Abstract
As biological and linguistic diversity, the world’s cultural diversity is on decline. However, to date there are no estimates of the rate at which the specific cultural traits of a group disappear, mainly because we lack empirical data to assess how the cultural traits of a given population change over time. Here we estimate changes in cultural traits associated to the traditional knowledge of wild plant uses among an Amazonian indigenous society. We collected data among 1151 Tsimane’ Amerindians at two periods of time. Results show that between 2000 and 2009, Tsimane’ adults experienced a net decrease in the report of plant uses ranging from 9% (for the female subsample) to 26% (for the subsample of people living close to towns), equivalent to a 1 to 3 % per year. Results from a Monte Carlo simulation show that the observed changes were not the result of randomness. Changes were more acute for men than for women and for informants living in villages close to market towns than for informants settled in remote villages. The Tsimane’ could be abandoning their traditional knowledge as they perceive that this form of knowledge do not equip them well to deal with the new socio-economic and cultural conditions they face nowadays.
Keywords: Bolivian Amazon; cultural change, acculturation; folk knowledge; Tsimane’
Introduction
Researchers agree that many drivers are potentially responsible for cultural change and that the rate at which such cultural traits change varies across cultures and time periods (Boyd & Richerson, 2005). Working under the framework of cultural evolutionary theory, several authors have attempted to explain the mechanisms and processes that drive cultural change. Such work has identified a set of factors that, working alone or in combination, provide pausible explainations for rates of cultural change at different points of time (Mace et al. 2005; Mesoudi et.al., in press). For example, Rogers’ and Ehrlich’s (2008) finding that functional features of Polynesian canoes change less rapidly than stylistic non-functional traits suggests that intrinsic characteristics of a given technology might affect rates of technological change. Differently, Henrich’s modelling work (2004) suggests that a reduction in population size was the main factor driving the technological loss in Tasmania. Other factors affecting rates of cultural change include external environmental change (Richerson et al. 2001), technology manufacturing costs (Martin 2000), fit between new and previous knowledge (Rogers 1995), external representation of knowledge (Leonti 2011), social network structure and social institutions (Henrich and Henrinch 2010, Tehrani and Collard 2009), or inter-group conflict (Di Cosmo 2002). Furthermore, random copying and cultural drift can also drive changes in cultural traits (Bentley, et al., 2004).
Besides providing explanations for the drivers of cultural change, researchers have also attempted to calculate the rate at which different cultural traits change. Generalizations from this body of research have proven challenging for two main reasons. First, when assessing cultural change, researchers have used different temporal scales and different units of analysis derived from different human bevavior, and including material culture, social organization and language (Aunger 2000a, Mace et al 2005). Although the scale and unit of analysis are probably related to the nature of the research (e.g., archaeologists are typically constrained to artifacts, whilst anthropologists focus on the measure knowledge and/or behavior), diversity in units of analysis hampers the comparability of research results. Second, the study of cultural change requires a diachronic perspective that is not necessarily implicit in all the disciplines addressing the study of cultural change (such as anthropology). While modelling frameworks (Boyd and Richerson 2005), incluging models of macroevolutionary change and cumulative cultural evolution (Enquist et al. 2011; Aoki et al. 2011), and phylogenetic methods (Mace et al. 2005; Lipo et al. 2006) stress the diachronic perspective, anthropologists attempting to asses cultural change in contemporary societies have often been less well-equiped. For example, previous anthropological research on the secular changes on traditional knowledge has relied on cross-sectional data comparing measures 1) among people of different ages and cohorts (Benz, et al., 2000; Demps, et al., 2012; Godoy, et al, 2009; Gomez-Baggethun, et al., 2010), or 2) among people living at different levels of cultural and economic isolation (Reyes-García et al., 2005). But anthropologists typically lack diachronic data for the study of cultural change.
Here we contribute to research on rates and factors affecting cultural change in contemporary indigenous societies by using a different methodological approach. We use contemporary data collected over two periods of time to assess changes in traditional knowledge among a highly autarkic foraging-farming society of native Amazonians. We focus on traditional knowledge, sensu Berkes (Berkes et al., 2000), because this form of knowledge seems to be declining in many parts of the world (Benz, et al., 2000; Maffi, 2002; Perales, et al., 2005; Gomez-Baggethun, et al., 2010). We address two main research questions. First, to what extent does contemporary traditional knowledge change over short periods of time? And second, what factors are likely to explain changes in traditional knowledge? In answering both questions we provide the first empirical estimate of the rate at which traditional knowledge change in a contemporaneous indigenous society, and the factors associated to such change.
Methods
Study population
The Tsimane’ are the third largest ethnic group in the Bolivian lowlands with about 10,000 people living in about 125 villages, mostly in the province of Beni. The Tsimane’ live in small communities of about 20 households, along riverbanks and logging roads. The Tsimane’ economy is primarily based on hunting, fishing, and slash-and-burn farming, with cash cropping of rice becoming a dominant form of monetary income (Vadez et al., 2008). The Tsimane’ also sell and barter agricultural, timber and non-timber forest products in nearby towns or to traveling traders who visit their villages.
