Abstract
AIM: To investigate the expression of insulin-like growth factor-1 (IGF-1)/insulin-like growth factor-1 receptor (IGF-1R) in colorectal cancer (CRC) tissues and to analyze their correlation with lymphangiogenesis and lymphatic metastasis.
METHODS: Immunohistochemistry was used to evaluate IGF-1 and IGF-1R expression and lymphatic vessel density (LVD) in 40 CRC specimens. The correlation between IGF-1/IGF-1R and LVD was investigated. Effects of IGF-1 on migration and invasion of CRC cells were examined using transwell chamber assays. A LoVo cell xenograft model was established to further detect the role of IGF-1 in CRC lymphangiogenesis in vivo.
RESULTS: Elevated IGF-1 and IGF-1R expression in CRC tissues was correlated with lymph node metastasis (r = 0.715 and 0.569, respectively, P < 0.05) and tumor TNM stage (r = 0.731 and 0.609, P < 0.05). A higher LVD was also found in CRC tissues and was correlated with lymphatic metastasis (r = 0.405, P < 0.05). A positive correlation was found between LVD and IGF-1R expression (r = 0.437, P < 0.05). Transwell assays revealed that IGF-1 increased the migration and invasion of CRC cells. In vivo mouse studies showed that IGF-1 also increased LVD in LoVo cell xenografts.
CONCLUSION: IGF-1/IGF-1R signaling induces tumor-associated lymphangiogenesis and contributes to lymphatic metastasis of CRC.
Keywords: Colorectal cancer, Insulin-like growth factor-1, Insulin-like growth factor-1 receptor, Lymphangiogenesis, Lymphatic metastasis
Core tip: Insulin-like growth factor-1 (IGF-1) and its receptor, insulin-like growth factor-1 receptor (IGF-1R), are frequently overexpressed in many types of tumors including colorectal cancer. A recent study (Björndahl et al, 2003) showed that both IGF-1 and IGF-2 could potently stimulate lymphatic vessel growth in the mouse cornea. However, equivalent evidence on IGF-1 in solid tumors is lacking. Here, we show that IGF-1/IGF-1R signaling induces tumor-associated lymphangiogenesis in vivo and contributes to lymphatic metastasis of colorectal cancer. Findings from the present study provide further evidence to support the involvement of IGF-1/IGF-1R signaling in lymphangiogenesis in solid tumors.
INTRODUCTION
Lymphatic metastasis is one of the most common metastatic routes of colorectal cancer (CRC). When cancer spreads, it is much harder to treat successfully. The presence or absence of lymph node involvement is one of the most important factors that determine the long-term outcome of cancer patients[1-3]. There is a complex process that a tumor cell must go through before metastasis can occur[4,5]. Excessive formation of new lymphatic vessels in CRC is a key step in metastatic progression[5,6]. However, the factors triggering lymphangiogenesis and the detailed molecular mechanisms are poorly understood.
Insulin-like growth factor-1 (IGF-1) and its receptor (IGF-1R) are frequently overexpressed in many types of tumors including CRC[7-10]. Increasing evidence suggests that amplified IGF-1/IGF-1R signaling not only is associated with an increased relative risk for cancer development, but also contributes to cancer cell survival, invasion, metastasis and resistance to chemotherapeutic drugs[7,10]. In addition to their roles in the development and progression of cancer, the IGF-1/IGF-1R signaling system can also induce lymphangiogenesis[11,12]. In a recent study, Björndahl et al[11] reported that both IGF-1 and IGF-2 could potently stimulate lymphatic vessel growth in the mouse cornea. However, direct evidence showing the involvement of IGF-1/IGF-1R signaling in lymphangiogenesis in solid tumors is lacking.
