Abstract
The use of specific terms under different meanings and varying definitions has always been a source of confusion in science. When we point our efforts towards an evidence based medicine for inflammatory bowel diseases (IBD) the same is true: Terms such as “mucosal healing” or “deep remission” as endpoints in clinical trials or treatment goals in daily patient care may contribute to misconceptions if meanings change over time or definitions are altered. It appears to be useful to first have a look at the development of terms and their definitions, to assess their intrinsic and context-independent problems and then to analyze the different relevance in present-day clinical studies and trials. The purpose of such an attempt would be to gain clearer insights into the true impact of the clinical findings behind the terms. It may also lead to a better defined use of those terms for future studies. The terms “mucosal healing” and “deep remission” have been introduced in recent years as new therapeutic targets in the treatment of IBD patients. Several clinical trials, cohort studies or inception cohorts provided data that the long term disease course is better, when mucosal healing is achieved. However, it is still unclear whether continued or increased therapeutic measures will aid or improve mucosal healing for patients in clinical remission. Clinical trials are under way to answer this question. Attention should be paid to clearly address what levels of IBD activity are looked at. In the present review article authors aim to summarize the current evidence available on mucosal healing and deep remission and try to highlight their value and position in the everyday decision making for gastroenterologists.
Keywords: Inflammatory bowel disease, Mucosal healing, Deep remission, Treatment targets, Clinical activity
Core tip: “Mucosal healing” and “deep remission” have been discussed heavily as “new” treatment goals in inflammatory bowel diseases patients in recent years. This was based on evidence that the long term disease behaviour appears to be better, when mucosal healing is achieved. Unfortunately, a definite proof that therapy escalation for patients in clinical remission not achieving mucosal healing will be beneficial is still lacking. Clinical trials are under way to answer this question. At the moment it appears to be helpful to summarize the current evidence available on mucosal healing and deep remission to support the everyday decision making for gastroenterologists.
INTRODUCTION
Assessing the activity of inflammatory bowel disease (IBD) is important for our daily practice treating patients with these chronic inflammatory diseases. The assessment of disease activity will guide our therapeutic decision and our choice of medication. Furthermore it is most important for clinical investigations of new treatment options and new drugs. The reduction of disease activity remains the most important endpoint in clinical trials.
However, the discussion on which parameters are most useful for this purpose is still ongoing and unresolved.
Assessment of activity of IBD can be performed on different levels such as clinical activity, biochemical activity (e.g. by measuring CRP or fecal calprotectin), endoscopy, and histology. Clinical remission in a given IBD patient does not necessarily imply biochemical, endoscopic, or histologic remission. To evaluate biochemical, endoscopic, and histologic activity, an increasing degree of invasive measures (blood sample, endoscopy, biopsies) is required. Assessing activity in IBD has thereby analogies to the iceberg phenomenon where the clinical assessment on the surface may show clinical remission, but inflammatory activity may still be present on biochemical, endoscopic, and histologic level (Figure 1).
Figure 1.
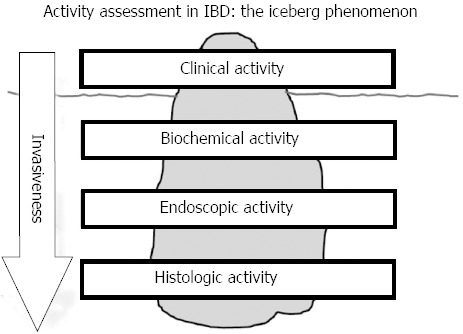
Activity assessment in inflammatory bowel disease: The iceberg phenomenon. IBD: inflammatory bowel disease.
HISTOLOGICAL REMISSION AS INITIAL DEFINITION OF MUCOSAL HEALING
One of the first scientists and clinicians that used the term “healing” or “mucosal healing” within the field of IBD was Burton I. Korelitz, past chief of the Division of Gastroenterology at Lenox Hill Hospital in New York[1]. However, he used this term exclusively with respect to histological changes of the mucosa[1]. So when the term “mucosal healing” was introduced into IBD clinic it meant the absence of histological alterations of the mucosa. Korelitz was well aware that healing of IBD is not regarded to be possible as both Crohn’s disease (CD) and ulcerative colitis (UC) are regarded to be chronic diseases without spontaneous healing[2]. There may be an absence of symptoms and flares over years but mucosal inflammation may re-occur after remission for years or even decades (Figure 1).
Histological healing is difficult to determine especially in Crohn’s disease as the inflammation may be patchy and a biopsy could miss an inflammatory infiltrate only a few millimeters away[3]. Similarly, in UC the histological evaluation of a biopsy may be misleading[4]. Histological alterations may be absent from the rectum and sigmoid due to effective topical therapy despite the presence of inflammation further proximal in the colon that may not be obvious to the endoscopist[4,5]. Histological healing would mean that we have to be sure that there had been an inflammatory infiltrate at a specific localization that completely disappeared upon therapy (or spontaneously). As is obvious this is hard or even impossible to prove as this would require frequent endoscopies with many biopsy samples and a labeling of former biopsy locations. Due to the impracticability of this approach the overall acceptance of the concept of “histological healing” was very limited[5]. Of note, newer techniques such as endomicroscopy suffer from the same shortcomings.
ENDOSCOPIC REMISSION AS A NEW CONCEPT FOR MUCOSAL HEALING
In contrast to the initial concept of “mucosal healing” as a “disappearance of inflammatory infiltrate”[2] recent original manuscripts and reviews on the topic have used the term under different meanings. The “newer” meanings of “mucosal healing” have been summarized again by Korelitz in a critical review[2]. One of the “newer meanings” of mucosal healing would be the absence of inflammation (“healed mucosa”) to the eye of the endoscopist, a definition that now has been applied in many clinical trials[6-16].
