Abstract
Dengue virus co-circulates as four serotypes and sequential infections with more than one serotype are common. One hypothesis for the increased severity seen in secondary infections is antibody dependent enhancement (ADE) leading to increased replication in Fc-receptor-bearing cells. In this study we have generated a panel of human monoclonal antibodies to dengue virus. Antibodies to the structural precursor-membrane protein (prM) dominate the response. These antibodies are highly cross-reactive among the dengue virus serotypes and, even at high concentrations, do not neutralise infection but potently promote ADE. We propose that the partial cleavage of prM from the viral surface reduces the density of antigen available for viral neutralisation, leaving dengue viruses susceptible to ADE by anti-prM, a finding which has implications for future vaccine design.
Dengue virus (DENV) is a mosquito borne virus infection found in tropical and subtropical areas of the world, with an estimated 50-100 million infections per annum (1). Sequence variation of 30-35% allows DENV to be divided into four serotypes, and infection with one serotype does not provide protection to infection with the other serotypes meaning secondary or sequential infections are common (2, 3). Serious complications of dengue haemorrhagic fever (DHF) are more likely during secondary versus primary infections (2, 3).
In 1977 Halstead suggested ADE to explain severe DENV infections (4). ADE has been widely studied and results from the high sequence divergence between DENV such that antibody to the first infection may not be of sufficient avidity to neutralise a secondary infection (5). The partial cross reactivity may cause a degree of opsonisation that promotes virus uptake into Fc bearing cells such as monocytes/macrophages, a major site of DENV replication in vivo, leading to increased virus replication.
DENV envelope contains 180 copies of the E glycoprotein, which can be found in either dimeric or trimeric (pre-fusion) conformation (6). prM is a 166 amino acid protein intimately associated in a 1:1 fashion with domain II of E (7), and is believed to act as a chaperone for the folding of E and prevent the premature fusion of virus to membranes inside the producing cell. prM contains a furin cleavage site, and is cleaved into a C-terminal M portion containing a transmembrane domain that remains associated with the virus particle, and an N-terminal 91 amino acid pr fragment that dissociates upon release of the virus from the infected cell.
B cells from seven DENV infected individuals (Table S1) were used to produce human mAb using the method of Traggiai (8). Culture supernatants were screened against structural antigens using whole virus and against non-structural protein 1 (NS1) by ELISA.
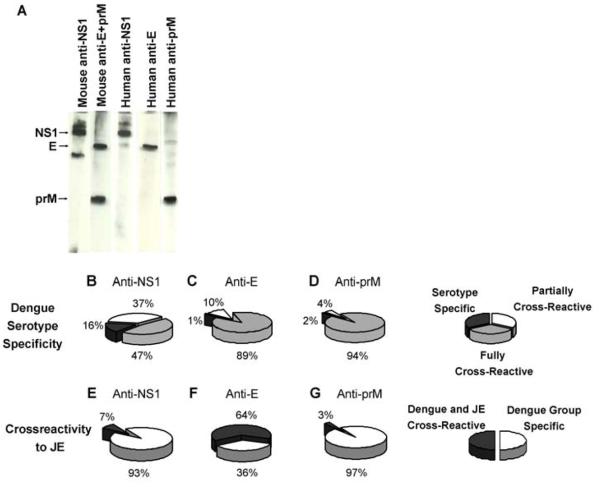
301 of 3020 cell lines screened positive; 73% reacted to the whole virus ELISA for structural antigens and 27% to NS1. Positive supernatants were tested for reactivity to specific DENV antigens by non-reducing Western blot (Fig. 1A). When the supernatants reacting to whole DENV were tested, 78% gave a positive signal by Western blot, all of these reacted to either E or prM with no reactivity to capsid. Interestingly, the anti-prM response was dominant (60% CI 67.3-52.2%) compared with the response to E (40%); subgroup analysis of each of the individual cases is shown in table S2.
Figure 1. Specificity of human antibodies (n=301).
