Abstract
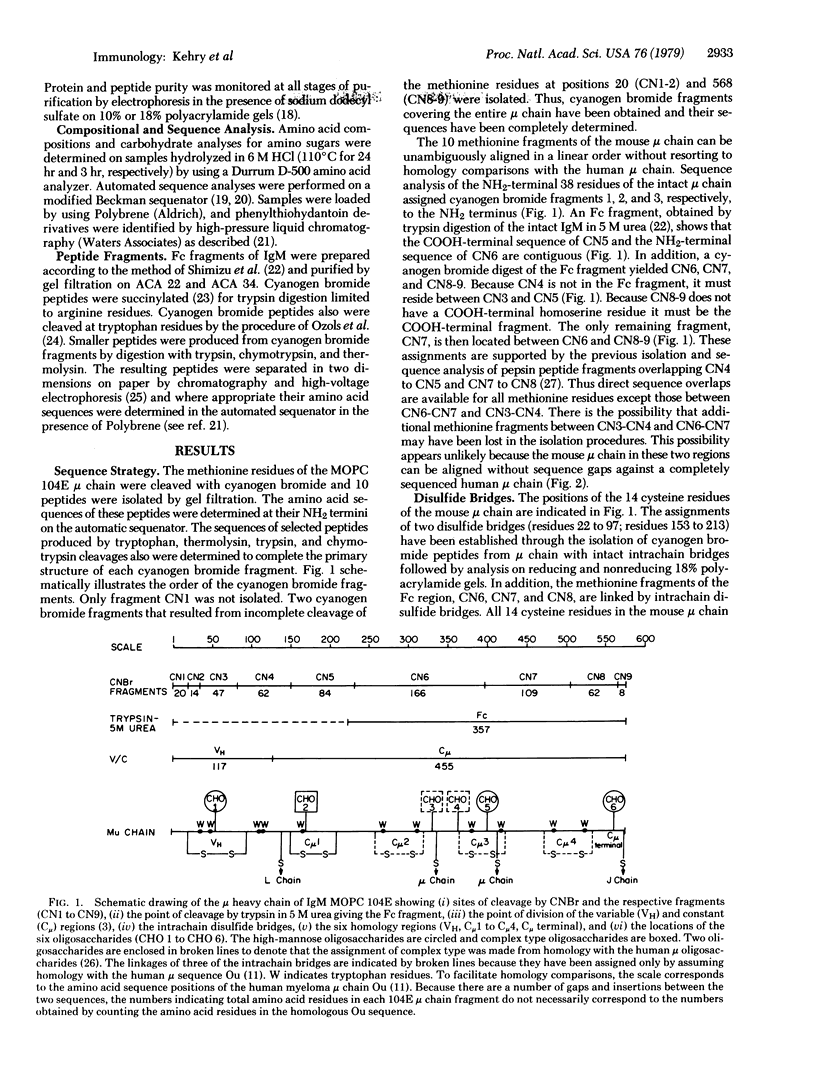
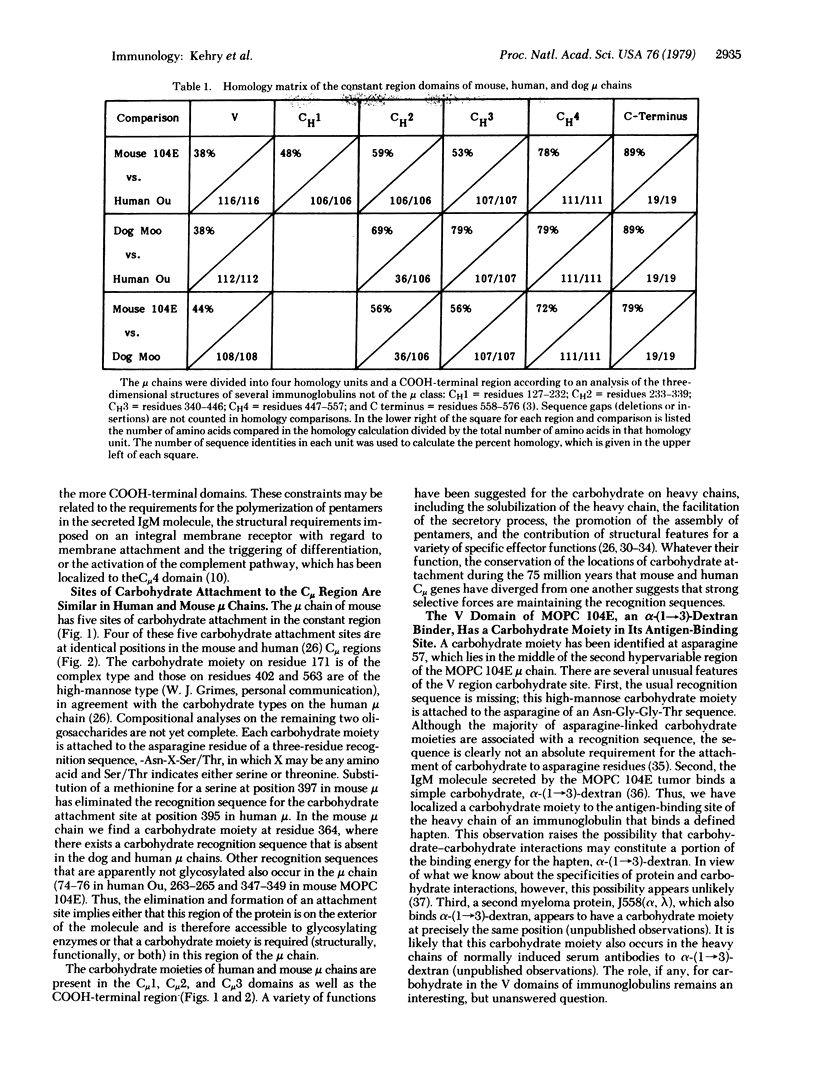
The complete amino acid sequence of the mouse mu chain from the BALB/c myeloma tumor MOPC 104E is reported. The C mu region contains four consecutive homology regions of approximately 110 residues and a COOH-terminal region of 19 residues. A comparison of this mu chain from mouse with a complete mu sequence from human (Ou) and a partial mu chain sequence from dog (Moo) reveals a striking gradient of increasing homology from the NH2-terminal to the COOH-terminal portion of these mu chains, with the former being the least and the latter the most highly conserved. Four of the five sites of carbohydrate attachment appear to be at identical residue positions when the constant regions of the mouse and human mu chains are compared. The mu chain of MOPC 104E has a carbohydrate moiety attached in the second hypervariable region. This is particularly interesting in view of the fact that MOPC 104E binds alpha-(1 leads to 3)-dextran, a simple carbohydrate. The structural and functional constraints imposed by these comparative sequence analyses are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Lafleur L., Melchers IgM in bone marrow-derived lymphocytes. Synthesis, surface deposition, turnover and carbohydrate composition in unstimulated mouse B cells. Eur J Immunol. 1974 Mar;4(3):170–180. doi: 10.1002/eji.1830040305. [DOI] [PubMed] [Google Scholar]
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D., Feinstein A. Structure and function of the constant regions of immunoglobulins. Q Rev Biophys. 1976 May;9(2):135–180. doi: 10.1017/s0033583500002390. [DOI] [PubMed] [Google Scholar]
- Guidotti G. The structure of intrinsic membrane proteins. J Supramol Struct. 1977;7(3-4):489–497. doi: 10.1002/jss.400070318. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Crumpton M. J. Immunoglobulin of T lymphoma cells is an integral membrane protein. Nature. 1974 Dec 13;252(5484):602–604. doi: 10.1038/252602a0. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Hurst M. M., Volanakis J. E., Stroud R. M., Bennett J. C. C1 fixation and classical complement pathway activation by a fragment of the Cmu4 domain of IgM. J Exp Med. 1975 Nov 1;142(5):1322–1326. doi: 10.1084/jem.142.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M., DREYER W. J., ANFINSEN C. B. Peptide separation by two-dimensional chromatography and electrophoresis. J Biol Chem. 1959 Nov;234:2897–2900. [PubMed] [Google Scholar]