There is an obvious problem with this definition. One must assume the location of endoscopically normal mucosa has previously been inflamed[2]. Certainly this is easier to assess with endoscopy rather than histology as the area of evaluation is larger and small local differences and a patchy pattern would play a less important role. Nevertheless it requires that two endoscopical examinations are compared.
The definition also ignores that in endoscopically normal appearing mucosa there still may be histological inflammation. Another problem of this definition of course is that the inter-observer reproducibility of endoscopical IBD scores usually is very poor[17] and depends on the experience of the endoscopist[18] regardless of the technique used[19,20] (it may be discussed whether a kappa between 0.7 and 0.8 is satisfying). Usually endoscopic findings are assessed on fixed point scales or described by dichotomous variables (present/absent)[18,21]. However, as outlined by de Lange and colleagues “endoscopic features of mucosal inflammation are continuous variables” for which dichotomous decisions are artificial and always require individual decisions[18]. The question arises how to interpret endoscopical findings indicating a clearly improved appearance of the mucosa in endoscopy with some or few remaining scattered erosions. A further important question arises with respect to endoscopical findings that cannot be interpreted as present inflammation but as residuals of former inflammation and a lack of complete normalization of the mucosa. Such findings would be pseudopolyps in an otherwise normal-appearing colon.
BIOCHEMICAL (FECAL MARKERS) REMISSION AS MUCOSAL HEALING
Fecal markers such as calprotectin or lactoferrin correlate very well with the degree and extent of infiltration of the mucosa by leukocytes. A good correlation between fecal calprotectin and the Crohn’s Disease Endoscopic Index of Severity (CDEIS) was reported in several studies[22,23]. There is also a good correlation of fecal calprotectin with the Simple Endoscopic Score for Crohn’s disease (SES-CD) which itself has a strong correlation with the CDEIS (correlation coefficient r = 0.920) and an excellent inter-observer reliability (κ coefficients 0.791-1.000)[24].
In ulcerative colitis calprotectin correlates well with disease activity as determined by histology and endoscopy[25,26].
It is a familiar experience to endoscopists that the mucosa may appear completely normal (healed) in patients that still have a markedly elevated fecal calprotectin. This would be an endoscopic remission but not biochemical remission, most likely reflecting a lack of histological remission with neutrophils still being present in the mucosal wall. It has been well established that calprotectin better correlates with histological findings (at least in UC) as compared to serum parameters or endoscopy[27-29].
MUCOSAL HEALING AND DEEP REMISSION: THE CONFUSED CLINICIAN
Surprisingly, some recent trials have reported a higher relative amount of patients with mucosal healing compared to the percentage of patients with clinical remission, especially in UC[30]. In those trials usually the endoscopist defined whether mucosal healing was present. How can this be explained? One reason could be that those patients had concomitant irritable bowel syndrome that was responsible for their complaints but no relevant remaining inflammation (“IBS superimposed on IBD”). The argument is straight forward and logical but it probably does not explain all cases. Firstly, little or no information is available on the histological remission in those patients. Histological remission - if evaluated by biopsies - again may be patchy and the evaluated biopsies may not be representative. Damage to deeper layers of the mucosa may have occurred that are not visible to the endoscopist’s eye. Therefore is has to be challenged whether healed mucosa to the eye of the endoscopist is indeed the “most satisfying objective confirmation to support the clinical response” as outlined by Korelitz[2]. As he states the endoscopic healing “might be satisfactory for comparison in time for response to therapy in an individual case, but not for mucosal healing as an entity and certainly not to be used as an index of response to therapy in trials.”[2]
To minimize the subjective component many clinical trials now apply the principle of a “central reader”. Not only does this make trials more complicated, more expensive and more time consuming. It substitutes the problem of a bias introduced by many subjective evaluations of the mucosal response to a bias introduced by one subjective interpretation of findings. The intra-observer agreement for many endoscopic scores is not satisfactory. It may well be argued that the subjective criteria used by a central reader may not be accepted by others and that there could be a reduction of bias by a “multi-subjective” view (as we assume is the case for multicenter trials as compared to monocentric studies). Of note, in a recent randomized-controlled trial in patients with UC the conclusion was significantly changed after blinded central review of endoscopic images, suggesting that central reading of endoscopy may be necessary for regulatory purposes[31]. However, the question about the best method of objective endoscopic assessment is far from being answered.
Korelitz[2] suggested that histological healing should be the “minimal criterion for mucosal healing and preferably this information should be derived from multiple biopsy sites of previous inflammation”. However, this would implicate that the evaluation of inflammation by a pathologist is objective. There have been studies on the inter-observer and intra-observer agreement of pathology findings[32]. Those results are not very encouraging. When a number of established criteria were used (excess of histiocytes in combination with a villous or irregular aspect of the mucosal surface and granulomas) experienced pathologists could correctly classify 70% of CD patients and 75% of UC patients[32]. Especially in mild disease, there is still dispute as to whether the presence of a “physiological (minor) inflammation” should be regarded as manifestation of IBD or not. Clinically unaffected siblings of IBD patients may show mild histological inflammation and increased cellular activation markers[33]. Cell counting will not solve the problem. The request for a “central pathology reader” also is not helpful as the same dilemma as for the central endoscopy reader will occur. Moreover, different pathologists have suggested different criteria to evaluate the presence or absence of “un-normal” inflammation (for an overview see[3,34-37]. There is no agreement on that. Geboes for example suggested that the presence of neutrophils in the intestinal epithelium is an important discriminator for the presence or absence of inflammation. He therefore suggested that a combination of endoscopy and histology should be used to evaluate the presence of inflammation in IBD patients to finally judge whether mucosal healing has been achieved (see above).
