateralis, and H. viridiflavus, based on the untransformed presence/absence of compounds (Table S1), we used non-metric multidimensional scaling (nMDS), based on Bray–Curtis dissimilarity matrices. To quantify the difference in compound composition between sympatric species we used a one-way analysis of similarity (ANOSIM) with Bray–Curtis distance measure (as in Russo et al., 2008). Statistical analyses were performed with SPSS 20.0 and PAST 2.17 (see Hammer, Harper & Ryan, 2001).
Results
Structure of gular patch and gular gland
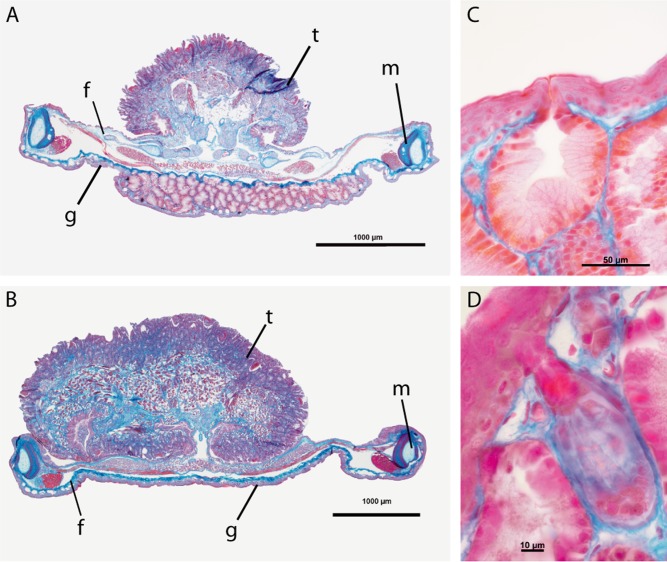
Histological analyses revealed that the conspicuous gular patch found on the vocal sac of male reed frogs is a gland complex consisting of different gland types. The hyperoliid gular patch is a hypertrophied area extending far into the vocal sac cavity, and is approximately ten times thicker than the surrounding vocal sac tissue (Fig. 2A, C and D). The gular patch is situated in the stratum spongiosum within the dermis, and comprises three different gland types. We hereafter use the term ‘gular gland’ to refer to this macrogland structure. A vast part of the tissue is made up of glands consisting of densely packed, highly prismatic cells with a central nucleus, which form tubular ducts leading to a basin directly underneath the skin surface (Fig. 2A, C and D). Secretions collected in the basin can be released onto the epidermis via a narrow duct. The collective basins are surrounded by myocytes, which most likely compress the gland to push the secretion through the narrow duct (Fig. 2A, C and D).The gular gland is made up by separate units of tubular glands, subdivided by thin layers of connective tissue, each with a separate secretion channel. Furthermore, there is a strand of connective tissue conjoining the gular patch with the diaphragma oris. The vocal sac tissue surrounding the gular patch contains both granular and mucous glands, but no tubular gland tissue.
Figure 2.
Cross sections (6-μm thick; AZAN stain) of the lower jaw of a male (A) and a female (B) Hyperolius viridiflavus, showing the tongue (t), the mandibular arch (m), the floor of the mouth (f), and the gular skin (g). The male's gular skin is extremely hypertrophied and forms a tubular gland complex, which is missing in the female. Details of tubular glands reveal collective pools near the surface of the gland complex with narrow ducts leading to the outside (C), and myocytes, probably used to discharge the gland (D). The tongue size difference between the male and the female is linked to the female's larger body size and the fact that the jaws of the two specimens were cut in slightly different areas.
Chemical compound screening in hyperoliid frogs
We performed a biochemical screening of members of four hyperoliid genera, all characterized by a prominent gular patch (Table 1995). The chemical survey revealed that sesquiterpenes are present in the gular patches of all genera sampled (Afrixalus spp., Heterixalus spp., Hyperolius spp., and Phlyctimantis sp.), with up to 13 different compounds per individual, suggesting that in all of these taxa the patches are made up by a gular gland structure. Fatty-acid esters were present in all Hyperolius samples, with 12–14 compounds per individual, and in Heterixalus alboguttatus (Boulenger, 1882) (seven compounds) and in Afrixalus lacteus Perret, 1976 (one compound). Furthermore, we found a small number of macrolides in two Hyperolius species and in Afrixalus paradorsalis Perret, 1960. In the samples of H. cinnamomeoventris and H. viridiflavus a number of unidentified alcohols were present. In Heterixalus spp. none of the three control samples of skin from the belly and skin from the vocal sac yielded any volatile compounds, confirming that these are produced and secreted by the gular gland only.
