Abstract
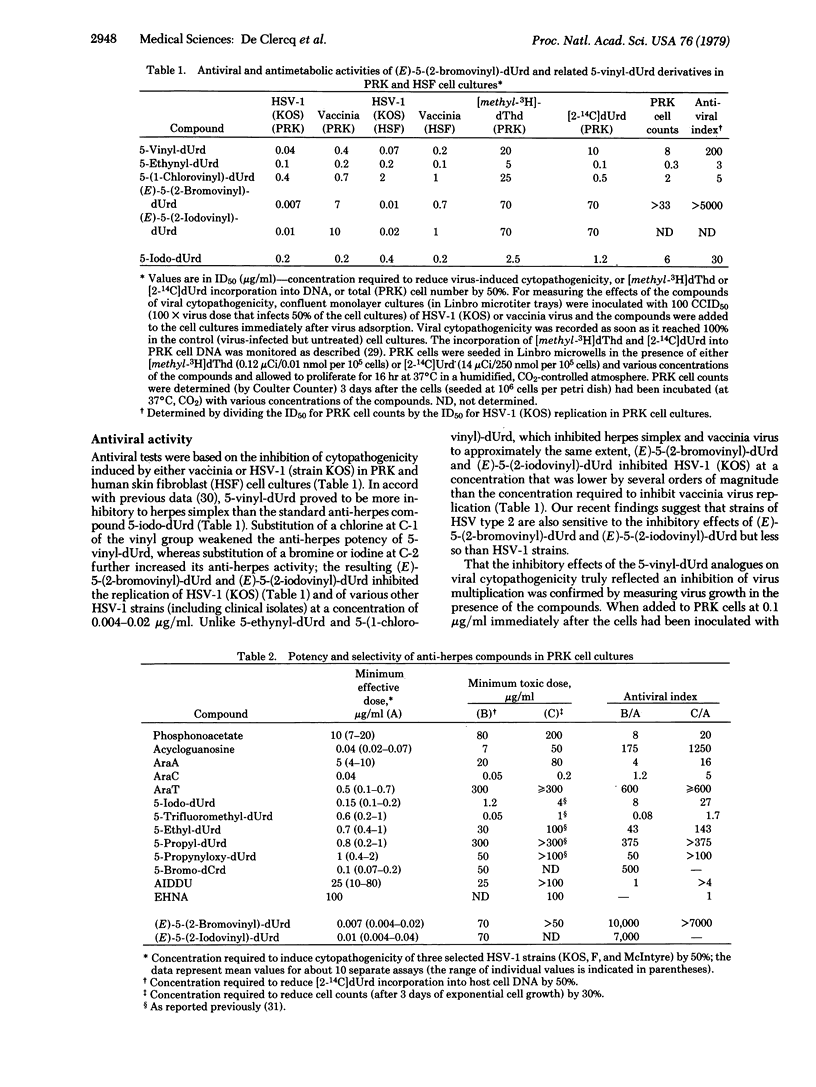
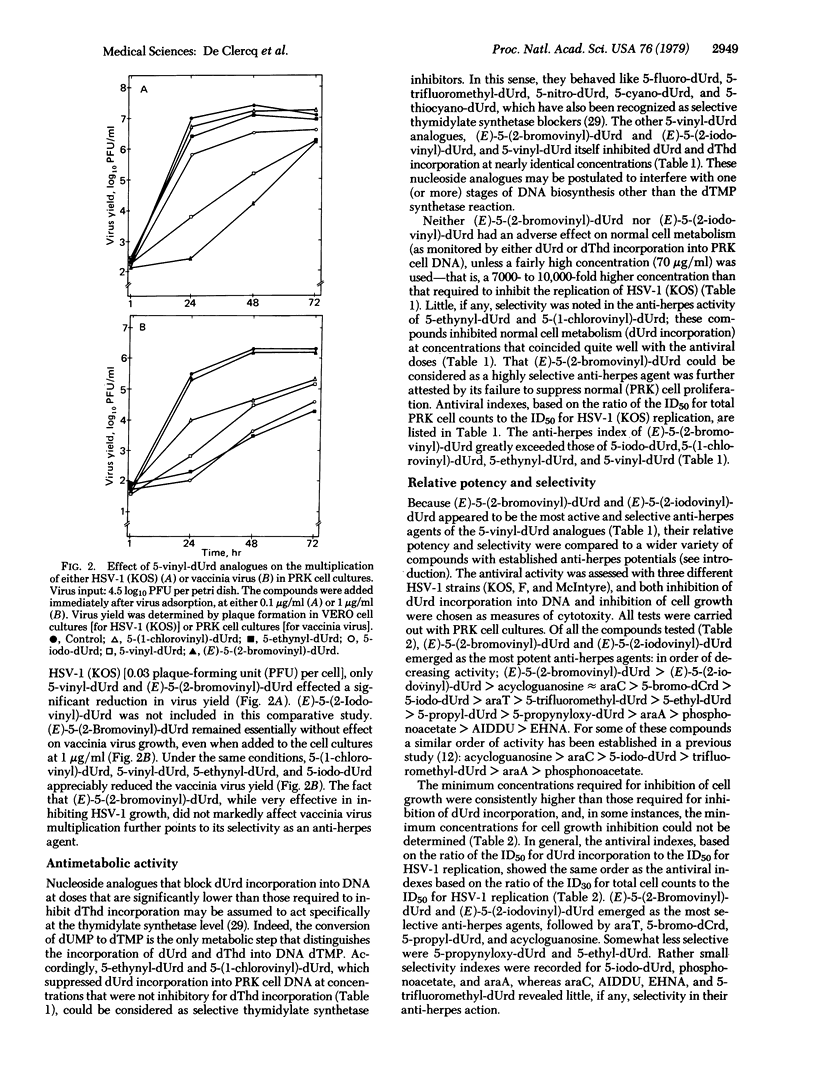
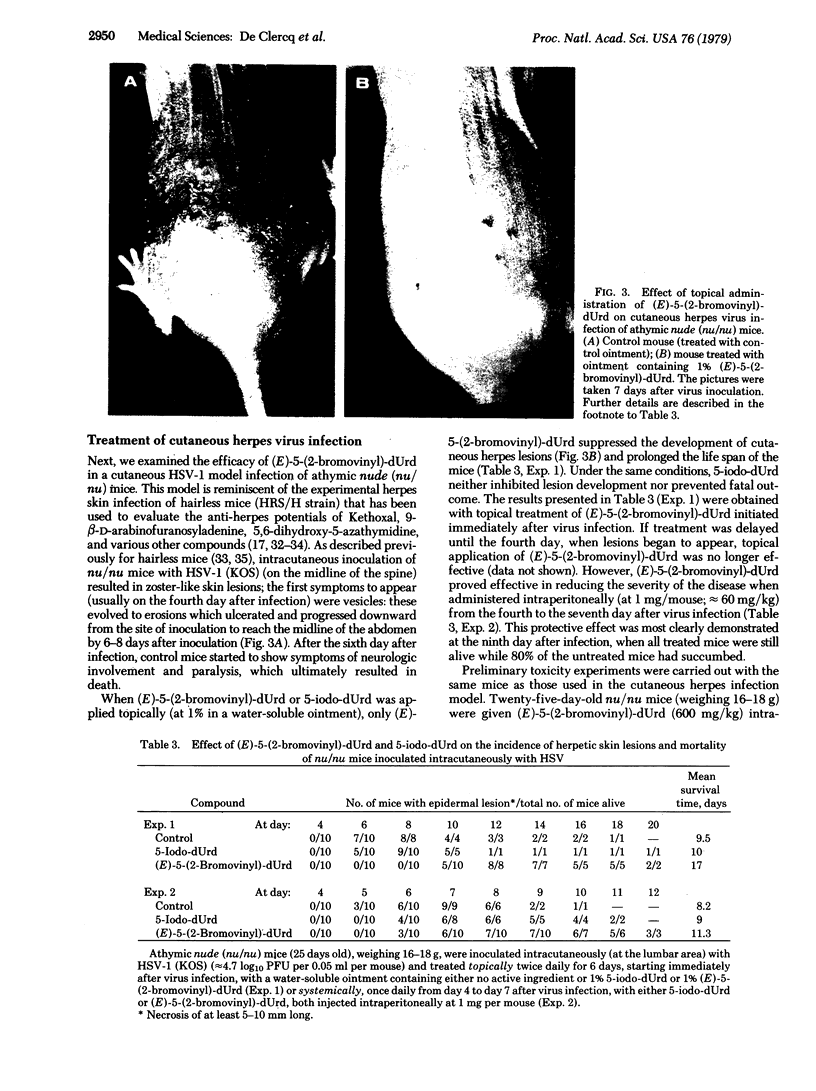
Of a series of five newly synthesized 2'-deoxyuridine derivatives, including 5-vinyl-dUrd, 5-ethynyl-dUrd, 5-(1-chlorovinyl)-dUrd, (E)-5-(2-bromovinyl)-dUrd, and (E)-5-(2-iodovinyl)-dUrd, the last two compounds were found to exert a marked inhibitory effect on the replication of herpes simplex virus type 1 [ID50 (mean inhibitory dose), 0.004-0.02 microgram/ml]. Both (E)-5-(2-bromovinyl)-dUrd and (E)-5-(2-iodovinyl)-dUrd were highly selective in their anti-herpes activity in that they did not affect the growth or metabolism of the host (primary rabbit kidney) cells unless drug concentrations were used that were 5,000- to 10,000-fold greater than those required to inhibit virus multiplication. In this sense (E)-5-(2-bromovinyl)-dUrd and (E)-5-(2-iodovinyl)-dUrd proved more selective in their activity against herpes simplex virus type 1 than all other anti-herpes compounds that have been described so far. In animal model systems (namely, cutaneous herpes infections of athymic nude mice), (E)-5-(2-bromovinyl)-dUrd suppressed the development of herpetic skin lesions and mortality therewith associated, whether the compound was administered topically or systemically. Under the same conditions, the standard anti-herpes drug 5-iodo-dUrd (Idoxuridine) offered little, if any, protection. Although the precise mechanism of action of (E)-5-(2-bromovinyl)-dUrd and (E)-5-(2-iodovinyl)-dUrd remains to be established, preliminary findings indicate that they do not specifically act at the thymidylate synthetase step.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., Meldrum B., Gupta V. S., Rouse B. T. Comparison of the antiviral effects of 5-methoxymethyl-deoxyuridine with 5-iododeoxyuridine, cytosine arabinoside, and adenine arabinoside. Antimicrob Agents Chemother. 1975 Dec;8(6):643–650. doi: 10.1128/aac.8.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976 Aug 25;251(16):4833–4838. [PubMed] [Google Scholar]
