Abstract
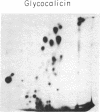
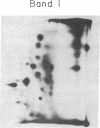
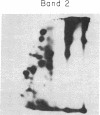
The glycoprotein I complex, consisting of two polypeptides of Mr 210,000 and 150,000, was isolated from human platelet membranes by wheat germ lectin affinity chromatography. Glycocalicin, a soluble loosely bound membrane glycoprotein of Mr 150,000 related to the glycoprotein I system, was also purified. The isolated polypeptides were radioiodinated in sodium dodecyl sulfate/polyacrylamide gels and digested with trypsin, and the labeled peptide digest was analyzed by two-dimensional high-voltage electrophoresis and thin-layer chromatography. The two polypeptides of Mr 210,000 and 150,000 in the glycoprotein I complex had essentially identical radioactive peptide maps. Glycocalicin had a completely different tryptic peptide map. These studies shed light on the molecular relationships of some of the components of the platelet membrane glycoprotein I system. The possibility is raised that the receptorlike function of the intrinsic platelet membrane glycoproteins may be related to the polymeric subunit associations of the constituent polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. Structural studies on human spectrin. Comparison of subunits and fragmentation of native spectrin. J Biol Chem. 1979 Feb 10;254(3):939–944. [PubMed] [Google Scholar]
- Bunting R. W., Peerschke E. I., Zucker M. B. Human platelet sialic acid content and tritium incorporation after ADP-induced shape change and aggregation. Blood. 1978 Oct;52(4):643–653. [PubMed] [Google Scholar]
- Clemetson K. J., Pfueller S. L., Luscher E. F., Jenkins C. S. Isolation of the membrane glycoproteins of human blood platelets by lectin affinity chromatography. Biochim Biophys Acta. 1977 Feb 4;464(3):493–508. doi: 10.1016/0005-2736(77)90025-6. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Jamieson G. A., Okumura T. Reduced thrombin binding and aggregation in Bernard-Soulier platelets. J Clin Invest. 1978 Mar;61(3):861–864. doi: 10.1172/JCI109000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C. S., Phillips D. R., Clemetson K. J., Meyer D., Larrieu M. J., Lüscher E. F. Platelet membrane glycoproteins implicated in ristocetin-induced aggregation. Studies of the proteins on platelets from patients with Bernard-Soulier syndrome and von Willebrand's disease. J Clin Invest. 1976 Jan;57(1):112–124. doi: 10.1172/JCI108251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Functional proteins of the human red blood cell membrane. Semin Hematol. 1979 Jan;16(1):3–20. [PubMed] [Google Scholar]
- Nachman R. L., Jaffe E. A., Weksler B. B. Immunoinhibition of ristocetin-induced platelet aggregation. J Clin Invest. 1977 Jan;59(1):143–148. doi: 10.1172/JCI108612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Tarasov E., Weksler B. B., Ferris B. Wheat germ agglutinin affinity chromatography of human platelet membrane glycoproteins. Thromb Res. 1978 Jan;12(1):91–104. doi: 10.1016/0049-3848(78)90088-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Membrane glycoproteins and human platelet function. Br J Haematol. 1978 Feb;38(2):155–160. doi: 10.1111/j.1365-2141.1978.tb01031.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975 Jun 26;255(5511):720–722. doi: 10.1038/255720a0. [DOI] [PubMed] [Google Scholar]
- Okumura I., Lombart C., Jamieson G. A. Platelet glycocalicin. II. Purification and characterization. J Biol Chem. 1976 Oct 10;251(19):5950–5955. [PubMed] [Google Scholar]
- Okumura T., Jamieson G. A. Platelet glycocalicin. I. Orientation of glycoproteins of the human platelet surface. J Biol Chem. 1976 Oct 10;251(19):5944–5949. [PubMed] [Google Scholar]
- Okumura T., Jamieson G. A. Platelet glycocalicin: a single receptor for platelet aggregation induced by thrombin or ristocetin. Thromb Res. 1976 May;8(5):701–706. doi: 10.1016/0049-3848(76)90250-4. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Solum N. O., Hagen I., Gjemdal T. Platelt membrane glycoproteins and the interaction between bovine factor VIII related protein and human platelets. Thromb Haemost. 1977 Dec 15;38(4):914–923. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]