Abstract
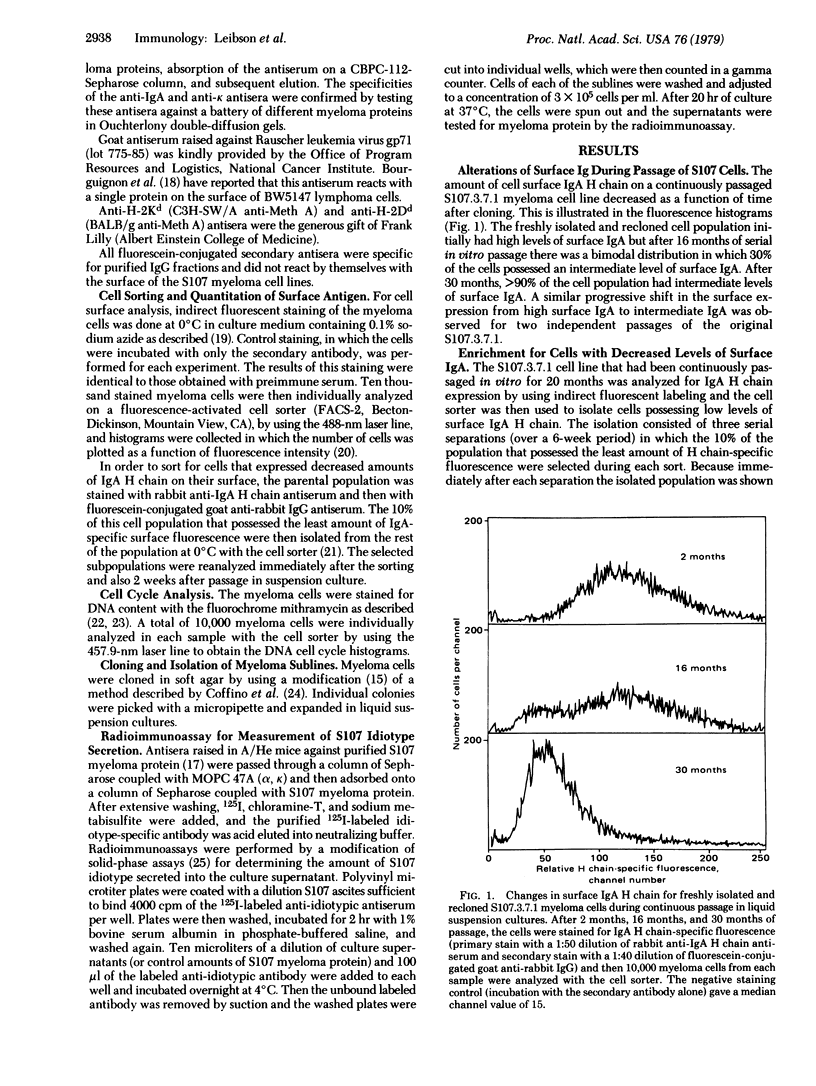
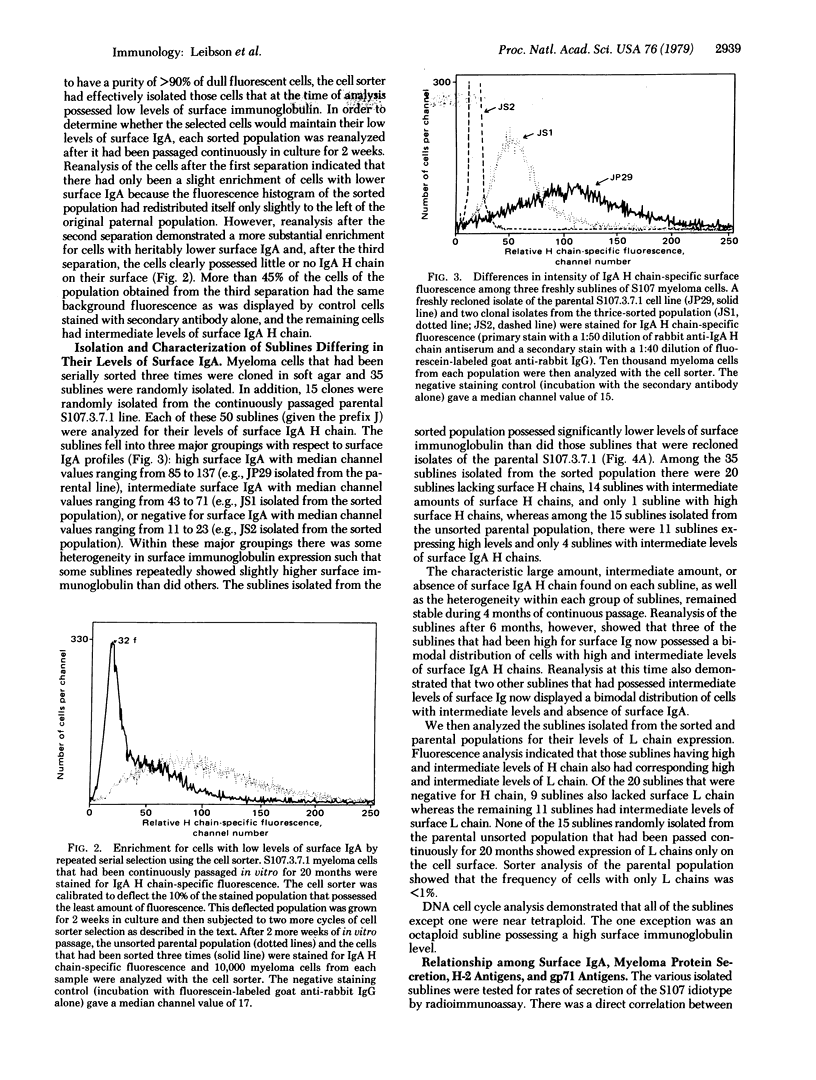
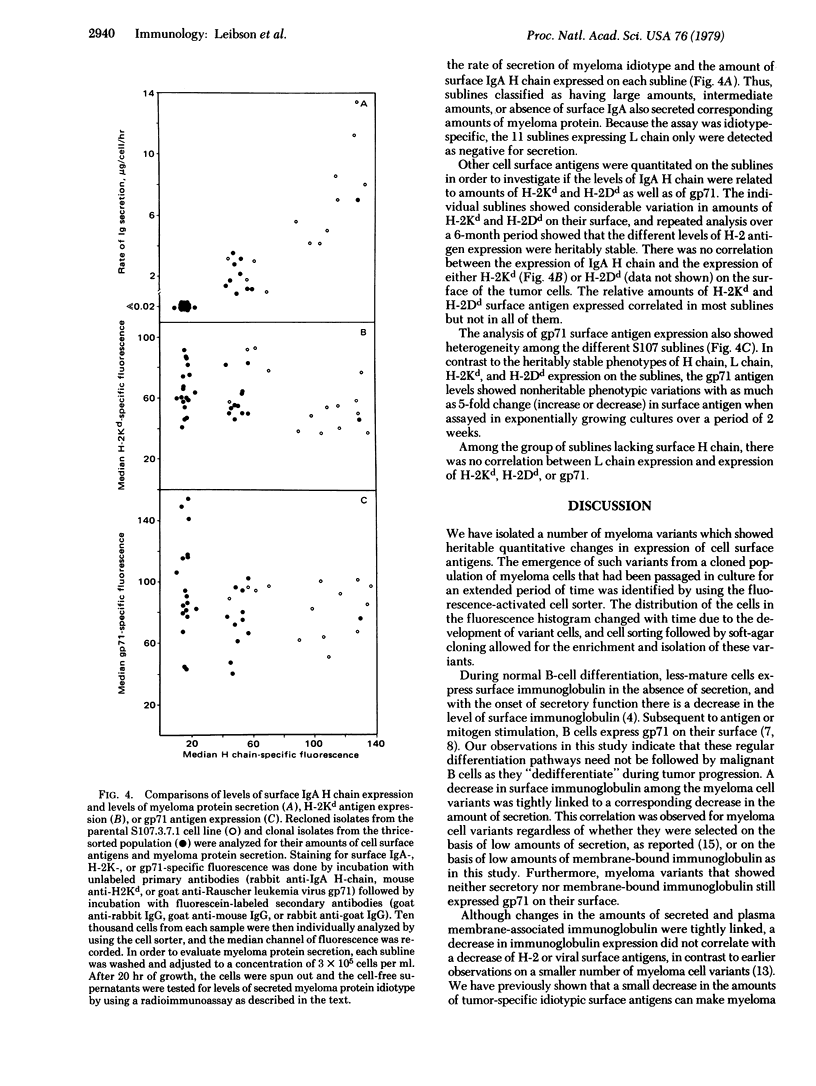
We report that a cloned population of tumor cells can rapidly produce variants that differ in their quantitative expression of surface proteins and in their rate of immunoglobulin secretion. A fresh clonal isolate of S107 myeloma cells possessing large amounts of surface IgA was continuously passaged in vitro for 2 years. During this period, fluorescence-activated cell sorter analysis indicated the development of subpopulations possessing decreased amounts of surface IgA. Cells from these variant subpopulations were isolated by first using the cell sorter to enrich for cells with decreased amounts of surface IgA and then cloning the selected population in soft agar. The 50 sublines that were isolated showed heritable differences in their levels of surface IgA and H-2 antigens and in their rates of myeloma protein secretion. Sublines having either large amounts, intermediate amounts, or absence of surface IgA also had corresponding large amounts, intermediate amounts, or absence of myeloma protein secretion. In contrast, a decrease or loss of surface Ig did not correlate with a decrease or loss of viral envelope glycoprotein gp71 and H-2 antigens. The variants did not resemble the phenotypes of less-differentiated normal lymphocyte populations of the B-cell lineage. The isolation and characterization of these variants allows us to explore the mechanisms and pathways of tumor cell differentiation as well as to study the regulation and function of cell surface proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Andersson J., Buxbaum J., Citronbaum R., Douglas S., Forni L., Melchers F., Pernis B., Stott D. IgM-producing tumors in the BALB-c mouse: a model for B-cell maturation. J Exp Med. 1974 Sep 1;140(3):742–763. doi: 10.1084/jem.140.