In the present study, we found that IGF-1 and IGF-1R were significantly overexpressed in CRC tissues compared with adjacent normal tissues using an immunohistochemical (IHC) assay. Using a lymphatic endothelial-specific antibody marker D2-40[3,13], we found that lymphatic vessel density (LVD) was significantly higher in CRC tissues. The levels of IGF-1, IGF-1R and LVD were all significantly correlated with lymphatic metastasis. In addition, a positive correlation was found between LVD and IGF-1R. These results suggest that the IGF-1/IGF-1R axis might promote lymph node metastasis of CRC by induction of lymphangiogenesis. To further explore its role in lymphangiogenesis, we created a LoVo cell (a human colon cancer cell line) xenograft model and showed that IGF-1 treatment resulted in an increase in the LVD in vivo. Together, our findings demonstrate that IGF-1/IGF-1R signaling can induce lymphangiogenesis in CRC and may facilitate lymphatic metastasis in CRC patients.
MATERIALS AND METHODS
Tissue samples
Forty CRC and adjacent normal tissue samples were obtained from randomly selected patients undergoing surgical resection without preoperative neoadjuvant chemoradiotherapy between January 2011 and June 2011 at Shaoxing People’s Hospital. Their average age was 68.5 years (range, 44 to 83 years). Of these patients, 9 had well-differentiated adenocarcinoma, 20 had moderately differentiated adenocarcinoma, and 11 had poorly differentiated adenocarcinoma. Twenty-five patients had stage I-II disease and 15 had stage III-IV disease according to the tumor-node-metastasis (TNM) classification defined by Union for International Cancer Control (UICC)[14]. D2 radical resection was performed in 3 patients and D3 radical resection was performed in 37 patients. The number of lymph nodes resected was 9-22.
Immunohistochemical staining
Tissue sections were stained using the Envision System (DakoCytomation, Carpinteria, CA, United States) according to manufacturer’s instructions. Mouse monoclonal antibodies against human IGF-1, IGF-1R, or D2-40 were obtained from Abcam (Cambridge, United Kingdom). Intensity of immunostaining signals was evaluated in 8 fields under a light microscope (Olympus Optical, Tokyo, Japan). Statistical analysis was carried out in accordance with a previous study[15]. In short, total staining of IGF-1 and IGF-1R were scored as the product of the staining intensity (on a scale of 0-3: negative = 0, weak = 1, moderate = 2, strong = 3) and the percentage of cells stained (0 = 0%, 1 = 1%-25%, 2 = 26%-50%, 3 = 51%-100%), which resulted in a scale of 0-9. Two independent pathologists scored each sample without prior knowledge of the patient’s clinical information and outcome.
Cell culture and IGF-1 treatment
The human CRC cell line LoVo was obtained from the American Type Culture Collection and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin in a 5% CO2 incubator at 37 °C. For IGF-1 treatment, media were replaced with fresh serum-free medium containing 50 ng/mL IGF-1 (PeproTech) when cells were grown to 30% confluency. An IGF-1 stock solution was prepared in PBS, and thus, PBS in serum-free medium was used as a control.
Migration and invasion analysis
The migration and invasion of CRC cells were examined using non-coated or matrigel-coated transwell culture chambers (8 μm pore size, Corning, NY, United States) 48 h after IGF-1 treatment. For migration assays, 1 × 105 cells were seeded in the top non-coated chamber and incubated at 37 °C for 8 h. For invasion assays, 5 × 104 cells were seeded in the top Matrigel-coated (BD Biosciences, Bedford, MA, United States) chambers and incubated at 37 °C for 24 h. In both assays, cells were suspended in serum-free Roswell Park Memorial Institute (RPMI)-1640 medium in the upper chamber, and the lower chamber was filled with RPMI-1640 medium containing 5% FBS. After incubation, the top chambers were wiped with cotton wool to remove the non-migratory or non-invasive cells. Cells on the underside of the membrane were fixed in methanol for 30 min, stained with 0.1% crystal violet, and counted under a microscope (Eclipse TS100, Nikon, Japan).
Xenograft and IGF-1 treatment
Four-week-old BALB/c nude mice (18-22 g) were used in this study. LoVo cells in the logarithmic phase of growth were adjusted to a density of 5 × 107/mL, and 0.2 mL of cell suspension was subcutaneously injected into the flank of the mice. On the second day after LoVo cell transplantation, the mice were randomized into either a treatment or a control group, with ten mice in each group. Referring to previous reports[16-19], IGF-1 (50 μg/kg) was administered every day by intraperitoneal injection in the treatment group. The mice in the control group received the same amount of normal saline. Three weeks after treatment, the cancer tissues were dissected from the nude mice for measurement of LVD.