Table 1.
Total number of compounds of each substance class present in the samples of the hyperoliid species examined
| Genus | Species | No. of samples | Sesquiterpenes | Fatty acid esters | Macrolides | Alcohols | Total no. of compounds |
|---|---|---|---|---|---|---|---|
| Hyperolius | kivuensis | 3 | 13 | 12 | 28 | ||
| Hyperolius | lateralis | 3 | 3 | 12 | 18 | ||
| Hyperolius | cinnamomeoventris | 3 | 7 | 12 | 2 | 1 | 25 |
| Hyperolius | viridiflavus bayoni (brown morph) | 5 | 2 | 14 | 1 | 2 | 24 |
| Hyperolius | viridiflavus bayoni (green morph) | 3 | 4 | 14 | 1 | 1 | 23 |
| Hyperolius | ademetzi | 1 | 11 | 12 | |||
| Afrixalus | lacteus | 3 | 1 | 1 | 5 | ||
| Afrixalus | laevis | 3 | 2 | 5 | |||
| Afrixalus | paradorsalis | 3 | 10 | 1 | 14 | ||
| Phlyctimantis | verrucosus | 1 | 10 | 11 | |||
| Heterixalus | alboguttatus | 6 | 13 | 7 | 26 | ||
| Heterixalus | betsileo | 3 | 6 | 9 |
Although the structures of most of the volatiles detected in the gular patch extracts are still unknown, some compounds could be identified. Figure 3 shows the mass spectra and structures of two sesquiterpenes detected in the gular patch extracts of H. kivuensis and H. cinnamomeoventris. The mass spectrometric data and the retention index of α-himachalene and 2-epi-(E)-β-caryophyllene matched those reported by (Adams1995). The identification of the structures of other sesquiterpenes and of the detected alcohols and macrolides will be pursued in the future.
Figure 3.
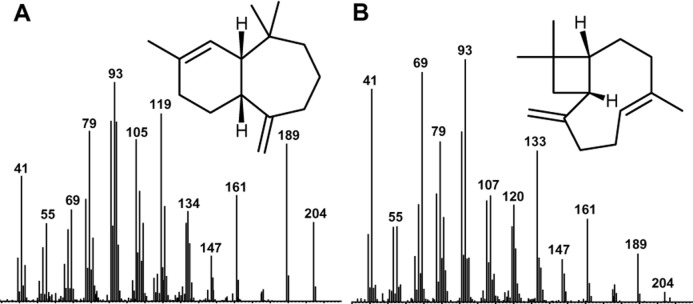
Mass spectra of the terpenes identified from Hyperolius cinnamomeoventris (α-himachalene, A) and from Hyperolius kivuensis [2-epi-(E)-β-caryophyllene, B]. The structures were confirmed by comparison of the mass spectrometric data and the retention index with those from the literature (Adams, 1995).