- Cheng Y. C., Domin B. A., Sharma R. A., Bobek M. Antiviral action and cellular toxicity of four thymidine analogues: 5-ethyl-,5-vinyl-, 5-propyl-, and 5-allyl-2'- deoxyuridine. Antimicrob Agents Chemother. 1976 Jul;10(1):119–122. doi: 10.1128/aac.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine V. S., Francis R. D., Mason B. H. Experimental zoster-like herpes simplex in hairless mice. J Invest Dermatol. 1971 Mar;56(3):193–199. doi: 10.1111/1523-1747.ep12260802. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Huang G. F., Torrence P. F. 5-Nitro-2'-deoxyuridine and 5-nitro-2'-deoxyuridine 5'-monophosphate: antiviral activity and inhibition of thymidylate synthetase in vivo. Mol Pharmacol. 1978 May;14(3):422–430. [PubMed] [Google Scholar]
- De Clercq E., Descamps J., Shugar D. 5-Propyl-2'-deoxyuridine: a specific anti-herpes agent. Antimicrob Agents Chemother. 1978 Mar;13(3):545–547. doi: 10.1128/aac.13.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Shugar D. Antiviral activity of 5-ethyl pyrimidine deoxynucleosides. Biochem Pharmacol. 1975 May 15;24(10):1073–1078. doi: 10.1016/0006-2952(75)90192-6. [DOI] [PubMed] [Google Scholar]
- Drach J. C., Shipman C., Jr The selective inhibition of viral DNA synthesis by chemotherapeutic agents: an indicator of clinical usefulness? Ann N Y Acad Sci. 1977 Mar 4;284:396–409. doi: 10.1111/j.1749-6632.1977.tb21976.x. [DOI] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry G. A., Aswell J. F. Inhibition of herpes simplex virus replication by araT. Virology. 1975 May;65(1):294–296. doi: 10.1016/0042-6822(75)90034-3. [DOI] [PubMed] [Google Scholar]