Measurement of LVD
LVD in tissue sections was quantitatively analyzed using the EnVision system with the specific lymphatic endothelial cell marker, D2-40, which allows for accurate discrimination of lymphatic vessels from blood vessels[3,13]. Five fields with the most abundant lymphatic regions (hot spots) were chosen by light microscopy at 40 × magnification. The LVD was then assessed by counting all stained vessels at 200 × magnification. Single immunoreactive endothelial cells or endothelial cell clusters separated from other microvessels were counted as a vessel according to previous procedures[20]. The average number of lymphatic vessels in five selected fields was taken as the LVD on the slide.
Statistical analysis
All statistical analyses were performed using SPSS (version 12.0, SPSS Inc, United States). Student’s t test test, Mann-Whitney U test and χ2 test were used for statistical analyses. Spearman’s rank correlation was used for correlation analysis. P values < 0.05 were considered statistically significant.
RESULTS
Up-regulation of IGF-1 and IGF-1R in colorectal cancer
Immunohistochemistry analysis was performed to examine the expression of IGF-1 and IGF-1R in CRC samples. IGF-1 and IGF-1R were weakly or negatively stained in tumor-adjacent normal tissues. In contrast, they were weakly or moderately stained in well-differentiated CRC tissues, and moderately or strongly stained in moderately and poorly differentiated CRC tissues (Table 1 and Figure 1). As expected, expression of IGF-1 was detected primarily in the cytoplasm and IGF-1R was detected on the membrane (Figure 1). Furthermore, the expression of IGF-1 and IGF-1R was correlated with lymph node metastasis (r = 0.715 and 0.569, respectively; P < 0.05) and TNM stage (r = 0.731 and 0.609, respectively; P < 0.05). These results indicate that elevated expression of IGF-1/IGF1R parallels CRC progression.
Table 1.
Expression of insulin-like growth factor-1 and insulin-like growth factor-1 receptor in colorectal cancer tissues
| n | IGF-1 | P value | IGF-1R | P value | |
| Tissue type | 0.001 | 0.002 | |||
| Adjacent normal tissues | 40 | 1.4 ± 0.6 | 1.3 ± 1.2 | ||
| Cancer tissue | 40 | 4.5 ± 2.5 | 4.7 ± 2.7 | ||
| Differentiation | 0.012 | 0.001 | |||
| Well | 9 | 3.0 ± 1.4 | 2.1 ± 0.9 | ||
| Moderately | 20 | 4.3 ± 2.6 | 4.6 ± 2.4 | ||
| Poorly | 11 | 6.1 ± 2.1 | 6.6 ± 2.1 | ||
| Lymph node metastasis | 0.003 | 0.002 | |||
| Yes | 15 | 6.8 ± 2.0 | 6.3 ± 2.2 | ||
| No | 25 | 3.1 ± 1.5 | 3.5 ± 2.2 |
IGF-I: Insulin-like growth factor-1; IGF-I R: Insulin-like growth factor-1 receptor.
Figure 1.
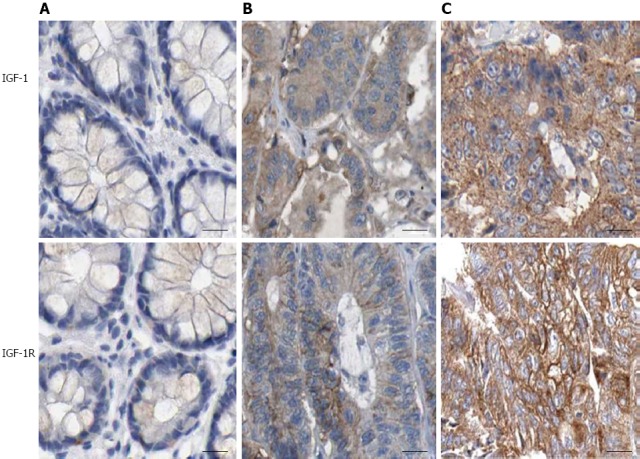
Immunohistochemical staining of insulin-like growth factor-1 and insulin-like growth factor-1 receptor in colorectal cancer tissues. A: Tumor-adjacent normal tissues; B: Moderately differentiated CRC and C, poorly differentiated CRC (× 200). IGF-1: Insulin-like growth factor-1. IGF-I R: Insulin-like growth factor-1 receptor; CRC: Colorectal cancer.