MUCOSAL HEALING AND DEEP REMISSION: THE CONFUSED SCIENTIST
CD and UC are regarded to be chronic diseases that never disappear. The concept of a healing of a part of the body affected by such a disease subsequently is surprising for scientists working on the elucidation of the pathophysiology of IBD.
However, there is another aspect that is disturbing. There have been reports that even in macroscopically and microscopically normal appearing mucosa specific changes can be found that are characteristic for inflammation or at least changes that could be associated with the pathophysiology[38-45].
Changes of the microbiota in the lumen of the gut have been described in IBD patients despite the absence of detectable inflammation[46-51]. Could a “complete deep remission” be possible without normalization of the intestinal microbiome? The mucus layer of the mucosa may be changed also in normal appearing mucosa in endoscopy[52-56]. The normal fixation procedure of biopsies and the subsequent H&E staining does not allow evaluation of the mucus layer as it is destroyed during this procedure. A reduced thickness of the mucus layer in UC in remission has been described[54,56,57] as well as a reduced secretion of mucin[52,53,58-60] or defensins[61-64]. The question arises whether the mucosa can be termed as “normal” or “healed” if those changes are still present.
Epithelial cells may have an impaired barrier function despite a lack of inflammatory signs. Cytokine expression and cytokine secretion by immune cells may still be significantly increased despite a normal appearing histology. A normalization of those changes has been termed biochemical healing[65-68]. There are no data available with respect to the predictive value of “biochemical healing” and whether this would correlate to a more favorable disease outcome.
The confused scientist, however, is able to imagine a further level of “healing”. In macroscopically normal appearing mucosa with microscopically normal appearing cells that display normal cytokine expression and secretion levels, epigenetic changes may still be present that may trigger pathological responses upon minor stimuli[69-76]. Can a persistence of epigenetic changes in otherwise normal mucosa be termed “mucosal healing”? Or do we have to achieve “epigenetic healing” to finally achieve the best outcome possible for our patients? These questions will have to be answered in the future. Currently we are just at the start of investigations into these aspects with the first interesting pieces of the puzzle being put together.
MUCOSAL HEALING AND DEEP REMISSION: THE CONFUSED "TRIALIST"
As mentioned above the terms “mucosal healing” and “deep remission” have been used in a number of trials with quite different meanings and definitions. The key confounder is the lack of unequivocal definition(s). Therefore, results and data from those trials with respect to mucosal healing cannot easily be compared. Nevertheless, this is done frequently. In most cases endoscopical investigation is used for the evaluation of “mucosal healing”. One crucial point is whether “mucosal healing” was defined simply as the absence of ulcers when ulcers had been seen previously or whether the absence of ulcerations and ulcers was investigated exactly at a place where those alterations had been found before.
The above is reflected in the way different trials have been reported. In the ACCENT 1 endoscopic sub-study the CDEIS was used for scoring and the complete absence of mucosal ulcerations that were observed at baseline was evaluated[77]. In the SONIC study in contrast no clearly defined score was used. Mucosal healing was defined as “complete absence of mucosal ulceration in the colon and terminal ileum”[78]. In the “Top-down versus step up” study by Gert D’Haens and coworkers SES-CD was used for the evaluation of mucosal healing which was a secondary endpoint[79,80]. Mucosal healing was defined as “absence of ulcers”. In the MUSIC trials again the CDEIS was applied. The definition of mucosal healing was “absence of ulcers and endoscopic remission defined as CDEIS < 6”. In the EXTEND study applying again SES-CD mucosal healing was seen as “absence of mucosal ulceration”[81]. As is obvious from those definitions, the question arises whether a few remaining aphthous lesions in a patient with severe and deep ulcers at the beginning of therapy also may be termed mucosal healing.
For UC the IOIBD attempted a consensus for mucosal healing in 2007: “absence of friability, blood, erosions and ulcers in all visualized segments of the gut mucosa”. According to the IOIBD experts the presence of an abnormal vascular pattern is still compatible with mucosal healing or “normal mucosa”. However, also in UC the definitions applied varied widely: In the ACT1 study mucosal healing was a secondary endpoint[82,83]. The Mayo endoscopic subscore was used and mucosal healing was defined as “absolute subscore for endoscopy of 0 or 1”[82,83]. The same definition was used for ULTRA 2[84].
In studies on the outcome of therapy with 5-aminosalicylic acid the definition of mucosal healing largely defined the number of patients achieving this endpoint (Table 1). As an example, Vecchi et al[85] compared mesalazine 4 g orally vs 2 + 2 g orally and enema in 2001 in patients with a clinical activity index (CAI) of 4-12 and used an endoscopic Rachmilewitz index < 4 as definition of mucosal healing leading to 58% vs 71% of patients achieving this endpoint[85]. In 2002 Malchow compared Mesalazine 4g enema vs 1g foam preparation in patients with a CAI > 4 for 4 wk and applied an endoscopic Rachmilewitz index < 2 as definition of mucosal healing leading to rates of 38% vs 37%[86]. As one would expect, the different definitions used cause huge variation in defined endoscopic mucosal healing rates in patients with UC, which makes the comparison of efficacy of different drugs or formulations extremely difficult.
Table 1.