Subject recruitment, study design, and sample
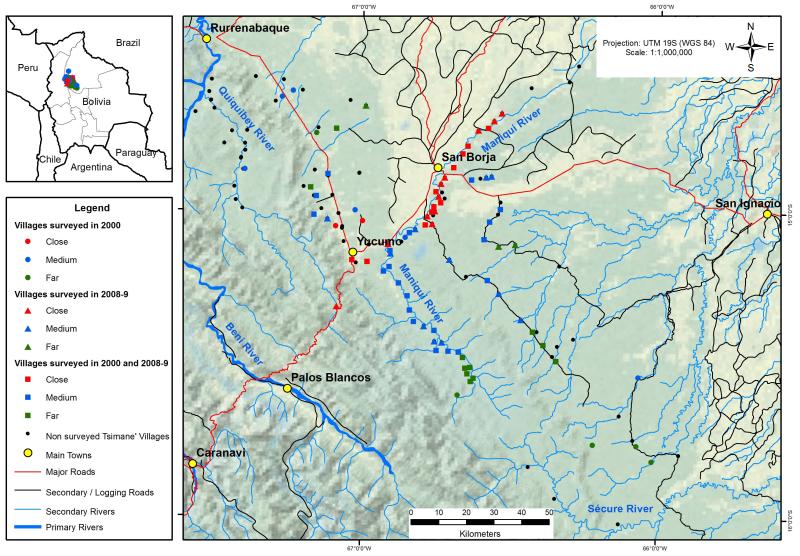
The Gran Consejo Tsimane’ (the Tsimane’ political organization) approved the study protocol and prior informed consent was obtained from each participant. Refusal to participate was low (<5%). We collected information twice: once during 2000 and once during 2008-2009 (hereafter 2009). In 2000 we selected 58 villages based on a census carried out by the Gran Consejo Tsimane’ (Reyes-García et al., 2003) and in 2009 we selected 66 villages using a census from our previous work in the area (Reyes-García et al., 2012). In both years we selected villages with different levels of distance from a main road or market town (Figure 1). In communities with 10 or less households we surveyed all the households; in communities with 11 to 40 households we randomly selected 10 households from a list provided by the highest-ranking authority, and in communities larger than 40 households we randomly selected 25% of the households. To adhere to cultural norms, within a household we interviewed the male household head (n=867) and the female (n=304) when the male was not available. During our 2009 visit to villages previously visited in 2000, we also sought the informants interviewed in 2000, though we were able to locate only a fraction of them. As several authors have suggested that the accumulation of traditional knowledge likely peaks in the late teens (e.g., Zarger, 2002), we restricted our analysis to people aged 20 years or older. Our final sample consists of 1151 individuals: 473 Tsimane’ adults from 58 villages interviewed in 2000 and 678 Tsimane’ adults from 66 villages interviewed in 2009. Forty-two villages were visited twice and 135 people were interviewed in both surveys.
Figure 1.
Map of study area with Tsimane’ villages categorized by year of the survey (non-surveyed, 2000, 2008-2009, or both) and by remoteness (close, medium, and remote).
Measure of traditional knowledge
We estimated an individual’s traditional knowledge using a test designed to quantify individual knowledge of wild plants uses. We used previous work in the area (Reyes-García et al., 2006) to identify cultural domains that correspond to emic semantic constructs that bear psychological reality for the Tsimane’. The specific cognitive domains identified correspond to five types of plant uses that seemed to be culturally valid: plants that can be used as medicine, food, firewood, canoe and house building.
We used responses to a free-listing exercise (n=50) on Tsimane’ useful plants to randomly select 20 plants for further evaluation. We constructed a multiple choice questionnaire with 20 plants and 5 plant uses to capture informant’s knowledge of 100 plant use reports. All survey respondents were asked to report on whether each plant could be used for each of the identified domains of plant uses. Multiple uses per plant were recorded (Reyes-García et al., 2005). Our measure of traditional knowledge consists of the sum of all possible distinctive uses (i.e., several medicinal uses of a plant are only counted as one – medicinal) of the plants in our questionnaire across the five categories of use. The same exact test was used in years 2000 and 2009.
Demographic survey
To capture individuals’ characteristics that might affect levels of traditional knowledge we conducted a demographic survey amongst all study participants. Participants were asked to estimate their age in years. As many adults did not know their exact age they guessed, thereby introducing a random measurement error in the age variable. We used the survey year and the participants’ self-reported age to create dummy variables for seven cohorts or birth decades (1920s to 1980s, both inclusive). Because several authors have argued that education affects the acquisition of traditional knowledge (Sternberg et al., 2001), we also included a control for the maximum education level of the person. Informant’s ability to communicate in Spanish (the language spoken by non-Tsimane’ in the area and a proxy for acculturation) was assessed at the time of the interview and included in our model as a dummy variable that took the value of 1 if the person could communicate fluently in Spanish and 0 otherwise.
The measure of traditional knowledge and the survey were developed after extensive ethnographic observation. In 1999-2000, prior to the first round of data collection, the instruments were tested during an 18-month stay in two communities with different levels of market integration. To ensure uniformity, the lead author trained interviewers in both survey rounds. We entered data into a database and conducted standard data cleaning procedures.
Data analysis
To analyze changes across decades of birth and survey years, for each cohort we run a t-test comparing data collected in 2000 and 2009. We tested whether observed changes were the result of randomness (i.e., copying or cultural drift processes (Boyd & Richerson, 1985; Hahn & Bentley, 2003; Herzog et al., 2004) by running Monte Carlo simulations with two indices: the mean number of plant uses known per individual and the diversity of plant uses, as defined by Neiman (1995) and Pérez-Losada & Fort (2011). While the measure of plant uses reported portrays changes across individuals, it does not reflect to what extent the knowledge is shared. Two samples may have the same mean but substantially differ as regards the number of specialized individuals and hence the diversity of knowledge.
To compute Monte Carlo simulations we first created a population about 20 times bigger than the initial population sampled in 2000 (on the assumption that about 5% of the total population was surveyed). Random changes were executed in the plant uses reports of every individual, with a probability defined at the beginning of the simulation. We calculated the diversity and mean plant uses reported per individual from a 5% of the virtual population and compared the results with data from the 2009 survey. As the probability of random cultural change was unknown, we repeated the process 10,000 times for 20 randomness values from 0 to 100% with lags of 0.05 (0.05, 0.10, …).
We also tested whether the observed changes were associated to informants’ proximity to market towns, a proxy for changes in economic, social, and environmental conditions. To do so, we first created an index of remoteness by adapting the formula proposed by Eisenberg et al. (2006). For each village we calculated the linear distance to the nearest market town using GPS readings collected at the village center. We also recorded the monetary cost of traveling to the nearest market town. We used those two values to calculate a rank of remoteness (Ri) for each village i, by averaging normalized values of distance (Di) and cost (Ci). Specifically,
where values close to 0 indicate proximity to a market town. In statistical analysis we used our index of remoteness in two different ways, as a continuous variable and as a categorical variable with three values (“close”, “medium”, and “remote”), each including 33% of the villages in the sample.