Chemical compound differentiation in four syntopic Hyperolius species
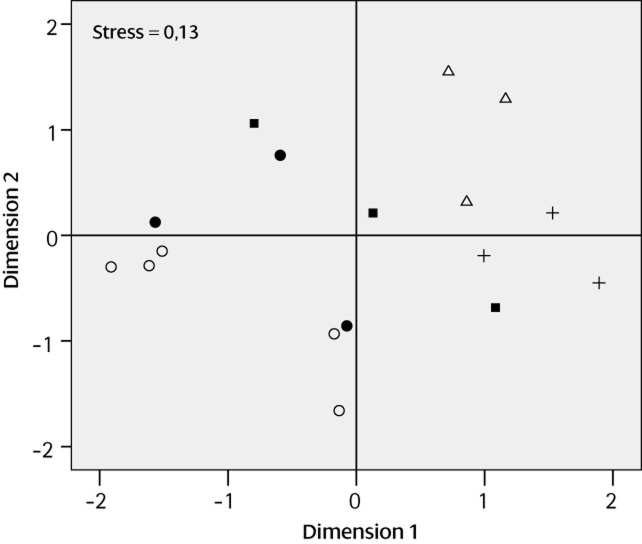
In total, 65 compounds were recorded from gular patch tissue of the 17 males examined from four Hyperolius species collected at Kibale. Most compounds could be assigned to a structural class, of which sesquiterpenes and fatty acid esters were the most abundant. Overall, the chemical profiles were different between species (ANOSIM: P = 0.0028, R = 0.5), which is illustrated by the fact that only a small portion of the terpenes was shared by the different syntopic species, and most of them were characteristic for one species (Table S2). Multidimensional scaling based on the presence/absence of compounds in the individual samples resulted in clustering (Fig. 4), confirming that each species has a characteristic cocktail of compounds in its gular glands, despite individual variation (nMDS: stress = 0.1). All four species were largely separated, but the chemical bouquets of H. cinnamomeoventris and H. viridiflavus differed the most (P = 0.012). Hyperolius cinnamomeoventris and H. lateralis had chemical cocktails that clustered in between the two other species, but still did not overlap. The highest variation of chemical composition between individuals was found in H. viridiflavus, with one individual even clustering with H. lateralis. The other specimens of H. viridiflavus formed two clusters that seem to correspond to body coloration (i.e. different colour morphs).
Figure 4.
Non-metric multidimensional scaling (nMDS) plot of Hyperolius chemical compound profiles among four sympatric species. The nMDS plot is based on the presence/absence of 65 compounds in the gular patch tissue samples (Table S1). Each symbol represents an individual frog. Symbol shape refers to species identity (△, Hyperolius kivuensis; +, Hyperolius cinnamomeoventris; ○, Hyperolius viridiflavus, brown morph; ●, H. viridiflavus, green morph; ■, Hyperolius lateralis).
Discussion
Stucture and function of the gular patch
The present study shows that the colourful gular patch found on the vocal sac of reed frogs (Hyperoliidae) is a gland complex (here named the gular gland), with a histological structure that is, in general, similar to other macroglands of anuran amphibians (Thomas et al., 1993), including the pheromone-secreting femoral glands of mantellid frogs (Vences et al., 2007). In urodeles, a similar structure has been described and analysed in detail from the cloacal gland of the salamander Eurycea lucifuga Rafinesque, 1822 (Sever, 1989; Hamlett, Strecker & Trauth, 1998). Eurycea males develop barrel-shaped caudal courtship glands surrounded by round mucous and granular glands, which considerably increase in size during the breeding season. The courtship glands of these salamanders consist of columnar cells with basal nuclei, which form two short tubular ducts leading directly to a duct opening ending on the surface of the epidermis. In the case of E. lucifuga, male pheromones are transmitted through the skin of the female during direct contact, as in other plethodontids. Because of the general structural similarity with other amphibian glands involved in pheromone production, the gular gland of hyperoliids is likely to serve a similar function; however, the mode of transmission remains unclear so far, and will be experimentally tested in future studies.
One possibility is direct transmission of chemical compounds from male to female during amplexus, when the male's gular region is tightly pressed onto the female's back, as in salamanders. For instance, in newts of the North American genus Notophthalmus, the male rubs his chin on the female's head to transmit courtship pheromones (Hilton, 1902). In this scenario, female reed frogs could chemically ascertain the conspecificity and potentially the attractiveness of the amplecting male, and potentially delay oviposition if amplected by a male deemed unsuitable.
A second possible mode of pheromone transmission would be the production of volatile substances that are emitted while a male is calling and circulate in the air to attract females, and/or to keep rival males at a distance. In Acanthixalus spinosus (Schmitz, Euskirchen & Böhme, 1999) and Acanthixalus sonjae (M.-O. Rödel, pers. observ.) there is a distinctive smell from the gular glands that is discernible to the human observer, and the presence of volatile sesquiterpenes among the compounds identified in reed frog gular glands makes this alternative conceivable, calling for future behavioural studies testing these hypotheses.
Chemical compound diversity in reed frogs
Our study confirmed the presence of volatile compounds in the gular patches of representatives of a subset of the 19 hyperoliid genera known at present. The data set includes members of four genera, including the most species-rich ones (Hyperolius, Afrixalus, and Heterixalus). As tissue samples of museum specimens conserved in ethanol or formalin cannot be used for the chemical screening, fresh additional samples are needed to ascertain the presence of such compounds in the remaining genera.