Association between the definitions of remission and mucosal healing and actual healing rates in patients with ulcerative colitis treated with mesalazine
| Author | Design | Study | Timing of endoscopy | Endoscopic index | Def. of MH | No of pat. Achieving MH |
| Vecchi (2001) | Mc, RCT | Mesalazine 4 g orally vs 2 + 2 g orally and enema | 6 wk | Rachmilewitz | Rachmilewitz < 4 | 58% vs 71% |
| Malchow (2002) | Mc, db, RCT | Mesalazine 4 g enema vs 1 g foam | 4 wk | Rachmilewitz | Rachmilewitz < 2 | 38% vs 37% |
| Mansfield (2002) | Mc, db, RCT | Balsalazide 6.75 g vs sulfasal. 3g | 8 wk | 4 point scale | Score of 0 = normal mucosa | 27% vs 25% |
| Hanauer (2007) Ascend | Mc, db, RCT | Asacol 4.8 g vs 2.4 g | 6 wk | Descriptive, no score | Normal endoscopic finding | 25% vs 20% |
| Kamm (2007) MMX | Mc, db, RCT | MMX mes. 4.8 g vs 2.4 g vs placebo | 8 wk | Mod. Sutherland index | Mod Sutherland index < 1 | 77% vs 69% vs 46% |
| Kruis (2009) | Mc, db, RCT | Mesalazine 3 g vs 1g x 3 | 8 wk | Rachmilewitz | Rachmilewitz < 4 | 71% vs 70% |
Mc: Multicenter; db: Double-blind; RCT: Randomized controlled trial; MH: Mesalazine.
One of the problems in endoscopic UC scores is the application of varying criteria (see Table 2). The reasons for such different definitions and endpoints may only be speculated. Unfortunately we lack an unequivocal definition; all of the scoring systems published so far have certain limitations, which have led to the introduction of several additional scoring systems. From a patient’s and physician’s perspective, however, the use of one single scoring system would be most desirable to enable valid comparisons among study outcomes.
Table 2.
One of the problems in endoscopic ulcerative colitis scores is the application of varying criteria
| Truelove | Baron | Powell-T (St Mark’s) | Levine | Rach-milewitz | Modified Baron | Mayo | Sutherland | |
| Erythema | + | + | ||||||
| Edema | + | |||||||
| Granularity | + | + | + | |||||
| Vascular pattern | + | + | + | + | + | |||
| Friability | + | + | + | + | + | + | + | + |
| Erosions | + | + | + | |||||
| Ulceration | + | + | + | + | ||||
| Exudate | + | + | ||||||
| Remission | 0 | 0-1 | 0-2 | 0-1 | 0-1 | 0 |
WHAT IS THE ADDITIVE VALUE OF DEEP REMISSION AS COMPARED TO MUCOSAL HEALING?
“Deep remission” is another term that has been discussed as a treatment target in recent years. The definition, however, is unfortunately not clearer than the one of mucosal healing. In the EXTEND study “deep remission” was defined as clinical remission (CDAI < 150) and complete mucosal healing as defined according to CDEIS[13]. It is worthwhile to look a bit closer at this definition. If a patient with CD achieves mucosal healing but still has increased CDAI (no clinical remission) this may be due to superimposed IBS symptoms or the fact that without the presence of inflammation there is some bowel damage such as a fibrotic stricture or an internal fistula which might contribute to increased bowel frequency. Subsequently the lack of clinical remission is important for the patient and his/her clinical management (e.g. surgery of the stricture) but not for the medical (anti-inflammatory) management of the disease. Thus, the term “deep remission” in the definition outlined above is not useful and does not provide more information than mucosal healing. In fact - it contributes to confusion of scientists, clinicians and “trialists”.
HOW CAN WE IMPROVE?
There should be standards on the definition of mucosal healing for clinical studies. It needs to be discussed - and finally decided - whether endoscopic mucosal healing, histologic mucosal healing or a combination of both can be standardized. Once agreement on definitions has been achieved, a given patient could be assessed by a -hopefully- simple binary coded tool that is oriented according to the TNM classification of oncology. A proposal for such a tool is illustrated in Table 3. The number “1” stands for “active”, “0” for “remission” and “x” for “not assessed”. Of note CD activity assessment would require, in contrast to UC, not only measuring clinical activity, biochemical, endoscopic and histologic activity, but also imaging modalities (presence of fistulas, strictures). This simple approach has the potential to reduce the amount of potentially confusing new definitions to describe different combinations of activities in IBD.
Table 3.
Proposal of the CBEHI classification to assess Crohn’s disease activity
| Activity level | Definition | Code |
| Clinical activity | Remission: CDAI < 150 | C0 |
| Active: CDAI ≥ 150 | C1 | |
| Biochemical activity | CRP normal | B0 (CRP) |
| Elevated CRP | B1 (CRP) | |
| Calprotectin < 200 μg/g | B0 (Calpro) | |
| Calprotectin ≥ 200 μg/g | B1 (Calpro) | |
| Endoscopic activity | Remission: SES-CD < 4 | E0 |
| Active: SES-CD ≥ 4 | E1 | |
| Histologic activity | Inactive | H0 |
| Active | H1 | |
| Imaging | Inactive: no fistulas, no stenoses | I0 |
| Active: presence of either fistula and/or stenosis | I1 |
Example: A Crohn’s disease (CD) patient with C0B0 (CRP) E1H1I0 would have clinical and biochemical remission, but endoscopic and histologic activity.
Other definitions of mucosal healing (such as “biological mucosal healing”, “epigenetic mucosal healing”, “mucus layer healing” or “microbiota mucosal healing”) require further studies and prospective trials. At this point they are purely investigational and should not be used in clinical trials.