Correlation between LVD and expression of
IGF-1/IGF-1R
It was reported that LVD is an indicator of lymphangiogenesis[3,21]. D2-40 is a specific lymphatic endothelial cell marker for the evaluation of LVD in human cancers[3,13]. Using IHC, we found that D2-40 was localized in the cytoplasm and membrane of lymphatic endothelial cells (Figure 2). LVD was significantly higher in CRC tissue as compared to non-tumor tissue (11.45 ± 3.03 vs 3.25 ± 1.28, P < 0.05). LVD in tumor tissues with lymph node metastasis was higher than that in tissues without metastasis (12.67 ± 4.09 vs 10.72 ± 1.90, P < 0.05) (Table 2 and Figure 2). Lymphatic vessel invasion was also detected in CRC tissue (Figure 2B and C). There was a significant correlation between LVD and lymphatic metastasis (R = 0.405, P < 0.05). In addition, a statistically significant correlation was also found between LVD and IGF-1R (R = 0.437, P < 0.05). These results suggest that the IGF-1/IGF-1R system might promote lymph node metastasis of CRC through induction of lymphangiogenesis.
Figure 2.
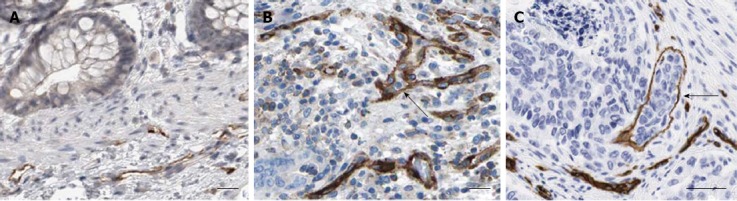
Morphological features of D2-40 positive lymphatic vessels in colorectal cancer. A: Tumor-adjacent normal tissues; B and C: Colorectal cancer tissues (× 200). Lymphatic vessel invasion of tumor cells was also detected in B and C (black arrow). IGF-1: Insulin-like growth factor-1.
Table 2.
Lymphatic vessel density in colorectal cancer tissues
| n | LVD | P value | |
| Tissue type | 0.001 | ||
| Adjacent normal tissues | 40 | 3.3 ± 1.3 | |
| Cancer tissue | 40 | 11.5 ± 3.0 | |
| Lymph node metastasis | 0.032 | ||
| Yes | 15 | 12.7 ± 4.1 | |
| No | 25 | 10.7 ± 3.9 |
LVD: Lymphatic vessel density.
IGF-1 increases migration and invasion of LoVo cells
In transwell migration assays, a significantly higher number of cells migrated through the chamber membrane after IGF-1 treatment (196.4 ± 21.6 vs 96.4 ± 11.2, P < 0.05, Figure 3A). Similar results were obtained in the transwell invasion assays. Cells which migrated through the matrigel and chamber membrane increased in the IGF-1-treated group (163.6 ± 19.4 vs 72.5 ± 9.1, P < 0.05, Figure 3B). These results indicated that IGF-1 increased the migration and invasion potential of CRC cells.
Figure 3.