The results from four syntopic Hyperolius species at Kibale Forest suggest that substances produced in the gular patch might play a role in species recognition. In our sampling of these four Hyperolius, sesquiterpenes were not usually shared among different species and thus could play a role in the chemical communication of the Hyperoliidae. Fatty acid esters were present throughout samples of all of the four species, and are therefore not species specific. This broad occurrence, their low volatility, and the fact that fatty acid esters are commonly found in anuran tissues makes their potential role in airborne chemical communication unlikely. The species-specific occurrence of the alcohols and the macrolides, only found in the samples of H. viridiflavus and H. cinnamomeoventris, are a good indication that those volatiles together with the terpenes may be used to create species-specific cocktails of chemicals. Because alcohols and macrolides are known to be used as volatile pheromones by Malagasy mantellid frogs (Poth et al., 2012), they may also play a role in the chemical communication and speciation of the Hyperoliidae. In H. viridiflavus the two colour morphs (brown and green) appear to form largely separated groups (Fig. 4). This result might point towards a possible incipient divergence within the so-far unresolved ‘viridiflavus’ species complex, although in our analysis, specimens of both morphs had identical haplotypes in the mitochondrial 16S gene.
Reed frogs might produce trimodal signals while calling
Reed frogs can often be found calling in mixed choruses together with closely related species (Schiøtz, 1999; e.g. Lötters et al., 2004). We hypothesize that for female reed frogs, advertisement calls alone might not be sufficient to precisely locate and identify a conspecific male in a mixed-species chorus, and in dense vegetation such as reeds. Hödl (1977) and Martins, Almeida & (Jim2006) showed that in the Neotropics, males in heterospecific choruses use calling site segregation (i.e. different plant species and different calling heights) to facilitate female approach to conspecific males. The four syntopic reed frog species studied herein called simultaneously in the same swamp, without any obvious spatial or temporal segregation, although spatial segregation is not uncommon in hyperoliids (Rödel, Lampert & Linsenmair, 2006; Sinsch et al., 2012). Wilbur, Rubenstein & (Fairchild1978) speculated that the ‘vocal sac pouch’ in the hyperoliid Kassina senegalensis (Dumeril & Bibron, 1841) plays a role in attracting females and/or in setting up breeding territories. From our data, we suggest that along with macrolides and alcohols, sesquiterpenes in particular might be the prime components in the chemical signalling of hyperoliids. Sesquiterpenes were found in the gular glands of all species examined, and as they are volatile they could therefore act as airborne pheromones released while calling.
As acoustic signals such as individual anuran advertisement calls are difficult to localize in a chorus situation (Bee & Micheyl, 2008; Richardson et al., 2010), or in environments (e.g. dense vegetation) that hamper the propagation and transmission of acoustic signals (Wells & Schwartz, 1982; Kime, Turner & Ryan, 2000; Boeckle, Preininger & Hödl, 2009), the differential colour of vocal sacs and gular patches of reed frogs might help in species recognition. A surprisingly high contrast between the gular patch and the surrounding vocal sac skin makes the gland stand out from its background, and might serve as a visual cue facilitating the localization of a male calling in dense vegetation (I. Starnberger, pers. observ.). In addition, ‘chemical cocktails’ might be used for close-range species identification. Such chemical signals might also contain cues on a male's ‘good genes’ and genetic compatibility, and might therefore influence a reproductive female in mate choice (reviewed in Johansson & Jones, 2007).
Conclusion
We propose that reed frogs use a combination of acoustic and chemical signals to enhance their ability to detect conspecifics within the dense multi-species breeding aggregations typical for hyperoliid frogs. Furthermore, it is likely that the vocal sac plays a role in visual signalling by its conspicuousness. Future experimental studies thus might characterize hyperoliids as a highly attractive model for multimodal communication. Nevertheless, there are several hyperoliid species where males lack an inflatable vocal sac but still have one or two gular glands, and several of these species seem to be mute and might therefore fully rely on chemical communication. Chemical cocktails found in the gular gland are species specific, and might even help to resolve taxonomic issues in this species complex.