To estimate changes in the number of plant uses reported by informants over the two survey years, we used a multivariate Ordinary Least Square regression model with the logarithm of the number of plant uses reported as the outcome variable and a dummy variable that took the value of 1 if the survey was conducted in the year 2009 as the main explanatory variable. As the outcome variable is in logarithms, the coefficient of the variable year 2009 can be read as the percentage change in plant use occurring during the two survey years (2000 and 2009). We use the coefficient of the variable year 2009 to estimate the annual rate of change. Regressions included individual-level controls for a) the sex of the person answering to the survey, b) the person’s age in years and the square of the variable age to control for non-linearity in the relation between age and traditional knowledge, c) the maximum school grade attained, d) the ability to speak Spanish, and e) a dummy variable that captures whether the person was interviewed in one or both survey rounds. Our model also includes two types of village-level controls. First, we used our remoteness index as a control. Second, we also included a set of dummy variables to control for village of residency. We also used village dummy variables to control for village attributes that most likely remained fix during 2000-2009 (e.g., physical infrastructure, resources availability). We clustered observations by village of residency to relax the assumption that individual observations are independent across villages. The procedure adjusts for the fact that individuals might share more knowledge with other people living close to them and provides robust (and more conservative) estimates of variance around regression parameters. In additional analysis, we a) substituted the variable age by our seven decade of birth dummy variables and b) included both variables (age and decade of birth) in the model. Models including age, birth cohort, and survey period are largely used in economics, sociology, and public health to study secular changes (Borjas 2005; Fienberg and Mason 1979; Rodgers 1982), as they allow one to estimate the effect of time while controlling for age and cohort effects – the first referring to the accumulation of knowledge over the life cycle (or aging) and the second to changes in knowledge between birth cohorts (Godoy et al., 2009a). We used STATA for the statistical analyses and R for the Monte Carlo simulations.
Results
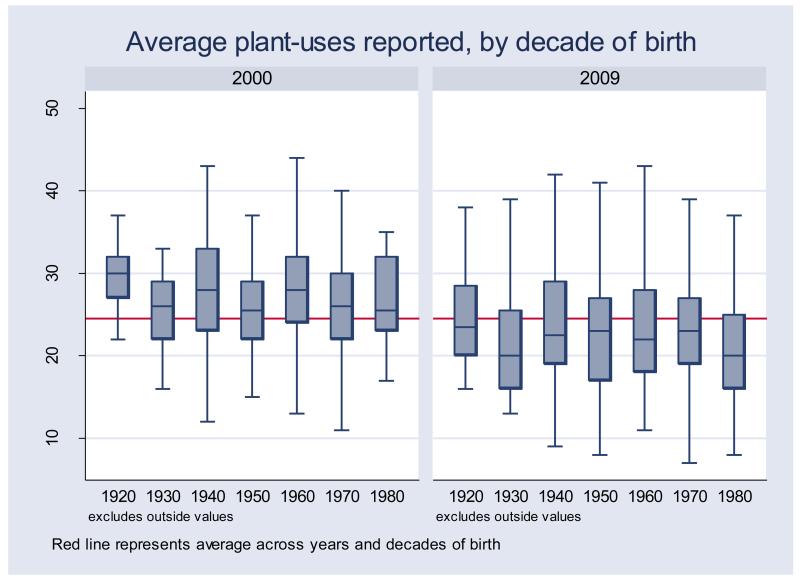
For every decade of birth, people who completed the survey in 2000 had higher scores than people who completed the survey in 2009 (Figure 2). The average number of plant uses reported by informants in 2000 was 27.28 (SD = 6.54, N = 476), whereas the average number of plant uses reported in 2009 was 22.62 (SD = 6.75, N = 678). For every decade of birth, differences were significant in a t-test comparison of means (P < 0.05). The largest difference was found among people born in the 1980s (t-value = 6.09, SE= 1.45), and the smallest among people born during the 1950s (t-value = 3.1, SE = 1.23). Data do not suggest a linear decrease in the number of plant uses reported across decades of birth either in the 2000 or in the 2009 samples. For example, in the 2000 survey, the average number of plant uses reported was lower in the 1930s cohort than in the 1940s cohort. The average number of plant uses reported was also lower in the 1950s cohort than in the 1960s cohort.
Figure 2.
Average number of plant uses reported by Tsimane’ adults (> 20 years of age, n=1151), by birth cohort and survey year.
Random copying processes
According to our simulations, the chance of obtaining a decrease in the mean number of plant uses reported that is similar to the one perceived between the 2000 and the 2009 surveys is almost non-existent (p < 0.0001 for observed results, Figure 3). For the diversity index, the comparison between observed data and simulation results also suggests that the chance of attaining a decrease in the diversity index that is similar to the one observed with our survey data is almost non-existent (p < 0.0001 for observed results, Figure 4).
Figure 3.
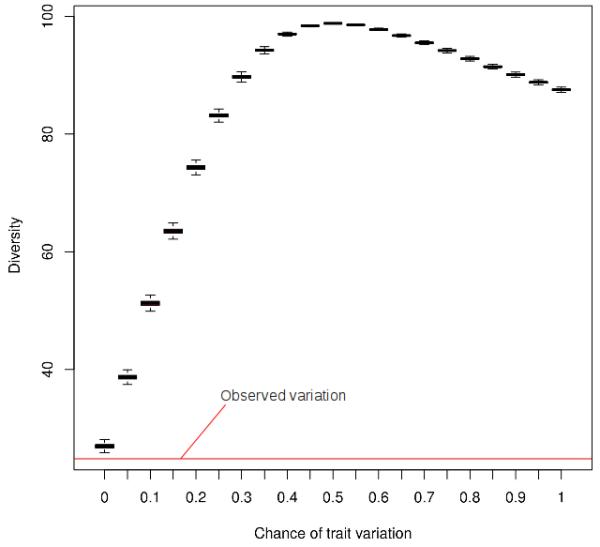
Boxplot depicting the variation of diversity on Monte Carlo Carlo simulations in relation to real observed variation. The figure shows the diversity distribution for each tested variation probability (from 0 to 1), as well as the 2009 observed variation (red line).