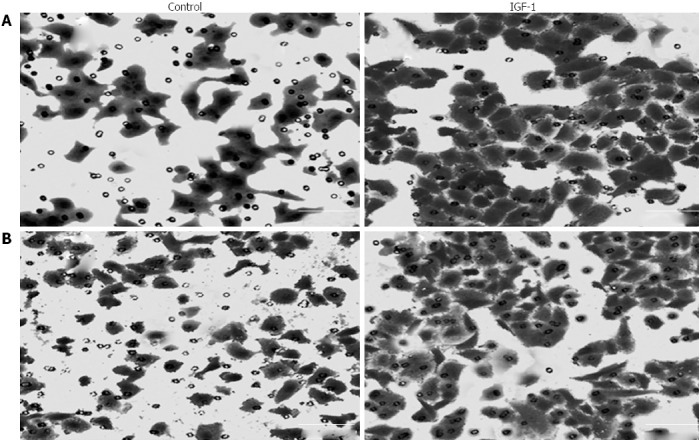
Effect of insulin-like growth factor-1 on the migration and invasion of LoVo cells after 48 h treatment. A: Cell migration; B: Cell invasion (× 200). IGF-1: Insulin-like growth factor-1.
Effects of IGF-1 on LVD of LoVo cell xenografts in nude mice
To assess whether IGF-1 affected lymphangiogenesis in CRC in vivo, LoVo cells were implanted subcutaneously into nude mice. Three weeks after daily IGF-1 or vehicle administration, tumor tissues were removed and measured. IHC analysis using anti-D2-40 antibodies revealed that LVD of LoVo cell xenografts was significantly higher in the IGF-1 group than in the control group (10.7 ± 3.3 vs 6.4 ± 2.9, P < 0.05, Figure 4).
Figure 4.
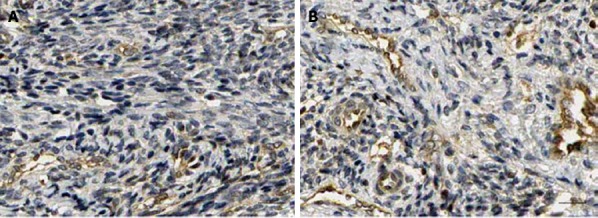
Effect of insulin-like growth factor-1 on lymphatic vessel density in LoVo cell xenografts in nude mice after treatment with insulin-like growth factor-1. A: Control group; B: 50 μg/kg IGF-1 group (× 200). IGF-1: Insulin-like growth factor-1; IGF-I R: Insulin-like growth factor-1 receptor.
DISCUSSION
The importance of lymph node metastasis in cancer progression has been well established and is considered one of the most important prognostic factors[1,2,22,23]. Recently, there is growing evidence that tumor lymphangiogenesis plays an important role in this process[5,6]. However, the detailed molecular mechanisms that regulate lymphangiogenesis remain largely unknown. In the present study, we have shown that IGF-1/IGF-1R signaling can induce tumor-associated lymphangiogenesis, and therefore, may facilitate further lymphatic metastasis of CRC.
IGF-1 and its receptor IGF-1R are frequently expressed in many solid tumors and have been implicated in cancer metastasis[7,24-26]. In 1999, Hakam et al[27] showed that IGF1-R plays a role in the evolution of colorectal adenoma to carcinoma and favors the metastasis of CRC. In a recent study, Wu et al[28] reported that IGF-1 is critical in activating and sustaining an inflammatory response in the liver which is needed for CRC hepatic metastasis. In the present study, we found that IGF-1 and IGF-1R protein expression was elevated in CRC tissues, and their expression was correlated with clinical stage and lymphatic metastasis, which is consistent with the reported data.
Lymphangiogenesis, the formation of new lymphatic vessels, is a key process in lymphatic invasion and metastasis[29]. Numerous studies have shown that LVD is an indicator of lymphangiogenesis and represents a tool to determine the metastatic risk of neoplasia[3,21,29]. A study by Cacchi et al[1] revealed that CRC could induce lymphangiogenesis and a higher LVD could increase the capability of cancer cells to invade the lymphatic system. In another study, Matsumoto et al[30] reported that both LVD and lymphatic vessel invasion were related to an adverse outcome in CRC. Similar results were obtained in this study. In addition, lymphatic vessel invasion was also detected in CRC tissue by IHC staining. Moreover, a statistically significant correlation was found between LVD and the level of IGF-1R in CRC tissue. This suggests that IGF-1/IGF-1R signaling probably facilitates metastasis of CRC by inducing tumor-associated lymphangiogenesis.