What would happen if such an agreement cannot be achieved? Then it would not make sense to discuss mucosal healing as a treatment target for IBD any further as this would be a treatment target that lacks a definition and subsequently is blurry, vague and indistinct.
ACKNOWLEDGMENTS
Gerhard Rogler has received in the last 2 years consultant fees from Abbott Switzerland and Abbott International, Tillotts International, FALK Germany, Essex/MSD Switzerland, Novartis, Roche, and Vifor Switzerland; GR has received speaker’s honoraria from Abbott, FALK, MSD, Phadia, Tillotts, UCB, and Vifor; GR has received educational grants and research grants from Abbott, Ardeypharm, Essex/MSD, FALK, Flamentera, Novartis, Tillotts, UCB and Zeller.
Stephan Vavricka has received in the last 2 years consultant fees from Abbvie, MSD, and UCB, and Tillotts. SV has received speaker’s honoraria from Abbott, MSD, Tillotts, UCB, and Vifor; SV has received educational grants and research grants from Abbot/Abbvie, Essex/MSD and UCB.
Alain Schoepfer has received in the last 2 years consultant fees from Abbvie, MSD, UCB, and Tillotts. AS has received speaker’s honoraria from Abbvie; AS has received educational grants and research grants from Essex/MSD, UCB, and Tillotts.
Peter L Lakatos has received in the last 2 years consultant and lecture fees from Abbott/Abbvie, MSD-Hungary, and Ferring
Footnotes
Supported by Grants from the Swiss National Science Foundation to Rogler G, Grant No. 310030-120312; to Schoepfer A, Grant No. 32003B_135665/1; to Vavricka S, Grant No. 320000-114009/3 and 32473B_135694/1; and to the Swiss IBD Cohort, Grant No. 33CS30_134274
P- Reviewers: Bessissow T, HIroshi N S- Editor: Wen LL L- Editor: A E- Editor: Wang CH
References
- 1.Korelitz BI, Sommers SC. Response to drug therapy in Crohn’s disease: evaluation by rectal biopsy and mucosal cell counts. J Clin Gastroenterol. 1984;6:123–127. doi: 10.1097/00004836-198404000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Korelitz BI. Mucosal healing as an index of colitis activity: back to histological healing for future indices. Inflamm Bowel Dis. 2010;16:1628–1630. doi: 10.1002/ibd.21268. [DOI] [PubMed] [Google Scholar]
- 3.Geboes K. Is histology useful for the assessment of the efficacy of immunosuppressive agents in IBD and if so, how should it be applied ? Acta Gastroenterol Belg. 2004;67:285–289. [PubMed] [Google Scholar]
- 4.Levine TS, Tzardi M, Mitchell S, Sowter C, Price AB. Diagnostic difficulty arising from rectal recovery in ulcerative colitis. J Clin Pathol. 1996;49:319–323. doi: 10.1136/jcp.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane S, Lu F, Kornbluth A, Awais D, Higgins PD. Controversies in mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2009;15:796–800. doi: 10.1002/ibd.20875. [DOI] [PubMed] [Google Scholar]
- 6.Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468; quiz 463-468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 7.Barreiro-de Acosta M, Lorenzo A, Mera J, Dominguez-Muñoz JE. Mucosal healing and steroid-sparing associated with infliximab for steroid-dependent ulcerative colitis. J Crohns Colitis. 2009;3:271–276. doi: 10.1016/j.crohns.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Laharie D, Reffet A, Belleannée G, Chabrun E, Subtil C, Razaire S, Capdepont M, de Lédinghen V. Mucosal healing with methotrexate in Crohn’s disease: a prospective comparative study with azathioprine and infliximab. Aliment Pharmacol Ther. 2011;33:714–721. doi: 10.1111/j.1365-2036.2010.04569.x. [DOI] [PubMed] [Google Scholar]
- 10.Levy LC, Siegel CA. Endoscopic mucosal healing in ulcerative colitis. Aliment Pharmacol Ther. 2011;33:1254–1255; author reply 1254-1255. doi: 10.1111/j.1365-2036.2011.04615.x. [DOI] [PubMed] [Google Scholar]
- 11.López-Palacios N, Mendoza JL, Taxonera C, Lana R, López-Jamar JM, Díaz-Rubio M. Mucosal healing for predicting clinical outcome in patients with ulcerative colitis using thiopurines in monotherapy. Eur J Intern Med. 2011;22:621–625. doi: 10.1016/j.ejim.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Mantzaris GJ, Christidou A, Sfakianakis M, Roussos A, Koilakou S, Petraki K, Polyzou P. Azathioprine is superior to budesonide in achieving and maintaining mucosal healing and histologic remission in steroid-dependent Crohn’s disease. Inflamm Bowel Dis. 2009;15:375–382. doi: 10.1002/ibd.20777. [DOI] [PubMed] [Google Scholar]
- 13.Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–1111.e2. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Jeen YT, Cho JY, Lee CK, Koo JS, Park DI, Lim JP, Park SJ, Kim YS, Kim TO, Lee SH, Jang BI, Kim JW, Park YS, Kim ES, Choi CH, Kim HJ; IBD study Group of Korean Association for the Study of Intestinal Diseases. Efficacy, Safety, and Predictors of Response to Infliximab Therapy for Ulcerative Colitis: a Korean Multicenter Retrospective Study. J Gastroenterol Hepatol. 2013 doi: 10.1111/jgh.12324. [DOI] [PubMed] [Google Scholar]