Figure 4.
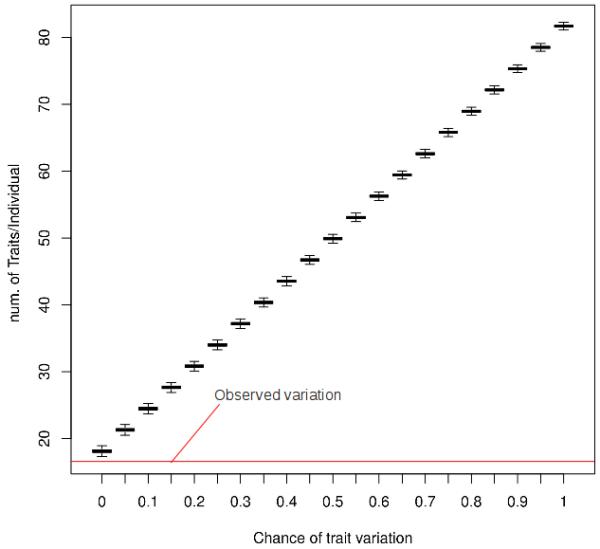
Boxplot depicting the variation of number of traits known per individual on Monte Carlo Carlo simulations in relation to real observed variation. The figure shows the number of traits/individual distribution for each tested variation probability (from 0 to 1), as well as the 2009 observed variation (red line).
Plant uses and remoteness
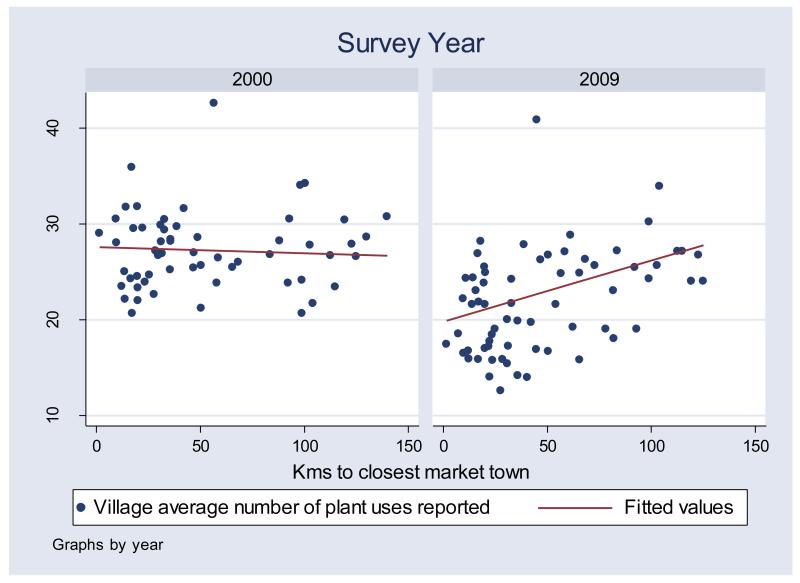
In both surveys the average number of plant uses reported was largest in remote villages (Table 1). For each remoteness category, the average number of plant uses reported in 2000 was higher than the average number of plant uses reported in 2009 (all differences were statistically significant in a t-test comparison of means P =< 0.003). Differences in close villages were larger than differences in remote villages (6.67 more plant uses reported in 2000 than in 2009 in close villages versus 4.28 in remote villages). A Pearson correlation between the index of village remoteness and the average number of plant uses reported showed a positive correlation in 2009 (r = 0.3, N=66, P = 0.01) but not in 2000 (r = 0.119, N = 58, P = 0.373) (Figure 5).
Table 1.
Sample size, distance to the closes market town, and average number of plant uses reported, by year of survey and remoteness
| Remoteness category |
Villages (n) |
Participants (n) |
Average distance to closest market town (Km) |
Average plant uses reported (%) |
||||
|---|---|---|---|---|---|---|---|---|
| Year: | 2000 | 2009 | 2000 | 2009 | 2000 | 2009 | 2000 | 2009 |
| Close | 16 | 25 | 149 | 270 | 12.52 | 14.23 | 26.44 | 19.77 |
| Medium | 19 | 22 | 157 | 250 | 32.23 | 31.07 | 27.13 | 22.80 |
| Remote | 23 | 19 | 167 | 158 | 58.78 | 53.39 | 27.98 | 23.69 |
| Overall | 58 | 66 | 473 | 678 | 37.32 | 31.12 | 27.28 | 21.91 |
Figure 5.
Relation between village remoteness and average number of plant uses reported, by survey year.
Rate of change
Results from Ordinary Least Square multivariate regressions with the logarithm of the number of plant uses reported as outcome variable and a dummy variable for year 2009 as the main explanatory variable suggest that during 2000-2009 the Tsimane’ experienced a significant decrease in the number of plant uses reported (Table 2). Irrespective of the remoteness of the settlement and of the individuals’ age, sex, schooling, Spanish fluency, and participation in one or both surveys, people interviewed in 2009 reported approximately 20% less plant uses than people interviewed in 2000 (p < 0.001, 95% Confidence Interval = [−0.293 to −0.114]). Since the variable year 2009 captures a nine-year period, assuming a linear change, our estimation suggests that the number of plant uses reported decreased by ~2.2%/year. This association was not altered if we substituted age by decades of birth (Model 2) nor if we included both variables: age and decade of birth (Model 3), although the coefficient of the variable year 2009 slightly decreased. From the control variables only remoteness showed a consistent and positive association with the number of plant uses reported.
Table 2.