- Kennel S. J., Lerner R. A. Isolation and characterization of plasma membrane associated immunoglobulin from cultured human diploid lymphocytes. J Mol Biol. 1973 Jun 5;76(4):485–502. doi: 10.1016/0022-2836(73)90487-7. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawton A. R., Kincade P. W., Cooper M. D. Sequential expression of germ line genes in development of immunoglobulin class diversity. Fed Proc. 1975 Jan;34(1):33–39. [PubMed] [Google Scholar]
- Leon M. A., Young N. M., McIntire K. R. Immunochemical studies of the reaction between a mouse myeloma macroglobulin and dextrans. Biochemistry. 1970 Feb 17;9(4):1023–1030. doi: 10.1021/bi00806a043. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. Conservatism in the evolution of immunoglobulin. Nat New Biol. 1972 Mar 22;236(64):84–86. doi: 10.1038/newbio236084a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Richardson N. E., Feinstein A. Evidence for a C-terminal tyrosine residue in human and mouse B-lymphocyte membrane mu chains. Nature. 1978 Apr 6;272(5653):555–557. doi: 10.1038/272555a0. [DOI] [PubMed] [Google Scholar]
- Melcher U., Eidels L., Uhr J. W. Are immunoglobulins integral membrane proteins? Nature. 1975 Dec 4;258(5534):434–435. doi: 10.1038/258434a0. [DOI] [PubMed] [Google Scholar]
- Melchers F. Difference in carbohydrate composition and a possible conformational difference between intracellular and extracellular immunoglobulin M. Biochemistry. 1972 May 23;11(11):2204–2208. doi: 10.1021/bi00761a031. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Schrohenloher R. E. Site of attachment of J chain to human immunoglobulin M. Nature. 1974 Jun 14;249(458):650–652. doi: 10.1038/249650a0. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Zikan J., Butler W. T. Immunoglobulin M and secretory immunoglobulin A: presence of a common polypeptide chain different from light chains. Science. 1971 Mar 19;171(3976):1163–1165. doi: 10.1126/science.171.3976.1163. [DOI] [PubMed] [Google Scholar]
- Milstein C. P., Richardson N. E., Dieverson E. V., Feinstein A. Interchain disulphide bridges of mouse immunoglobulin M. Biochem J. 1975 Dec;151(3):615–624. doi: 10.1042/bj1510615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Gerard C. Cleavage of tryptophanyl peptide bonds in cytochrome b5 by cyanogen bromide. J Biol Chem. 1977 Sep 10;252(17):5986–5989. [PubMed] [Google Scholar]
- Parkhouse R. M., Melchers F. Biosynthesis of the carbohydrate portions of immunoglobulin M. Biochem J. 1971 Nov;125(1):235–240. doi: 10.1042/bj1250235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Florent G., Paul C., Shinoda T., Shimizu A. Complete amino acid sequence of the Mu heavy chain of a human IgM immunoglobulin. Science. 1973 Oct 19;182(4109):287–291. doi: 10.1126/science.182.4109.287. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. Changes in the three-dimensional structure of concanavalin A upon demetallization. Proc Natl Acad Sci U S A. 1978 May;75(5):2286–2290. doi: 10.1073/pnas.75.5.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Putnam F. W., Paul C., Clamp J. R., Johnson I. Structure and role of the five glycopeptides of human IgM immunoglobulins. Nat New Biol. 1971 May 19;231(20):73–76. doi: 10.1038/newbio231073a0. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Watanabe S., Yamamura Y., Putnam F. W. Tryptic digestion of immunoglobulin M in urea: conformational lability of the middle part of the IgM molecule. Immunochemistry. 1974 Nov;11(11):719–727. doi: 10.1016/0019-2791(74)90271-7. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Baur S., Uhr J. W. Cell surface immunoglobulin. II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. J Exp Med. 1971 Jul 1;134(1):242–264. doi: 10.1084/jem.134.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh F. S., Crumpton M. J. Orientation of cell-surface antigens in the lipid bilayer of lymphocyte plasma membrane. Nature. 1977 Sep 22;269(5626):307–311. doi: 10.1038/269307a0. [DOI] [PubMed] [Google Scholar]
- Warner N. L. Membrane immunoglobulins and antigen receptors on B and T lymphocytes. Adv Immunol. 1974;19(0):67–216. doi: 10.1016/s0065-2776(08)60252-7. [DOI] [PubMed] [Google Scholar]
- Wasserman R. L., Capra J. D. Amino acid sequence of the Fc region of a canine immunoglobulin M: interspecies homology for the IgM class. Science. 1978 Jun 9;200(4346):1159–1161. doi: 10.1126/science.653360. [DOI] [PubMed] [Google Scholar]
- Wasserman R. L., Capra J. D. Primary structure of the variable regions of two canine immunoglobulin heavy chains. Biochemistry. 1977 Jul 12;16(14):3160–3168. doi: 10.1021/bi00633a019. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Williams P. B., Kubo R. T., Grey H. M. mu-Chains from a nonsecretor B cell line differ from secreted mu-chains at the C-terminal end. J Immunol. 1978 Dec;121(6):2435–2439. [PubMed] [Google Scholar]
- Wittmann-Liebold B. Amino acid sequence studies on ten ribosomal proteins of Escherichia coli with an improved sequenator equipped with an automatic conversion device. Hoppe Seylers Z Physiol Chem. 1973 Oct-Nov;354(10-11):1415–1431. doi: 10.1515/bchm2.1973.354.2.1415. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Graffunder H., Kohls H. A device coupled to a modified sequenator for the automated conversion of anilinothiazolinones into PTH amino acids. Anal Biochem. 1976 Oct;75(2):621–633. doi: 10.1016/0003-2697(76)90117-2. [DOI] [PubMed] [Google Scholar]