Acknowledgments
We thank A. Amézquita, N. L. Gonwouo, M. Hirschfeld, A. Lembens, H. C. Liedtke, and I. Maiditsch for their help in the field, and F. and M. Kamoga and the Tropical Biology Association for helping us with logistics and the acquisition of permits. D. Preininger and M. Sztatecsny helped with equipment and valuable advice. Our study received financial support from the Austrian Science Fund (FWF): W1234 and P25612, a seed grant of the Zukunftsfonds (Programmpauschale) of TU Braunschweig, the Deutsche Forschungsgemeinschaft (grant no. Schu984/10-1), and was co-financed by a research grant of the University of Vienna, 2011. Specimen collection and export was authorized by the Uganda Wildlife Authority, the Uganda National Council for Science and Technology (permit NS 374). Specimen collection in Cameroon was conducted within the research project 0603/P/MINFOF/SG approved by the Cameroonian Ministries MINFOF and MINRESI. The collection of samples of Heterixalus in Madagascar was carried out in the framework of a collaboration between T.U. Braunschweig and the Université d'Antananarivo. We thank D.C. Blackburn and three anonymous reviewers for their constructive criticism and their encouraging comments.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site
(a) First part of list of absence and presence data for chemical compounds in tissue samples of 17 specimens belonging to four reed frog species (Hyperolius). (b) Second part of list of absence and presence data for chemical compounds in tissue samples of 17 specimens belonging to four reed frog species (Hyperolius).
Table S2. Occurence of volatile compounds in the gular patch extracts from 17 individuals belonging to four Hyperolius species.
Table S3. Voucher numbers of specimens used in the biochemical survey.
References
- Adams RP. Identification of essential oil components by gas chromatography-mass spectrometry. Carol Stream: Allured Publishing Corporation; 1995. [Google Scholar]
- Amiet JL. Notes faunistiques, biologiques et écologiques sur quelques amphibiens anoures du Cameroun. Annals de la Faculté des Sciences du Cameroun. 1972;9:127–153. [Google Scholar]
- Amiet JL. Les rainettes du Cameroun Nyons & Saint Nazaire: Edition J.-L. Amiet & La Nef des Livres. 2012.
- Bee MA, Micheyl C. The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? Journal of Comparative Psychology. 2008;122:235–251. doi: 10.1037/0735-7036.122.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger R, Corkum L. Review of aquatic sex pheromones and chemical communication in anurans. Journal of Herpetology. 2009;43:184–191. [Google Scholar]
- Boeckle M, Preininger D, Hödl W. Communication in noisy environments I: acoustic signals of Staurois latopalmatus Boulenger 1887. Herpetologica. 2009;65:154–165. [Google Scholar]
- Bucher TL, Ryan MJ, Bartholomew GA. Oxygen-consumption during resting, calling, and nest building in the frog Physalaemus pustulosus. Physiological Zoology. 1982;55:10–22. [Google Scholar]
- Byrne P, Keogh J. Terrestrial toadlets use chemosignals to recognize conspecifics, locate mates and strategically adjust calling behaviour. Animal Behaviour. 2007;74:1155–1162. [Google Scholar]
- Caldwell MS, Johnston GR, McDaniel JG, Warkentin KM. Vibrational signaling in the agonistic interactions of red-eyed treefrogs. Current Biology. 2010;20:1012–1017. doi: 10.1016/j.cub.2010.03.069. [DOI] [PubMed] [Google Scholar]
- Carson FL. Histotechnology: a self-instructional text. Chicago: American Society for Clinical Pathology; 1997. [Google Scholar]
- Coleman S. Taxonomic and sensory biases in the mate-choice literature: there are far too few studies of chemical and multimodal communication. Acta Ethologica. 2009;12:45–48. [Google Scholar]
- Dorcas ME, Price SJ, Walls SC, Barichivich WJ. Amphibian ecology and conservation: a handbook of techniques. In: Dodd KC, editor. Auditory monitoring of anuran populations. Oxford: Oxford University Press; 2010. pp. 281–298. [Google Scholar]
- Drewes R. A phylogenetic analysis of the Hyperoliidae (Anura): treefrogs of Africa, Madagascar, and the Seychelles Islands. Occasional Papers of the California Academy of Sciences. 1984;139:1–70. [Google Scholar]
- Duellman WE, Trueb L. Biology of Amphibians. New York: McGraw-Hill Publishing Company; 1986. [Google Scholar]
- Endler JA, Thery M. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three Neotropical forest-dwelling birds. American Naturalist. 1996;148:421–452. [Google Scholar]
- Fleishman L, McClintock WJ, D'Eath R, Brainard D, Endler JA. Colour perception and the use of video playback experiments in animal behaviour. Animal Behaviour. 1998;56:1035–1040. doi: 10.1006/anbe.1998.0894. [DOI] [PubMed] [Google Scholar]
- Flowers MA, Graves BM. Juvenile toads avoid chemical cues from snake predators. Animal Behaviour. 1997;53:641–646. [Google Scholar]
- Forester DC, Thompson KJ. Gauntlet behaviour as a male sexual tactic in the American toad (Amphibia: Bufonidae) Behaviour. 1998;135:99–119. [Google Scholar]
- Frost DR. Amphibian Species of the World: an online reference. Version 5.6 (1 October 2012). 2012. Electronic Database accessible at http://research.amnh.org/herpetology/amphibia/index.html. American Museum of Natural History, New York, USA.