Results of Ordinary Least Square models predicting the rate of change on the logarithm of the number of plant uses reported.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coef | CI | Coef | CI | Coef | CI | |
| Year 2009 | −0.204*** | (−0.293, −0.114) | −.181*** | (−0.267, −0.094) | −.203*** | (−0.308, −0.099) |
| Age | 0.009** | (0.003, 0.015) | ^ | ^ | .008 | (0.002, 0.018) |
| Age square | −0.0001* | (−.0001, −0.00001) | ^ | ^ | −.00001 | (−0.0002, 0.00005) |
| Male | 0.035 | (0.010, 0.080) | .035 | (0.009, 0.079) | .035 | (0.009, 0.079) |
| Maximum education level | 0.007 | (−0.001, 0.014) | .007 | (−0.0004, 0.014) | .007* | (−0.0002, 0.014) |
| Spanish | 0.039 | (−0.013, 0.090) | .037 | (−0.012, 0.087) | .036 | (−0.013, 0.086) |
| Remoteness | 0.118*** | (0.087, 0.149) | .119*** | (0.086, 0.152) | .118*** | (0.084, 0.151) |
| Interviewed twice | 0.005 | (−0.044, 0,054) | .001 | (−0.047, 0,049) | .001 | (−0.049, 0,047) |
| Decade of birth (1920 excluded category) | ||||||
| 1930s | ^ | ^ | −.0.094 | (−0.228, 0.039) | −.0.099 | (−0.258, 0.059) |
| 1940s | ^ | ^ | −.0.032 | (−0.171, 0.107) | −.0.037 | (−0.265, 0.190) |
| 1950s | ^ | ^ | −.0.071 | (−0.184, 0.042) | −.0.064 | (−0.318, 0.191) |
| 1960s | ^ | ^ | −.0.047 | (−0.158, 0.063) | −.0.012 | (−0.315, 0.291) |
| 1970s | ^ | ^ | −.0.077 | (−0.195, 0.040) | −.0.005 | (−0.352, 0.341) |
| 1980s | ^ | ^ | −.0.153** | (−0.273, −0.034) | −.0.046 | (−0.423, 0.332) |
| R2 | 0.418 | 0.421 | 0.423 | |||
| N | 1151 | 1151 | 1151 | |||
Coef: Coefficient, CI: Confidence Interval.
P ≤ 0.05
P ≤ 0.01
P≤ 0.001. Ordinary least squares regressions with clustering by village of residency.
=variable intentionally omitted.
To test whether those results were an artifact of methodological differences between both surveys (i.e., sampling strategy or more inquisitive enumerators in one of the rounds), we did three additional robustness tests. Robustness models resemble the regression model presented in Table 2, except for the changes explained below. First, we run the regressions for the subsamples of men and women (Table 3, Rows [1] and [2]). In both models the variable year 2009 shows the expected negative sign, but the coefficients differ in magnitude and statistical significance. Men interviewed in 2009 reported approximately 22% less plant uses than men interviewed in 2000 (p<0.001), whereas women interviewed in 2009 reported only about 9% less plant uses than women interviewed in 2000. Furthermore, differences in the female subsample were not statistically significant. Second, we next ran our model using only the subsample of people who were interviewed only once (Row [3]) and then using only the subsample of people who were interviewed during both survey years (n = 135*2 = 270) (Row [4]). We observe a 24% decrease in the number of plant uses reported by people interviewed once (p < 0.001), but only a 12% decrease by people who were interviewed in the two survey rounds (p < 0.001, 95% CI = [−0.214 to −0.033]).
Table 3.
Robustness analysis: Results of Ordinary Least Square models predicting the rate of change on the logarithm of the number of plant uses reported.
| Model | Subsample | Coef | 95% CI | R2 | N |
|---|---|---|---|---|---|
| [1] | Males | −.224*** | (−0.319, −0.129) | 0.51 | 849 |
| [2] | Females | −.092 | (−0.211, 0.027) | 0.32 | 302 |
| [3] | People interviewed once | −.245*** | (−0.362,−0.127) | 0.47 | 881 |
| [4] | People interviewed twice | −.123*** | (−0.213, −0.033) | 0.24 | 270 |
| [5] | Close villages’ residents | −.262*** | (−0.412, −0.113) | 0.48 | 419 |
| [6] | Medium distance villages’ residents | −.207*** | (−0.353, −0.061) | 0.39 | 407 |
| [7] | Remote villages’ residents | −.117 | (−0.310, 0.075) | 0.32 | 325 |
Coef: Coefficient of the variable Year 2009, CI: Confidence Interval.
P≤ 0.001. Ordinary least squares regressions with clustering by village of residency. Regressions include the same controls reported in Table 2.
In our last robustness test we separated the sample in the three remoteness categories (Table 3, Rows [5]-[7]). People living in villages close to market towns reported 26% less plant uses in 2009 than people living in similar villages did in 2000 (P < 0.001), representing a decline of ~3%/year. The difference was lower (21%, P < 0.001) for people living in villages in the medium category, and lowest and statistically 5 non-significant (12%, P = 0.221) for people living in remote villages.
Discussion
We organize the discussion around the two main theoretical questions that motivated this research: 1) what is the rate of change of traditional knowledge in a contemporary indigenous society? and 2) what could be the drivers of such a change? We conclude by addressing the contributions of this work to cultural evolution theory and to policy-making.
Rate of change
Our results suggest that between 2000 and 2009, Tsimane’ adults experienced a net decrease in the report of plant uses ranging from 9% (for the female subsample) to 26% (for the subsample of people living close to towns), equivalent to a 1% to 3 % per year. As those figures suggest, changes do not equally affect the sampled population: changes were more acute for men than for women and for informants living in villages close to market towns than for informants settled in remote villages.