- 15.Tursi A, Elisei W, Giorgetti GM, Penna A, Picchio M, Brandimarte G. Factors Influencing Mucosal Healing in Crohn’s Disease during Infliximab Treatment. Hepatogastroenterology. 2013;60:1041–1046. doi: 10.5754/hge11514. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical Study of the Relation between Mucosal Healing and Long-Term Outcomes in Ulcerative Colitis. Gastroenterol Res Pract. 2013;2013:192794. doi: 10.1155/2013/192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlandi F, Brunelli E, Feliciangeli G, Svegliati-Baroni G, Di Sario A, Benedetti A, Guidarelli C, Macarri G. Observer agreement in endoscopic assessment of ulcerative colitis. Ital J Gastroenterol Hepatol. 1998;30:539–541. [PubMed] [Google Scholar]
- 18.de Lange T, Larsen S, Aabakken L. Inter-observer agreement in the assessment of endoscopic findings in ulcerative colitis. BMC Gastroenterol. 2004;4:9. doi: 10.1186/1471-230X-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen HB, Huang Y, Chen SY, Deng DY, Gao LH, Xie JT, Yang LN, Huang C, He S, Li XL. A comparative study of two kinds of small bowel cleaning score system for capsule endoscopy. Acta Gastroenterol Belg. 2012;75:342–348. [PubMed] [Google Scholar]
- 20.Bessho R, Kanai T, Hosoe N, Kobayashi T, Takayama T, Inoue N, Mukai M, Ogata H, Hibi T. Correlation between endocytoscopy and conventional histopathology in microstructural features of ulcerative colitis. J Gastroenterol. 2011;46:1197–1202. doi: 10.1007/s00535-011-0439-1. [DOI] [PubMed] [Google Scholar]
- 21.Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lémann M, Lichtenstein GR, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS) Gut. 2012;61:535–542. doi: 10.1136/gutjnl-2011-300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 23.Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 24.Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 25.Røseth AG, Aadland E, Grzyb K. Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. 2004;39:1017–1020. doi: 10.1080/00365520410007971. [DOI] [PubMed] [Google Scholar]
- 26.Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176–180. doi: 10.1159/000201441. [DOI] [PubMed] [Google Scholar]
- 27.Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, Rossini LG, Candelária PA. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. doi: 10.1186/1756-0500-2-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Licata A, Randazzo C, Cappello M, Calvaruso V, Butera G, Florena AM, Peralta S, Cammà C, Craxì A. Fecal calprotectin in clinical practice: a noninvasive screening tool for patients with chronic diarrhea. J Clin Gastroenterol. 2012;46:504–508. doi: 10.1097/MCG.0b013e318248f289. [DOI] [PubMed] [Google Scholar]
- 29.Kok L, Elias SG, Witteman BJ, Goedhard JG, Muris JW, Moons KG, de Wit NJ. Diagnostic accuracy of point-of-care fecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: the Cost-Effectiveness of a Decision Rule for Abdominal Complaints in Primary Care (CEDAR) study. Clin Chem. 2012;58:989–998. doi: 10.1373/clinchem.2011.177980. [DOI] [PubMed] [Google Scholar]
- 30.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–265.e1-3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Feagan BG, Sandborn WJ, D’Haens G, Pola S, McDonald JW, Rutgeerts P, Munkholm P, Mittmann U, King D, Wong CJ, et al. The role of centralized reading of endoscopy in a randomized controlled trial of mesalamine for ulcerative colitis. Gastroenterology. 2013;145:149–157.e2. doi: 10.1053/j.gastro.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Seldenrijk CA, Morson BC, Meuwissen SG, Schipper NW, Lindeman J, Meijer CJ. Histopathological evaluation of colonic mucosal biopsy specimens in chronic inflammatory bowel disease: diagnostic implications. Gut. 1991;32:1514–1520. doi: 10.1136/gut.32.12.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhulina Y, Hahn-Strömberg V, Shamikh A, Peterson CG, Gustavsson A, Nyhlin N, Wickbom A, Bohr J, Bodin L, Tysk C, et al. Subclinical inflammation with increased neutrophil activity in healthy twin siblings reflect environmental influence in the pathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1725–1731. doi: 10.1097/MIB.0b013e318281f2d3. [DOI] [PubMed] [Google Scholar]
- 34.Geboes K, Dalle I. Influence of treatment on morphological features of mucosal inflammation. Gut. 2002;50 Suppl 3:III37–III42. doi: 10.1136/gut.50.suppl_3.iii37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bressenot A, Geboes K, Vignaud JM, Guéant JL, Peyrin-Biroulet L. Microscopic features for initial diagnosis and disease activity evaluation in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1745–1752. doi: 10.1097/MIB.0b013e318281f2e8. [DOI] [PubMed] [Google Scholar]
- 36.Geboes K, Colombel JF, Greenstein A, Jewell DP, Sandborn WJ, Vatn MH, Warren B, Riddell RH. Indeterminate colitis: a review of the concept--what’s in a name? Inflamm Bowel Dis. 2008;14:850–857. doi: 10.1002/ibd.20361. [DOI] [PubMed] [Google Scholar]
- 37.Geboes K, Villanacci V. Terminology for the diagnosis of colitis. J Clin Pathol. 2005;58:1133–1134. doi: 10.1136/jcp.2005.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamora SA, Hilsden RJ, Meddings JB, Butzner JD, Scott RB, Sutherland LR. Intestinal permeability before and after ibuprofen in families of children with Crohn’s disease. Can J Gastroenterol. 1999;13:31–36. doi: 10.1155/1999/457315. [DOI] [PubMed] [Google Scholar]