- Gerhardt H, Huber F. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Hamer R, Lemckert FL, Banks PB. Adult frogs are sensitive to the predation risks of olfactory communication. Biology Letters. 2011;7:361–363. doi: 10.1098/rsbl.2010.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlett BE, Strecker AG, Trauth SE. Caudal courtship glands in the cave salamander, Eurycea lucifuga (Caudata: Plethodontidae) Journal of the Arkansas Academy of Science. 1998;52:124–128. [Google Scholar]
- Hammer O, Harper DAT, Ryan PD. PAST: paleontological Statistics software package for education and data analysis. Paleontologica Electronica. 2001;4:9. [Google Scholar]
- Hedin PA, Maxwell FG, Jenkins JN. Insect plant attractants, feeding stimulants, repellents, deterrents and other related factors affecting insect behavior. In: Maxwell FG, Harris FA, editors. Proceedings, summer institute on biological control of plants and diseases. Jackson: Mississipi University Press; 1974. pp. 494–527. [Google Scholar]
- Hilton WA. A structural feature connected with the mating of Diemyctulus viridescens. American Naturalist. 1902;34:643–651. [Google Scholar]
- Hirschmann W, Hödl W. Visual signaling in Phrynobatrachus krefftii Boulenger, 1909 (Anura: Ranidae) Herpetologica. 2006;62:18–27. [Google Scholar]
- Hödl W. Call differences and calling site segregation in anuran species from Central Amazonian floating meadows. Oecologia. 1977;28:351–363. doi: 10.1007/BF00345990. [DOI] [PubMed] [Google Scholar]
- Hödl W, Amézquita A. Visual signaling in anuran amphibians. In: Ryan MJ, editor. Anuran communication. Washington: Smithsonian Institution Press; 2001. pp. 121–141. [Google Scholar]
- Houck L. Pheromone communication in amphibians and reptiles. Annual Review of Physiology. 2009;71:161–176. doi: 10.1146/annurev.physiol.010908.163134. [DOI] [PubMed] [Google Scholar]
- Johansson BG, Jones TM. The role of chemical communication in mate choice. Biological Reviews. 2007;82:265–289. doi: 10.1111/j.1469-185X.2007.00009.x. [DOI] [PubMed] [Google Scholar]
- Kime N, Turner W, Ryan M. The transmission of advertisement calls in Central American frogs. Behavioral Ecology. 2000;11:71–83. [Google Scholar]
- Korbeck RG, McRobert SP. Home area recognition via olfactory cues in the tropical poison frog Dendrobates auratus. Russian Journal of Herpetology. 2005;12:161–166. [Google Scholar]
- Lenzi-Mattos R, Antoniazzi M, Haddad C, Tambourgi D, Rodrigues M, Jared C. The inguinal macroglands of the frog Physalaemus nattereri (Leptodactylidae): structure, toxic secretion and relationship with deimatic behaviour. Journal of Zoology. 2005;266:385–394. [Google Scholar]
- Lewis E, Narins P, Cortopassi K, Yamada W, Poinar E, Moore W, Yu X. Do male white-lipped frogs use seismic signals for intraspecific communication? Integrative and Comparative Biology. 2001;41:1185–1193. [Google Scholar]
- Lötters S, Schick S, Scheelke K, Teege P, Kosuch J, Rotich D, Veith M. Bio-sketches and partitioning of sympatric reed frogs, genus Hyperolius (Amphibia; Hyperoliidae), in two humid tropical African forest regions. Journal of Natural History. 2004;38:1969–1997. [Google Scholar]
- Malacarne G, Giacoma C. Chemical signals in European newt courtship. Italian Journal of Zoology. 1986;53:79–83. [Google Scholar]
- Martins IA, Almeida SC, Jim J. Calling sites and acoustic partitioning in species of the Hyla nana and rubicundula groups (Anura, Hylidae) The Herpetological Journal. 2006;16:239–247. [Google Scholar]