Recently, a number of lymphangiogenic factors which stimulate the proliferation of lymphatic vessels and lymphangiogenesis have been identified, including vascular endothelial growth factor (VEGF)-A, VEGF-C, VEGF-D, fibroblast growth factor 2, and platelet-derived growth factors[11,31]. Most of these lymphangiogenic factors were shown to be directly or indirectly regulated by IGF-1[11,12,32]. These findings prompted us to hypothesize that IGF-1/IGF-1R signaling promotes lymphangiogenesis. In 2005, Björndahl et al[11] reported that both IGF-1 and IGF-2 could stimulate lymphatic vessel growth in the mouse cornea. However, to date, it is still unclear whether a similar correlation exists in solid tumors. Here, we showed that IGF-1 could also induce lymphangiogenesis in CRC. The spread of metastasis then occurs through the new lymphatic vessel system in the tumor.
Tumor lymphangiogenesis and metastasis to lymph nodes are a complex process and a number of growth factors are involved in these events. IGFs are particularly interesting regulators due to their multiple roles in promoting tumor growth[33-35]. Here, the in vivo studies provided direct evidence that the IGF-1/IGF-1R axis can also induce lymphangiogenesis in solid tumors. However, so far, it is not known whether the IGF-1/IGF-1R axis directly acts on lymphatic endothelial cells to induce lymphangiogenesis or indirectly via other growth factor/receptor systems. In addition, the detailed mechanisms underlying lymphangiogenesis associated with the IGF-1/IGF-1R system are largely unknown. Therefore, additional efforts are warranted to study the relationship between IGF-1/IGF-1R and lymphangiogenesis in cancer.
In summary, we found that the IGF-1/IGF-1R system can induce tumor-associated lymphangiogenesis and facilitate lymphatic metastasis of CRC. Findings from the present study provide further evidence supporting the involvement of IGF-1/IGF-1R signaling in lymphangiogenesis in solid tumors. In addition, we also found that increased expression of IGF-1 and IGF-1R is correlated with tumor differentiation in human CRC. Our findings and others suggest that the IGF-1/IGF-1R axis plays critical and diverse roles in promoting tumor progression. Taken together, the IGF-1/IGF-1R axis is a potentially useful target for the treatment of cancer and metastasis.
COMMENTS
Background
Lymphangiogenesis plays an important role in lymphatic metastasis. Insulin-like growth factor-1 (IGF-1) and insulin-like growth factor-1 receptor (IGF-1R) are frequently overexpressed in many types of tumors including colorectal cancer. In addition to their role in the development and progression of cancer, the IGF-1/IGF-1R signaling system can also induce lymphangiogenesis.
Research frontiers
A recent study (Björndahl et al, 2003) showed that both IGF-1 and IGF-2 potently stimulated lymphatic vessel growth in the mouse cornea. However, equivalent evidence in solid tumors is lacking.
Innovations and breakthroughs
The authors of this paper demonstrate that the IGF-1/IGF-1R system can induce tumor-associated lymphangiogenesis in colorectal cancer (CRC) and contributes to its lymphatic metastasis, thus providing a novel mechanism for lymphangiogenesis in solid tumors.
Applications
The findings of this study are of value in further explaining the molecular mechanisms of lymphangiogenesis in CRC. The IGF-1/IGF-1R axis may be a potential target for the treatment of cancer and metastasis.
Terminology
IGF-1 is a hormone similar in molecular structure to insulin. It plays an important role in child growth and continues to have anabolic effects in adults. The role of IGF-1 in promoting cancer has been investigated for many years. Increasing evidence suggests that IGF-1 not only is associated with an increased relative risk for the development of cancer, but also contributes to cancer cell survival, invasion, metastasis and resistance to chemotherapeutic drugs.
Peer review
The authors examined the expression of IGF-1/IGF-1R in CRC tissues and its correlation with lymphangiogenesis and lymphatic metastasis. The study revealed that IGF-1/IGF-1R signaling system induces lymphangiogenesis in CRC and contributes to lymphatic metastasis of CRC. The results are interesting and provide further evidence that IGF-1/IGF-1R signaling is involved in lymphangiogenesis in solid tumors.