- 39.Fries W, Renda MC, Lo Presti MA, Raso A, Orlando A, Oliva L, Giofré MR, Maggio A, Mattaliano A, Macaluso A, et al. Intestinal permeability and genetic determinants in patients, first-degree relatives, and controls in a high-incidence area of Crohn’s disease in Southern Italy. Am J Gastroenterol. 2005;100:2730–2736. doi: 10.1111/j.1572-0241.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 40.Secondulfo M, de Magistris L, Fiandra R, Caserta L, Belletta M, Tartaglione MT, Riegler G, Biagi F, Corazza GR, Carratù R. Intestinal permeability in Crohn's disease patients and their first degree relatives. Dig Liver Dis. 2001;33:680–685. doi: 10.1016/s1590-8658(01)80045-1. [DOI] [PubMed] [Google Scholar]
- 41.Hollander D. Intestinal permeability in patients with Crohn’s disease and their relatives. Dig Liver Dis. 2001;33:649–651. doi: 10.1016/s1590-8658(01)80038-4. [DOI] [PubMed] [Google Scholar]
- 42.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn’s disease. Gastroenterology. 1997;113:802–807. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 43.Howden CW, Gillanders I, Morris AJ, Duncan A, Danesh B, Russell RI. Intestinal permeability in patients with Crohn’s disease and their first-degree relatives. Am J Gastroenterol. 1994;89:1175–1176. [PubMed] [Google Scholar]
- 44.Munkholm P, Langholz E, Hollander D, Thornberg K, Orholm M, Katz KD, Binder V. Intestinal permeability in patients with Crohn’s disease and ulcerative colitis and their first degree relatives. Gut. 1994;35:68–72. doi: 10.1136/gut.35.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollander D. Permeability in Crohn’s disease: altered barrier functions in healthy relatives? Gastroenterology. 1993;104:1848–1851. doi: 10.1016/0016-5085(93)90668-3. [DOI] [PubMed] [Google Scholar]
- 46.Midtvedt T, Zabarovsky E, Norin E, Bark J, Gizatullin R, Kashuba V, Ljungqvist O, Zabarovska V, Möllby R, Ernberg I. Increase of faecal tryptic activity relates to changes in the intestinal microbiome: analysis of Crohn’s disease with a multidisciplinary platform. PLoS One. 2013;8:e66074. doi: 10.1371/journal.pone.0066074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pérez-Brocal V, García-López R, Vázquez-Castellanos JF, Nos P, Beltrán B, Latorre A, Moya A. Study of the viral and microbial communities associated with Crohn’s disease: a metagenomic approach. Clin Transl Gastroenterol. 2013;4:e36. doi: 10.1038/ctg.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papa E, Docktor M, Smillie C, Weber S, Preheim SP, Gevers D, Giannoukos G, Ciulla D, Tabbaa D, Ingram J, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One. 2012;7:e39242. doi: 10.1371/journal.pone.0039242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varela E, Manichanh C, Gallart M, Torrejón A, Borruel N, Casellas F, Guarner F, Antolin M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38:151–161. doi: 10.1111/apt.12365. [DOI] [PubMed] [Google Scholar]
- 50.Vigsnaes LK, van den Abbeele P, Sulek K, Frandsen HL, Steenholdt C, Brynskov J, Vermeiren J, van de Wiele T, Licht TR. Microbiotas from UC patients display altered metabolism and reduced ability of LAB to colonize mucus. Sci Rep. 2013;3:1110. doi: 10.1038/srep01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemoto H, Kataoka K, Ishikawa H, Ikata K, Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T, Yasutomo K. Reduced diversity and imbalance of fecal microbiota in patients with ulcerative colitis. Dig Dis Sci. 2012;57:2955–2964. doi: 10.1007/s10620-012-2236-y. [DOI] [PubMed] [Google Scholar]
- 52.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fyderek K, Strus M, Kowalska-Duplaga K, Gosiewski T, Wedrychowicz A, Jedynak-Wasowicz U, Sładek M, Pieczarkowski S, Adamski P, Kochan P, et al. Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol. 2009;15:5287–5294. doi: 10.3748/wjg.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 56.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dörffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Habib NA, Dawson PM, Krausz T, Blount MA, Kersten D, Wood CB. A study of histochemical changes in mucus from patients with ulcerative colitis, Crohn’s disease, and diverticular disease of the colon. Dis Colon Rectum. 1986;29:15–17. doi: 10.1007/BF02555277. [DOI] [PubMed] [Google Scholar]
- 59.Dorofeyev AE, Vasilenko IV, Rassokhina OA, Kondratiuk RB. Mucosal barrier in ulcerative colitis and Crohn’s disease. Gastroenterol Res Pract. 2013;2013:431231. doi: 10.1155/2013/431231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperber K, Shim J, Mehra M, Lin A, George I, Ogata S, Mayer L, Itzkowitz S. Mucin secretion in inflammatory bowel disease: comparison of a macrophage-derived mucin secretagogue (MMS-68) to conventional secretagogues. Inflamm Bowel Dis. 1998;4:12–17. doi: 10.1097/00054725-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Wehkamp J, Stange EF, Fellermann K. Defensin-immunology in inflammatory bowel disease. Gastroenterol Clin Biol. 2009;33 Suppl 3:S137–S144. doi: 10.1016/S0399-8320(09)73149-5. [DOI] [PubMed] [Google Scholar]
- 62.Bevins CL, Stange EF, Wehkamp J. Decreased Paneth cell defensin expression in ileal Crohn’s disease is independent of inflammation, but linked to the NOD2 1007fs genotype. Gut. 2009;58:882–883; discussion 882-883. [PubMed] [Google Scholar]