- Partan S, Marler P. Issues in the classification of multimodal communication signals. The American Naturalist. 2005;166:231–245. doi: 10.1086/431246. [DOI] [PubMed] [Google Scholar]
- Pauly GB, Bernal XE, Rand AS, Ryan M. The vocal sac increases call rate in the túngara frog Physalaemus pustulosus. Physiological and Biochemical Zoology. 2006;79:708–719. doi: 10.1086/504613. [DOI] [PubMed] [Google Scholar]
- Pearl C, Cervantes M, Chan M, Ho U, Shoji R, Thomas E. Evidence for a mate-attracting chemosignal in the dwarf African clawed frog Hymenochirus. Hormones and Behavior. 2000;38:67–74. doi: 10.1006/hbeh.2000.1609. [DOI] [PubMed] [Google Scholar]
- Perret JL. La biologie d'Acanthixalus spinosus (Amphibia Salientia) Recherches et Etudes camerounaises. 1961;1:90–101. [Google Scholar]
- Poth D, Peram PS, Vences M, Schulz S. Macrolides and alcohols as scent gland constituents of the Madagascan frog Mantidactylus femoralis and their intraspecific diversity. Journal of Natural Products. 2013;76:1548–1558. doi: 10.1021/np400131q. [DOI] [PubMed] [Google Scholar]
- Poth D, Wollenberg KC, Vences M, Schulz S. Volatile amphibian pheromones: macrolides from mantellid frogs from Madagascar. Angewandte Chemie International Edition. 2012;51:2187–2190. doi: 10.1002/anie.201106592. [DOI] [PubMed] [Google Scholar]
- Rajchard J. Sex pheromones in amphibians: a review. Veterinary Medicine–Czech. 2005;50:385–389. [Google Scholar]
- Rand A, Dudley R. Frogs in helium: the anuran vocal sac is not a cavity resonator. Physiological Zoology. 1993;66:793–806. [Google Scholar]
- Richardson C, Gomez D, Durieux R, Thery M, Joly P, Léna J, Plenet S, Lengagne T. Hearing is not necessarily believing in nocturnal anurans. Biology Letters. 2010;6:633–635. doi: 10.1098/rsbl.2010.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödel MO, Kosuch J, Veith M, Ernst R. First record of the genus Acanthixalus Laurent, 1944 from the Upper Guinean rain forest, West Africa, with the description of a new species. Journal of Herpetology. 2003;37:43–52. [Google Scholar]
- Rödel MO, Lampert KP, Linsenmair KE. Reproductive biology of the West African savannah frog Hyperolius nasutus Günther, 1864. Herpetozoa. 2006;19:3–12. [Google Scholar]
- Rosenthal G, Rand A, Ryan M. The vocal sac as a visual cue in anuran communication: an experimental analysis using video playback. Animal Behaviour. 2004;68:55–58. [Google Scholar]
- Russo T, Pulcini D, O'Leary Á, Cataudella S, Mariani S. Relationship between body shape and trophic niche segregation in two closely related sympatric fishes. Journal of Fish Biology. 2008;73:809–828. [Google Scholar]
- Ryan MJ. The Túngara frog: a study in sexual selection and communication. Chicago: University of Chicago Press; 1985. [Google Scholar]
- Schiøtz A. The treefrogs (Rhacophoridae) of West Africa. Spolia Zoologica Musei Hauniensis. 1967;25:1–346. [Google Scholar]
- Schiøtz A. Treefrogs of Africa. Frankfurt am Main: Edition Chimaira; 1999. [Google Scholar]
- Schmitz A, Euskirchen O, Böhme W. Zur Herpetofauna einer montanen Regenwaldregion in SW-Kamerun (Mt. Kupe und Bakossi-Bergland) herpetofauna. 1999;21:5–17. [Google Scholar]
- Schulte LM, Yeager J, Schulte R, Veith M, Werner P, Beck LA, Lötters S. The smell of success: choice of larval rearing sites by means of chemical cues in a Peruvian poison frog. Animal Behaviour. 2011;81:1147–1154. [Google Scholar]