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Bonner W. A., Hulett H. R., Sweet R. G., Herzenberg L. A. Fluorescence activated cell sorting. Rev Sci Instrum. 1972 Mar;43(3):404–409. doi: 10.1063/1.1685647. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Hyman R., Trowbridge I., Singer S. J. Participation of histocompatibility antigens in capping of molecularly independent cell surface components by their specific antibodies. Proc Natl Acad Sci U S A. 1978 May;75(5):2406–2410. doi: 10.1073/pnas.75.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. Mutations affecting adenine phosphoribosyl transferase activity in Chinese hamster cells. Cell. 1974 May;2(1):37–41. doi: 10.1016/0092-8674(74)90006-3. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Crissman H. A., Tobey R. A. Cell-cycle analysis in 20 minutes. Science. 1974 Jun 21;184(4143):1297–1298. doi: 10.1126/science.184.4143.1297. [DOI] [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars R. Sex Chromatin Patterns and the Lyon Hypothesis. Science. 1963 Aug 16;141(3581):649–650. doi: 10.1126/science.141.3581.649. [DOI] [PubMed] [Google Scholar]
- Hyman R., Ralph P., Sarkar S. Cell-specific antigens and immunoglobulin synthesis of murine myeloma cells and their variants. J Natl Cancer Inst. 1972 Jan;48(1):173–184. [PubMed] [Google Scholar]
- Jones G. E., Sargent P. A. Mutants of cultured chinese hamster cells deficient in adenine phosphoribosyl transferase. Cell. 1974 May;2(1):43–54. doi: 10.1016/0092-8674(74)90007-5. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E., Haughton G. Variation of antigenic characteristics between different mouse lymphomas induced by the Moloney virus. J Natl Cancer Inst. 1966 Apr;36(4):607–621. doi: 10.1093/jnci/36.4.607. [DOI] [PubMed] [Google Scholar]