Footnotes
Supported by Technological Research Project for Public Welfare from Science and Technology Department of Zhejiang Province, No. 2010C33099
P- Reviewers: Da MX, Lee SY, Natsugoe S, Shibata MA S- Editor: Cui XM L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Cacchi C, Arnholdt HM, Jähnig H, Anthuber M, Probst A, Oruzio DV, Märkl B. Clinical significance of lymph vessel density in T3 colorectal carcinoma. Int J Colorectal Dis. 2012;27:721–726. doi: 10.1007/s00384-011-1373-7. [DOI] [PubMed] [Google Scholar]
- 2.Gao J, Knutsen A, Arbman G, Carstensen J, Frånlund B, Sun XF. Clinical and biological significance of angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver Dis. 2009;41:116–122. doi: 10.1016/j.dld.2008.07.315. [DOI] [PubMed] [Google Scholar]
- 3.Holmqvist A, Gao J, Adell G, Carstensen J, Sun XF. The location of lymphangiogenesis is an independent prognostic factor in rectal cancers with or without preoperative radiotherapy. Ann Oncol. 2010;21:512–517. doi: 10.1093/annonc/mdp486. [DOI] [PubMed] [Google Scholar]
- 4.Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- 5.Wang TB, Chen ZG, Wei XQ, Wei B, Dong WG. Serum vascular endothelial growth factor-C and lymphoangiogenesis are associated with the lymph node metastasis and prognosis of patients with colorectal cancer. ANZ J Surg. 2011;81:694–699. doi: 10.1111/j.1445-2197.2010.05539.x. [DOI] [PubMed] [Google Scholar]
- 6.Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J Gastroenterol. 2010;16:4003–4012. doi: 10.3748/wjg.v16.i32.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fürstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3:298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 8.Hermani A, Shukla A, Medunjanin S, Werner H, Mayer D. Insulin-like growth factor binding protein-4 and -5 modulate ligand-dependent estrogen receptor-α activation in breast cancer cells in an IGF-independent manner. Cell Signal. 2013;25:1395–1402. doi: 10.1016/j.cellsig.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Neuhouser ML, Platz EA, Till C, Tangen CM, Goodman PJ, Kristal A, Parnes HL, Tao Y, Figg WD, Lucia MS, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Prev Res (Phila) 2013;6:91–99. doi: 10.1158/1940-6207.CAPR-12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107:873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 11.Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da MX, Wu Z, Tian HW. Tumor lymphangiogenesis and lymphangiogenic growth factors. Arch Med Res. 2008;39:365–372. doi: 10.1016/j.arcmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Yan G, Zhou XY, Cai SJ, Zhang GH, Peng JJ, Du X. Lymphangiogenic and angiogenic microvessel density in human primary sporadic colorectal carcinoma. World J Gastroenterol. 2008;14:101–107. doi: 10.3748/wjg.14.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C, editors . TNM classification of malignant tumors. 7th ed. Weinheim, Germany: Wiley; 2009. [Google Scholar]
- 15.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt (Ser473) is an excellent predictor of poor clinical outcome in prostate cancer. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi C, Bott LC, Chen KL, Harmison GG, Katsuno M, Sobue G, Pennuto M, Fischbeck KH. Insulinlike growth factor (IGF)-1 administration ameliorates disease manifestations in a mouse model of spinal and bulbar muscular atrophy. Mol Med. 2012;18:1261–1268. doi: 10.2119/molmed.2012.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali SG, Brinkman AS, Solverson P, Pun W, Pintar JE, Ney DM. Exogenous GLP-2 and IGF-I induce a differential intestinal response in IGF binding protein-3 and -5 double knockout mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G794–G804. doi: 10.1152/ajpgi.00372.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zacharakis E, Demetriades H, Pramateftakis MG, Lambrou I, Zacharakis E, Zaraboukas T, Koliakos G, Kanellos I, Betsis D. Effect of IGF-I on healing of colonic anastomoses in rats under 5-FU treatment. J Surg Res. 2008;144:138–144. doi: 10.1016/j.jss.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Zacharakis E, Demetriades H, Kanellos D, Sapidis N, Zacharakis E, Mantzoros I, Kanellos I, Koliakos G, Zaraboukas T, Topouridou K, et al. Contribution of insulin-like growth factor I to the healing of colonic anastomoses in rats. J Invest Surg. 2007;20:9–14. doi: 10.1080/08941930601126074. [DOI] [PubMed] [Google Scholar]