- 63.Wehkamp J, Koslowski M, Wang G, Stange EF. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn’s disease. Mucosal Immunol. 2008;1 Suppl 1:S67–S74. doi: 10.1038/mi.2008.48. [DOI] [PubMed] [Google Scholar]
- 64.Wehkamp J, Stange EF. A new look at Crohn’s disease: breakdown of the mucosal antibacterial defense. Ann N Y Acad Sci. 2006;1072:321–331. doi: 10.1196/annals.1326.030. [DOI] [PubMed] [Google Scholar]
- 65.Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR, Wolf JL, Cheifetz AS, Robson SC, Moss AC. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19:779–784. doi: 10.1097/MIB.0b013e3182802b0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenberg L, Nanda KS, Zenlea T, Gifford A, Lawlor GO, Falchuk KR, Wolf JL, Cheifetz AS, Goldsmith JD, Moss AC. Histologic markers of inflammation in patients with ulcerative colitis in clinical remission. Clin Gastroenterol Hepatol. 2013;11:991–996. doi: 10.1016/j.cgh.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.D’Incà R, Sturniolo GC, Martines D, Di Leo V, Cecchetto A, Venturi C, Naccarato R. Functional and morphological changes in small bowel of Crohn’s disease patients. Influence of site of disease. Dig Dis Sci. 1995;40:1388–1393. doi: 10.1007/BF02065556. [DOI] [PubMed] [Google Scholar]
- 68.Riis P. Inflammation as a diagnostic keystone and its clinical implications, exemplified by the inflammatory bowel diseases. Agents Actions. 1990;29:4–7. doi: 10.1007/BF01964705. [DOI] [PubMed] [Google Scholar]
- 69.Tahara T, Shibata T, Nakamura M, Okubo M, Yamashita H, Yoshioka D, Yonemura J, Kmiya Y, Ishizuka T, Fujita H, et al. Host genetic factors, related to inflammatory response, influence the CpG island methylation status in colonic mucosa in ulcerative colitis. Anticancer Res. 2011;31:933–938. [PubMed] [Google Scholar]
- 70.Adamik J, Henkel M, Ray A, Auron PE, Duerr R, Barrie A. The IL17A and IL17F loci have divergent histone modifications and are differentially regulated by prostaglandin E2 in Th17 cells. Cytokine. 2013;64:404–412. doi: 10.1016/j.cyto.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293–308. doi: 10.1053/j.gastro.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stylianou E. Epigenetics: the fine-tuner in inflammatory bowel disease? Curr Opin Gastroenterol. 2013;29:370–377. doi: 10.1097/MOG.0b013e328360bd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenke AC, Zilbauer M. Epigenetics in inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28:577–584. doi: 10.1097/MOG.0b013e328357336b. [DOI] [PubMed] [Google Scholar]
- 74.Häsler R, Feng Z, Bäckdahl L, Spehlmann ME, Franke A, Teschendorff A, Rakyan VK, Down TA, Wilson GA, Feber A, et al. A functional methylome map of ulcerative colitis. Genome Res. 2012;22:2130–2137. doi: 10.1101/gr.138347.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooke J, Zhang H, Greger L, Silva AL, Massey D, Dawson C, Metz A, Ibrahim A, Parkes M. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2128–2137. doi: 10.1002/ibd.22942. [DOI] [PubMed] [Google Scholar]
- 76.Archanioti P, Gazouli M, Theodoropoulos G, Vaiopoulou A, Nikiteas N. Micro-RNAs as regulators and possible diagnostic bio-markers in inflammatory bowel disease. J Crohns Colitis. 2011;5:520–524. doi: 10.1016/j.crohns.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 77.Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, Bao W, Patel K, Wolf DC, Safdi M, Colombel JF, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433–442; quiz 464. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 79.D’Haens G, Baert F, van Assche G, Caenepeel P, Vergauwe P, Tuynman H, De Vos M, van Deventer S, Stitt L, Donner A, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 80.D’Haens GR. Top-down therapy for IBD: rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. 2010;7:86–92. doi: 10.1038/nrgastro.2009.222. [DOI] [PubMed] [Google Scholar]
- 81.Schreiber S. Certolizumab pegol for the treatment of Crohn’s disease. Therap Adv Gastroenterol. 2011;4:375–389. doi: 10.1177/1756283X11413315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sandborn WJ. Mucosal healing with infliximab: results from the active ulcerative colitis trials. Gastroenterol Hepatol (N Y) 2012;8:117–119. [PMC free article] [PubMed] [Google Scholar]
- 83.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 84.Sandborn WJ, Colombel JF, D’Haens G, Van Assche G, Wolf D, Kron M, Lazar A, Robinson AM, Yang M, Chao JD, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther. 2013;37:204–213. doi: 10.1111/apt.12145. [DOI] [PubMed] [Google Scholar]
- 85.Vecchi M, Meucci G, Gionchetti P, Beltrami M, Di Maurizio P, Beretta L, Ganio E, Usai P, Campieri M, Fornaciari G, et al. Oral versus combination mesalazine therapy in active ulcerative colitis: a double-blind, double-dummy, randomized multicentre study. Aliment Pharmacol Ther. 2001;15:251–256. doi: 10.1046/j.1365-2036.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 86.Malchow H, Gertz B. A new mesalazine foam enema (Claversal Foam) compared with a standard liquid enema in patients with active distal ulcerative colitis. Aliment Pharmacol Ther. 2002;16:415–423. doi: 10.1046/j.1365-2036.2002.01199.x. [DOI] [PubMed] [Google Scholar]