- Leibson P. J., Schreiber H., Loken M. R., Panem S., Rowley D. A. Time-dependent resistance or susceptibility of tumor cells to cytotoxic antibody after exposure to a chemotherapeutic agent. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6202–6206. doi: 10.1073/pnas.75.12.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Dennert G. Effect of antigen density on complement-mediated lysis, T-cell-mediated killing, and antigenic modulation. J Natl Cancer Inst. 1974 Dec;53(6):1759–1765. [PubMed] [Google Scholar]
- Loken M. R., Herzenber L. A. Analysis of cell populations with a fluorescence-activated cell sorter. Ann N Y Acad Sci. 1975 Jun 30;254:163–171. doi: 10.1111/j.1749-6632.1975.tb29166.x. [DOI] [PubMed] [Google Scholar]
- MOLLER E., MOLLER G. Quantitative studies of the sensitivity of normal and neoplastic mouse cells to the cytotoxic action of isoantibodies. J Exp Med. 1962 Mar 1;115:527–553. doi: 10.1084/jem.115.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Delovitch T. L., Press J. L. Functional and genetic analysis of Ia antigens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):489–496. doi: 10.1101/sqb.1977.041.01.056. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Okumura K., Herzenberg L. A., Herzenberg L. A., McDevitt H. O. Selective expression of separate I-region loci in functionally different lymphocyte subpopulations. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):497–504. doi: 10.1101/sqb.1977.041.01.057. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Lewis H. Variation in accessible cell surface immunoglobulin among antibody-firming cells. J Exp Med. 1972 Jun 1;135(6):1416–1422. doi: 10.1084/jem.135.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Klinman N. R. Allogeneic carrier-specific enhancement of hapten-specific secondary B-cell responses. J Exp Med. 1976 Nov 2;144(5):1254–1262. doi: 10.1084/jem.144.5.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan T. V., Nathenson S. G., Scharff M. D. Regulatory variants for the expression of H-2 antigens. I. Isolation and characterization. J Natl Cancer Inst. 1976 Jun;56(6):1221–1227. doi: 10.1093/jnci/56.6.1221. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Seligmann M. B-cell neoplasia in man. Lancet. 1974 Nov 23;2(7891):1230–1233. doi: 10.1016/s0140-6736(74)90748-x. [DOI] [PubMed] [Google Scholar]
- Schreiber H., Leibson P. Suppression of myeloma growth in vitro by anti-idiotypic antibodies: inhibition of DNA synthesis and colony formation. J Natl Cancer Inst. 1978 Jan;60(1):225–233. doi: 10.1093/jnci/60.1.225. [DOI] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Immunosuppressive activity of antibody directed against endogenous C-type virus interferes with early events of the immune response. J Immunol. 1978 Jun;120(6):1913–1916. [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriel J. Cancer, retrodifferentiation, and the myth of Faust. Cancer Res. 1976 Nov;36(11 Pt 2):4269–4275. [PubMed] [Google Scholar]
- Watson J., Ralph P., Sarkar S., Cohn M. Leukemia viruses associated with mouse myeloma cells. Proc Natl Acad Sci U S A. 1970 Jun;66(2):344–351. doi: 10.1073/pnas.66.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T. Expression of MuLV GP71-like antigen in normal mouse spleen cells induced by antigenic stimulation. Nature. 1977 Oct 13;269(5629):598–600. doi: 10.1038/269598a0. [DOI] [PubMed] [Google Scholar]
- Weitzman S., Margulies D. H., Scharff M. D. Mutations in mouse myeloma cells: implications for human multiple myeloma and the production of immunoglobulins. Ann Intern Med. 1976 Jul;85(1):110–116. doi: 10.7326/0003-4819-85-1-110. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. The concept that surveillance of self is mediated via the same set of genes that determines recognition of allogenic cells. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):505–510. doi: 10.1101/sqb.1977.041.01.058. [DOI] [PubMed] [Google Scholar]