We do not have similar data on rates of cultural change from other contemporary indigenous societies. Recent phylogenetic work investigating groups belonging to the Tupi language family in lowland South America suggests that, although there is a historical trend towards losing cultural features, cultural change has historically been very slow, on the order of only a few changes per 10,000 years (Walker et al. 2012). The findings presented here are not directly comparable to phylogenetic work, as our unit of analysis (number of wild plant uses reported) is less specific that the cultural traits examined in Walker’s et al work (e.g. traditional warfare patterns, post-marital residence, presence of canoes, tattooing). However, it seems plausible to argue that the rate of change found in this work largely surpasses estimates derived from the study of past societies, which raises the issue of the drivers of change.
Drivers of change
Results from a Monte Carlo simulation show that the observed changes were not the result of randomness. When changes occur as a result of random copying, the probability of acquiring a new cultural trait for a given population is similar to the probability of losing one already known (as defined by Enquist et al., 2011). Since changes observed in our data during the studied period of time exceed by far the variation of knowledge that could be expected to occur due to random drift, we could reject the hypothesis that random variations of knowledge are responsible for the observed decrease in the number of plant uses reported. The observed changes in plant uses reported could be then explained as a consequence of Tsimane’ adaptation to the new economic, social, political, and environmental conditions influencing them.
The Tsimane’ remained relatively isolated until the 1970s but they have since undergone a rapid process of acculturation and integration to the market economy that has rapidly transformed their economy and society (Godoy et al., 2009b). Over the last decades, the Tsimane’ have increasingly engaged in wage labor and cash cropping (Reyes-García et al., 2012) and have replaced many plant-made items with market goods (Godoy et al., 2007). Recent changes, such as the establishment of schools or the election of political representatives to defend their interest, have also affected the traditional learning processes and the traditional leadership role of elders (Reyes-García et al., 2008b). Furthermore, the arrival to the area of colonist farmers, logging companies, illegal loggers and cattle ranchers has also resulted in fast environmental degradation over the last few decades, especially in areas close to market towns (Gueze et al., 2012). Thus, Tsimane’ adaptation to those new socio-economic, political and environmental conditions (new economic activities, learning system, political representatives, legal autonomy, and an increasingly degraded-environment) are likely to explain the observed changes in plant uses know.
This explanation also fits well with the fact that changes in levels of traditional knowledge do not affect equally to the whole studied population. For example, the fact that changes are more acute among men than among women meshes well with the previous explanation, as Tsimane’ men are much more likely to abandon their home villages in search of new economic activities than Tsimane’ women. The explanation also fits well with the sharper decrease of knowledge in villages closer to market-towns than in remote villages, as people in remote villages continue to depend on their traditional knowledge to sustain their livelihoods, whereas people in closer villages have different economic opportunities and a more degraded environment that is becoming increasingly unsuitable for their traditional livelihood activities.
Contributions to cultural evolution theory
The research presented here provides more than an empirical case study on rates and drivers of cultural change among contemporary indigenous societies. At the methodological level, our research adds to the tool-kit of methods used to study cultural change, specifically to those commonly utilized by anthropological field-workers interested in cultural evolution. At the theoretical level, results from our research provide insights into individual-level processes that are responsible for population-level patterns of cultural change. Researchers have argued that cultural change occurs through different individual- and group-level innovation processes, different types of social learning processes, and different cultural transmission processes (Mesoudi et al. in press). Within this context, researchers have argued that new cultural variants can occur within one generation through individual innovation (Cavalli-Sforza and Feldman 1981), but less attention has been paid to loss of cultural traits within a generation. Our analysis of a subsample of people interviewed twice indicates that, under rapidly changing socio-economic, political, and environmental conditions, cultural loss can occur within a single generation, and not only during the transmission process, as one generation neglects to transmit knowledge to the next generation as it has been previously argued (Aunger, 2000b; Casagrande, 2002; Gomez-Baggethun et al., 2010; Reyes-García et al., 2009).
Policy implications
Because traditional knowledge contributes to conservation (Gadgil et al., 1993; Gomez-Baggethun et al., 2010; Turner & Berkes, 2006), provides multiple benefits to the holders of such knowledge (e.g., McDade et al. 2007), and partially encodes human cultural diversity (Maffi, 2005), researchers and policy makers have shown increasing concern for its loss. The research presented here provides a first estimate of the rate at which this process occurs in a contemporary indigenous society. Findings from this work might not be directly transferable to other societies but, as many contemporary indigenous societies are exposed to changing conditions similar to those described here (Godoy et al., 2005), our estimates could be taken as an indication of the general trend of cultural knowledge loss among contemporary indigenous societies.
Our research suggests that the Tsimane’, and probably other contemporary indigenous societies, could be abandoning their traditional knowledge as they perceive that this form of knowledge do not equip them well to deal with the new socio-economic and cultural conditions they face nowadays. Policy-makers interested in the well-being of indigenous peoples should then assess what other forms of knowledge are substituting traditional knowledge and to what degree how those other forms of knowledge actually contribute to improve the quality of life of contemporary indigenous peoples. Policy makers should also be concerned about the apparent parallel decline of the world’s biological (Sutherland, 2003; Thomas et al., 2004), linguistic (Harmon & Loh, 2010), and cultural diversity.
Acknowledgments
NSF-Anthropology, the BBVA Foundation (BIOCON_06_106-07), and the ERC (FP7-261971-LEK) funded the research. Rubio-Campillo acknowledges financial support of a CONSOLIDER-INGENIO2010 (CSD2010-00034) and Reyes-García acknowledges logistical support of Resilient Dryland Systems, ICRISAT. We thank the Tsimane’ for their patience and J. Boesch, K. Demps, R. Godoy, E. Gomez-Bagetthun, O. Heffetz., G. Shively, J. van den Berg, and two anonymous reviewers for comments to previous versions.