- 20.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barresi V, Reggiani-Bonetti L, Di Gregorio C, De Leon MP, Barresi G. Lymphatic vessel density and its prognostic value in stage I colorectal carcinoma. J Clin Pathol. 2011;64:6–12. doi: 10.1136/jcp.2010.083550. [DOI] [PubMed] [Google Scholar]
- 22.Yuan Y, Li MD, Hu HG, Dong CX, Chen JQ, Li XF, Li JJ, Shen H. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World J Gastroenterol. 2013;19:2650–2659. doi: 10.3748/wjg.v19.i17.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SR, Kim HO, Son BH, Yoo CH, Shin JH. Prognostic factors associated with long-term survival and recurrence in pancreatic adenocarcinoma. Hepatogastroenterology. 2013;60:358–362. doi: 10.5754/hge12727. [DOI] [PubMed] [Google Scholar]
- 24.Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y, Yoshikawa H, Rosen CJ, Mundy GR, Yoneda T. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res. 2012;72:4238–4249. doi: 10.1158/0008-5472.CAN-11-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang F, Xu LA, Khambata-Ford S. Correlation between gene expression of IGF-1R pathway markers and cetuximab benefit in metastatic colorectal cancer. Clin Cancer Res. 2012;18:1156–1166. doi: 10.1158/1078-0432.CCR-11-1135. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Hao L, Wang L, Xiao Y, Ge H, Zhu Z, Luo Y, Zhang Y, Zhang Y. Elevated IGFIR expression regulating VEGF and VEGF-C predicts lymph node metastasis in human colorectal cancer. BMC Cancer. 2010;10:184. doi: 10.1186/1471-2407-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakam A, Yeatman TJ, Lu L, Mora L, Marcet G, Nicosia SV, Karl RC, Coppola D. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Brodt P, Sun H, Mejia W, Novosyadlyy R, Nunez N, Chen X, Mendoza A, Hong SH, Khanna C, et al. Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res. 2010;70:57–67. doi: 10.1158/0008-5472.CAN-09-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M, Ma SP, Lin HZ, Ji P, Xie D, Yu JX. Intratumoral as well as peritumoral lymphatic vessel invasion correlates with lymph node metastasis and unfavourable outcome in colorectal cancer. Clin Exp Metastasis. 2010;27:123–132. doi: 10.1007/s10585-010-9309-0. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto K, Nakayama Y, Inoue Y, Minagawa N, Katsuki T, Shibao K, Tsurudome Y, Hirata K, Nagata N, Itoh H. Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum. 2007;50:308–314. doi: 10.1007/s10350-006-0792-y. [DOI] [PubMed] [Google Scholar]
- 31.Stefansson IM, Salvesen HB, Akslen LA. Vascular proliferation is important for clinical progress of endometrial cancer. Cancer Res. 2006;66:3303–3309. doi: 10.1158/0008-5472.CAN-05-1163. [DOI] [PubMed] [Google Scholar]
- 32.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arcaro A. Targeting the insulin-like growth factor-1 receptor in human cancer. Front Pharmacol. 2013;4:30. doi: 10.3389/fphar.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruchim I, Werner H. Targeting IGF-1 signaling pathways in gynecologic malignancies. Expert Opin Ther Targets. 2013;17:307–320. doi: 10.1517/14728222.2013.749863. [DOI] [PubMed] [Google Scholar]
- 35.Martin JL, Baxter RC. Signalling pathways of insulin-like growth factors (IGFs) and IGF binding protein-3. Growth Factors. 2011;29:235–244. doi: 10.3109/08977194.2011.614237. [DOI] [PubMed] [Google Scholar]