- Greer S., Schildkraut I., Zimmerman T., Kaufman H. 5-Halogenated analogs of deoxycytidine as selective inhibitors of the replication of herpes simplex viruses in cell culture and related studies of intracranial herpes simplex virus infections in mice. Ann N Y Acad Sci. 1975 Aug 8;255:359–365. doi: 10.1111/j.1749-6632.1975.tb29243.x. [DOI] [PubMed] [Google Scholar]
- Helgstrand E., Eriksson B., Johansson N. G., Lannerö B., Larsson A., Misiorny A., Norén J. O., Sjöberg B., Stenberg K., Stening G. Trisodium phosphonoformate, a new antiviral compound. Science. 1978 Sep 1;201(4358):819–821. doi: 10.1126/science.210500. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Brady E. Herpes simplex virus skin infection in hairless mice: treatment with antiviral compounds. Antimicrob Agents Chemother. 1974 Mar;5(3):318–322. doi: 10.1128/aac.5.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Schafer T. W., Came P. E. Chemotherapy of cutaneous herpesvirus infection of hairless mice. J Invest Dermatol. 1973 Apr;60(4):203–206. doi: 10.1111/1523-1747.ep12724476. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Erythro-9-(2-hydroxy-3-nonyl)adenine as a specific inhibitor of herpes simplex virus replication in the presence and absence of adenosine analogues. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4684–4688. doi: 10.1073/pnas.75.10.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliafito C. A., Robinson N. L., Albert D. M., Pavan-Langston D., Lin T. S., Ward D. C., Prusoff W. H. Therapy of experimental herpes simplex keratitis in rabbits with 5-lodo-5'-amino-2',5'-dideoxyuridine (39882). Proc Soc Exp Biol Med. 1977 Oct;156(1):92–96. doi: 10.3181/00379727-156-39882. [DOI] [PubMed] [Google Scholar]
- Renis H. E. 5,6-Dihydro-5-azathymidine: in vitro antiviral properties against human herpesviruses. Antimicrob Agents Chemother. 1978 Apr;13(4):613–617. doi: 10.1128/aac.13.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reno J. M., Lee L. F., Boezi J. A. Inhibition of herpesvirus replication and herpesvirus-induced deoxyribonucleic acid polymerase by phosphonoformate. Antimicrob Agents Chemother. 