References
- Aoki K, Lehmann L, Feldman MW. Rates of cultural change and patterns of cultural accumulation in stochastic models of social transmission. Theor. Popul. Biol. 2011;79:192–202. doi: 10.1016/j.tpb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Aunger R, editor. Darwinizing culture. Oxford University Press; Oxford: 2000a. [Google Scholar]
- Aunger R. The life history of culture learning in a face-to-face society. Ethos. 2000b;28(2):1–38. [Google Scholar]
- Bentley RA, Hahn MW, Shennan SJ. Random drift and culture change. Proceedings of the Royal Society B-Biological Sciences. 2004;271(1547):1443–1450. doi: 10.1098/rspb.2004.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz BF, Cevallos J, Santana F, Rosales J, Graf S. Losing knowledge about plant use in the sierra de Manantlan Biosphere Reserve, Mexico. Economic Botany. 2000;54(2):183–191. [Google Scholar]
- Berkes F, Colding J, Folke C. Rediscovery of traditional ecological knowledge as adaptive management. Ecological Applications. 2000;10(5):1251–1262. [Google Scholar]
- Borjas GJ. Labor economics. third ed. McGraw-Hill; New York: 2005. [Google Scholar]
- Boyd R, Richerson P. Culture and the Evolutionary Process. University of Chicago Press; Chicago: 1985. [Google Scholar]
- Boyd R, Richerson P. The origin and evolution of cultures. Oxford University Press; Oxford: 2005. [Google Scholar]
- Casagrande DG. Ecology, Cognition, and Cultural Transmission of Tzeltal Maya Medicinal Plant Knowledge. University of Georgia; 2002. [Google Scholar]
- Cavalli-Sforza LL, Feldman MW. Cultural transmission and evolution. Princeton University Press; Princeton: 1981. [PubMed] [Google Scholar]
- Demps K, Zorondo-Rodriguez F, García C, Reyes-García V. Social learning across the lifecycle: Cultural knowledge acquisition for honey hunting among the Jenu Kuruba, India. Evolution and Human Behavior. 2012;33(5):460–470. [Google Scholar]
- Di Cosmo N. Ancient China and its enemies: the rise of nomadic power in East Asian history. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Eisenberg JNS, Cevallos W, Ponce K, Levy K, Bates SJ, Scott JC, Hubbard A, Vieira N, Endara P, Espinel M, Trueba G, Riley LW, Trostle J. Environmental change and infectious disease: How new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(51):19460–19465. doi: 10.1073/pnas.0609431104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist M, Ghirlanda S, Eriksson K. Modelling the evolution and diversity of cumulative culture. Philosophical Transactions of the Royal Society B-Biological Sciences. 2011;1563:412–423. doi: 10.1098/rstb.2010.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg SE, Mason WM. Identification and estimation of age-period-cohort models in the analysis of discrete archival data. Sociological Methodology. 1979;10:1–67. [Google Scholar]
- Gadgil M, Berkes F, Folke C. Indigenous Knowledge for Biodiversity Conservation. Ambio. 1993;22(2-3):151–156. doi: 10.1007/s13280-020-01478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy R, Reyes-García V, Broesch J, Fitzpatrick IC, Giovarmini P, Rodriguez MRM, Jha N, Huanca T, Leonard WR, McDade TW, Tanner S, TAPS Bolivia Study Team Long-Term (Secular) Change of Ethnobotanical Knowledge of Useful Plants Separating Cohort and Age Effects. Journal of Anthropological Research. 2009a;65(1):51–67. [Google Scholar]
- Godoy R, Reyes-García V, Byron E, Leonard WR, Vadez V. The effect of market economies on the well-being of indigenous peoples and on their use of renewable natural resources. Annual Review of Anthropology. 2005;34:121–138. [Google Scholar]
- Godoy R, Reyes-García V, Gravlee CC, Huanca T, Leonard WR, McDade TW, Tanner S. Moving beyond a Snapshot to Understand Changes in the Well-Being of Native Amazonians Panel Evidence (2002-2006) from Bolivia. Current Anthropology. 2009b;50(4):560–570. [Google Scholar]
- Godoy R, Reyes-García V, Huanca T, Leonard WR, McDade T, Tanner S, Seyfried C. Signaling by consumption in a native Amazonian society. Evolution and Human Behavior. 2007;28(2):124–134. [Google Scholar]
- Gomez-Baggethun E, Mingorria S, Reyes-García V, Calvet L, Montes C. Traditional Ecological Knowledge Trends in the Transition to a Market Economy: Empirical Study in the Donana Natural Areas. Conservation Biology. 2010;24(3):721–729. doi: 10.1111/j.1523-1739.2009.01401.x. [DOI] [PubMed] [Google Scholar]
- Gueze M, Paneque-Galvez J, Luz AC, Pino J, Orta-Martínez M, Reyes-García V, Macía M. Determinants of tree species turnover in a southern Amazonian rainforest. Journal of Vegetation Science. 2012 DOI: 10.1111/j.1654-1103.2012.01461.x. [Google Scholar]
- Hahn MW, Bentley RA. Drift as a mechanism for cultural change: an example from baby names. Proceedings of the Royal Society B-Biological Sciences. 2003;270:S120–S123. doi: 10.1098/rsbl.2003.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon D, Loh J. The Index of Linguistic Diversity: A New Quantitative Measure of Trends in the Status of the World’s Languages. Language Documentation and Conservation. 2010;4:97–151. [Google Scholar]
- Herzog HA, Bentley RA, Hahn MW. Random drift and large shifts in popularity of dog breeds. Proceedings of the Royal Society B-Biological Sciences. 2004;271:S353–S356. doi: 10.1098/rsbl.2004.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J. Demography and cultural evolution: How adaptive cultural processes can produce maladaptive losses - The Tasmanian case. American Antiquity. 2004;69:197–214. [Google Scholar]
- Henrich J, Henrich N. The evolution of cultural adaptations: Fijian food taboos protect against dangerous marine toxins. Proc. R. Soc. B. 2010;277:3715–3724. doi: 10.1098/rspb.2010.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonti M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. Journal of Ethnopharmacology. 2011;134:542–555. doi: 10.1016/j.jep.2011.01.017. [DOI] [PubMed] [Google Scholar]