- Sever DM. Caudal hedonic glands in salamanders of the Eurycea bislineata complex (Amphibia, Plethodontidae) Herpetologica. 1989;45:322–329. [Google Scholar]
- Sinsch U. Migration and orientation in anuran amphibians. Ethology Ecology & Evolution. 1990;2:65–79. [Google Scholar]
- Sinsch U, Lümkemann K, Rosar K, Schwarz C, Dehling M. Acoustic niche partitioning in an anuran community inhabiting an Afromontane wetland (Butare, Rwanda) African Zoology. 2012;47:60–73. [Google Scholar]
- Taylor R, Buchanan B, Doherty J. Sexual selection in the squirrel treefrog Hyla squirella: the role of multimodal cue assessment in female choice. Animal Behaviour. 2007;74:1753–1763. [Google Scholar]
- Taylor R, Klein B, Stein J, Ryan M. Faux frogs: multimodal signalling and the value of robotics in animal behaviour. Animal Behaviour. 2008;76:1089–1097. [Google Scholar]
- Thomas EO, Tsang L, Licht P. Comparative histochemistry of the sexually dimorphic skin glands of anuran amphibians. Copeia. 1993;1993:133–143. [Google Scholar]
- Treer D, Van Bocxlaer I, Matthijs S, Four Du D, Janssenswillen S, Willaert B, Bossuyt F. Love is blind: indiscriminate female mating responses to male courtship pheromones in newts (Salamandridae) PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0056538. e56538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences M, Kosuch J, Glaw F, Böhme W, Veith M. Molecular phylogeny of hyperoliid treefrogs: biogeographic origin of Malagasy and Seychellean taxa and re-analysis of familial paraphyly. Journal of Zoological Systematics and Evolutionary Research. 2003;41:205–215. [Google Scholar]
- Vences M, Wahl-Boos G, Hoegg S, Glaw F, Spinelli Oliveira E, Meyer A, Perry S. Molecular systematics of mantelline frogs from Madagascar and the evolution of their femoral glands. Biological Journal of the Linnean Society. 2007;92:529–539. [Google Scholar]
- Wabnitz P, Bowie JH, Tyler M, Wallace J, Smith B. Animal behaviour: aquatic sex pheromone from a male tree frog. Nature. 1999;401:444–445. doi: 10.1038/46724. [DOI] [PubMed] [Google Scholar]
- Waldman B, Bishop PJ. Chemical communication in an archaic anuran amphibian. Behavioral Ecology. 2004;15:88–93. [Google Scholar]
- Wells KD, Schwartz J. The effect of vegetation on the propagation of calls in the neotropical frog Centrolenella fleischmanni. Herpetologica. 1982;38:449–455. [Google Scholar]
- Wilbur HM, Rubenstein DI, Fairchild L. Sexual selection in toads: the roles of female choice and male body size. Evolution. 1978;32:264–270. doi: 10.1111/j.1558-5646.1978.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Woodley S. Pheromonal communication in amphibians. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 2010;196:713–727. doi: 10.1007/s00359-010-0540-6. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Mate selection: a selection for a handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) First part of list of absence and presence data for chemical compounds in tissue samples of 17 specimens belonging to four reed frog species (Hyperolius). (b) Second part of list of absence and presence data for chemical compounds in tissue samples of 17 specimens belonging to four reed frog species (Hyperolius).
Table S2. Occurence of volatile compounds in the gular patch extracts from 17 individuals belonging to four Hyperolius species.
Table S3. Voucher numbers of specimens used in the biochemical survey.