1978 Feb;13(2):188–192. doi: 10.1128/aac.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schildkraut I., Cooper G. M., Greer S. Selective inhibition of the replication of herpes simplex virus by 5-halogenated analogues of deoxycytidine. Mol Pharmacol. 1975 Mar;11(2):153–158. [PubMed] [Google Scholar]
- Sharma R. A., Bobek M. Synthesis of 5-vinyluridine and 5-vinyl-2'-deoxyuridine as new pyrimidine nucleoside analogs. J Org Chem. 1975 Aug 8;40(16):2377–2379. doi: 10.1021/jo00904a025. [DOI] [PubMed] [Google Scholar]
- Shen T. Y., McPherson J. F., Linn B. O. Nucleosides. 3. Studies on 5-methylamino-2'-deoxyuridine as a specific antiherpes agent. J Med Chem. 1966 May;9(3):366–369. doi: 10.1021/jm00321a025. [DOI] [PubMed] [Google Scholar]
- Swierkowski M., Shugar D. A nonmutagenic thymidine analog with antiviral activity. 5-Ethyldeoxyuridine. J Med Chem. 1969 May;12(3):533–534. doi: 10.1021/jm00303a617. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Spencer J. W., Bobst A. M. 5-O-Alkylated derivatives of 5-hydroxy-2'-deoxyuridine as potential antiviral agents. Anti-herpes activity of 5-propynyloxy-2'-deoxyuridine. J Med Chem. 1978 Feb;21(2):228–231. doi: 10.1021/jm00200a018. [DOI] [PubMed] [Google Scholar]
- Underwood G. E. Kethoxal for treatment of cutaneous herpes simplex. Proc Soc Exp Biol Med. 1968 Oct;129(1):235–239. doi: 10.3181/00379727-129-33294. [DOI] [PubMed] [Google Scholar]
- Underwood G. E., Weed S. D. Efficacy of 5,6-dihydro-5-azathymidine against cutaneous herpes simplex virus in hairless mice. Antimicrob Agents Chemother. 1977 Apr;11(4):765–767. doi: 10.1128/aac.11.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Dolin R., Galasso G. J., Ch'ien L. T., Alford C. A. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious Diseases collaborative antiviral study. N Engl J Med. 1977 Aug 11;297(6):289–294. doi: 10.1056/NEJM197708112970601. [DOI] [PubMed] [Google Scholar]