- McDade T, Reyes-García V, Leonard W, Tanner S, Huanca T. Maternal ethnobotanical knowledge is associated with multiple measures of child health in the Bolivian Amazon. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6134–6139. doi: 10.1073/pnas.0609123104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace R, Holden CJ, Shennan S. The Evolution of Cultural Diversity: A Phylogenetic Approach. Left Coast Press; Walnut Creek, California: 2005. [Google Scholar]
- Maffi L. Endangered languages, endangered knowledge. International Social Science Journal. 2002;54(173):385–393. [Google Scholar]
- Maffi L. Linguistic, Cultural, and Biological Diversity. Annual Review of Anthropology. 2005;34:599–618. [Google Scholar]
- Martin G. Stasis in complex artefacts. In: Ziman J, editor. Technological Innovation as an Evolutionary Process. Cambridge University Press; Cambridge: 2000. pp. 90–100. [Google Scholar]
- Mesoudi A, Laland KN, Boyd R, Buchanan B, Flynn E, Garrod S, McCauley RN, Renn J, Reyes-García V, Shennan S, Stout D, Tennie C. The Cultural Evolution of Technology and Science. In: Richersond P, editor. Cultural evolution. In press. [Google Scholar]
- Neiman FD. Stylistic Variation in Evolutionary Perspective: Inferences from Decorative Diversity and Interassemblage Distance in Illinois Woodland Ceramic Assemblages. American Antiquity. 1995;60(1):7–36. [Google Scholar]
- Perales HR, Benz BF, Brush SB. Maize diversity and ethnolinguistic diversity in Chiapas, Mexico. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):949–954. doi: 10.1073/pnas.0408701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Losada J, Fort J. Spatial dimensions increase the effect of cultural drift. Journal of Archaeological Science. 2011;38(6):1294–1299. [Google Scholar]
- Reyes-García V, Broesch J, Calvet-Mir L, Fuentes-Pelaez N, McDade TW, Parsa S, Tanner S, Huanca T, Leonard WR, Martinez-Rodriguez MR. Cultural transmission of ethnobotanical knowledge and skills: an empirical analysis from an Amerindian society. Evolution and Human Behavior. 2009;30(4):274–285. [Google Scholar]
- Reyes-García V, Godoy R, Vadez V, Apaza L, Byron E, Huanca T, Leonard WR, Pérez E, Wilkie D. Ethnobotanical knowledge shared widely among Tsimane’ Amerindians, Bolivia. Science. 2003;299(5613):1707–1707. doi: 10.1126/science.1080274. [DOI] [PubMed] [Google Scholar]
- Reyes-García V, Huanca T, Vadez V, Leonard W, Wilkie D. Cultural, practical, and economic value of wild plants: A quantitative study in the Bolivian Amazon. Economic Botany. 2006;60(1):62–74. [Google Scholar]
- Reyes-García V, Ledezma JC, Paneque-Galvez J, Orta M, Gueze M, Lobo A, Guinard D, Huanca T, Luz AC, TAPS Bolivia Study Team Presence and purpose of non-indigenous peoples on indigenous lands. A descriptive account from the Bolivian Lowlands. Society & Natural Resources. 2012;25(3):270–284. [Google Scholar]
- Reyes-García V, Molina JL, Broesch J, Calvet L, Huanca T, Saus J, Tanner S, Leonard WR, McDade TW, TAPS study team Do the aged and knowledgeable men enjoy more prestige? A test of predictions from the prestige-bias model of cultural transmission. Evolution and Human Behavior. 2008b;29(4):275–281. [Google Scholar]
- Reyes-García V, Vadez V, Byron E, Apaza L, Leonard WR, Perez E, Wilkie D. Market economy and the loss of folk knowledge of plant uses: Estimates from the Tsimane’ of the Bolivian Amazon. Current Anthropology. 2005;46(4):651–656. [Google Scholar]
- Richerson PJ, Boyd R, Bettinger RL. Was agriculture impossible during the Pleistocene but mandatory during the Holocene? A climate change hypothesis. American Antiquity. 2001;66:387–411. [Google Scholar]
- Rodgers WL. Estimable functions of age, period, and cohort effects. American Sociological Review. 1982;47:774–87. [Google Scholar]
- Rogers E. The diffusion of innovations. Free Press; New York: 1995. [Google Scholar]
- Rogers DS, Ehrlich PR. Natural selection and cultural rates of change. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3416–3420. doi: 10.1073/pnas.0711802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg R, Nokes C, Geissler P, Prince R, Okatcha F, Bundy D, Grigorenko E. The relationship between academic and practical intelligence: a case study in Kenya. Intelligence. 2001;29:401–418. [Google Scholar]
- Sutherland WJ. Parallel extinction risk and global distribution of languages and species. Nature. 2003;423(6937):276–279. doi: 10.1038/nature01607. [DOI] [PubMed] [Google Scholar]
- Tehrani JJ, Collard M. On the relationship between interindividual cultural transmission and population-level cultural diversity: a case study of weaving in Iranian tribal populations. Evolution and Human Behaviour. 2009;30:286–300. [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, de Siqueira MF, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE. Extinction risk from climate change. Nature. 2004;427(6970):145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Turner NJ, Berkes F. Developing resource management and conservation. Human Ecology. 2006;34(4):475–478. [Google Scholar]
- Vadez V, Reyes García V, Godoy R, Leonard W, Huanca T, Byron E. Income diversification of rural households: What role for agriculture? Household evidence from the Tsimane’ Amerindians of the Bolivian Amazon. Human Organization. 2008;67(4):384–396. [Google Scholar]
- Walker RS, Wichmann S, Mailund T, Atkisson CJ. Cultural Phylogenetics of the Tupi Language Family in Lowland South America. PLoS ONE. 2012;7(4):e35025. doi: 10.1371/journal.pone.0035025. doi:10.1371/journal.pone.0035025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarger R. Acquisition and Transmission of Subsistence Knowledge by Q’eqchi’ Maya in Belize. In: Stepp JR, Wyndham FS, Zarger R, editors. Ethnobiology and Biocultural Diversity. International Society of Ethnobiology; Athens, GA: 2002. pp. 592–603. [Google Scholar]