Western Blot of infected cell lysates (non-reduced) showing reactivity of antibodies with dengue NS1, E and prM proteins (A). Cross-reactivity of human mAb within the DENV serotypes (B-D) or between the DENV group and JEV (E-G)
We next assessed the serotype specificity of the human antibodies by dot blot against the four viral serotypes, which showed a divergence in the cross reactivity between the anti-NS1 and structural (anti-E and anti-prM) groups of antibodies. Half of the anti-NS1 showed limited cross reactivity among DENV, while most of the structural antibodies showed full cross reactivity against all virus serotypes (Fig. 1B-D). As these antibodies were made from secondary cases of DENV we investigated primary anti-prM responses. Western blotting of DENV infected cell lysates demonstrate that cross-reactive anti-prM responses are made during the primary infection (Fig. S1) although as has been reported before, the anti-prM response is amplified following secondary infection (9).
Finally, we tested cross reactivity to the related flavivirus Japanese encephalitis virus (JEV) which co circulates with DENV in some parts of SE Asia (Fig. 1E-G). Interestingly, only 3% of the anti-prM antibodies cross reacted with JEV in contrast to the antibodies recognizing envelope which showed 64% cross reactivity. The relative specificity of anti-prM to the DENV may reflect the lower sequence conservation between prM sequences (35% DENV vs. JEV) compared with E (50%); a comparison of sequence conservation among other members of the family flaviviridae can be found in Table S3.
Six monoclonal anti-prM mAbs were produced, Western blotting showed that at least 5/6 react with the cleaved pr peptide and reactivity was lost to reduced antigen implying that they recognize conformational epitopes (Fig. S2).
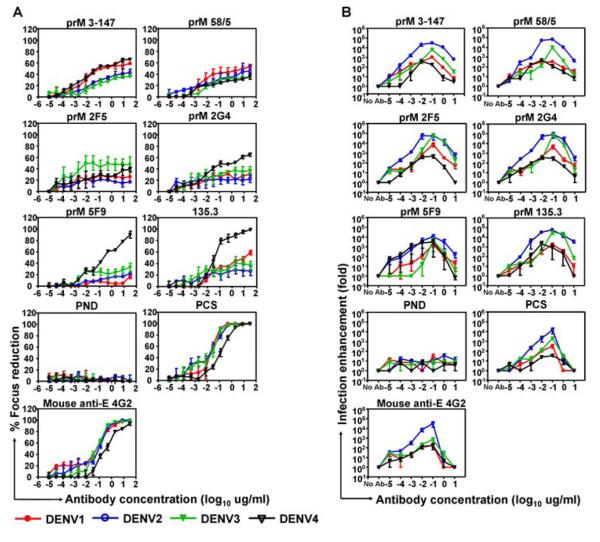
In general, the anti-prM antibodies were unable to completely neutralise infection (Fig. 2A). Instead, neutralisation plateaued between 10 and 60% and the partial neutralisation was largely cross-reactive among the four virus serotypes, the only exceptions being mAb 5F9 and 135.3 which showed almost 100% neutralisation of DENV4 at high antibody concentrations. This partial neutralisation was in contrast to results seen with pooled convalescent dengue serum (PCS) or anti-envelope monoclonal antibodies, where neutralisation approached or reached 100%.
Figure 2. Partial neutralisation but potent enhancement by human anti-prM monoclonal antibodies.
Neutralisation assays (A) and enhancement assays (B) were performed with the six human anti-prM mAbs (clones 3-147, 58/5, 2F5, 2G4, 5F9 and 135.3), murine anti-E mAb (4G2) and purified Ig from pooled dengue convalescent serum (PCS) and pooled non dengue immune serum (PND) were used as controls; mean±SE from three independent experiments.
Next, we performed ADE assays using U937 cells as targets where virus was pre-incubated with an increasing titre of antibody before addition to the Fc-receptor-bearing cells. Enhancement of infection was seen with all six human anti-prM monoclonal antibodies with a peak of nearly 105 fold (Fig. 2B) which is consistent with a report of ADE with murine anti-prM mAb (10).
To ascertain whether the results with these six anti-prM mAbs were representative we tested the enhancing and neutralising capacity of a further 20 anti-prM and 20 anti-E cell lines as well as two irrelevant human antibodies (Fig. S3A&B). None of the anti-prM antibodies showed a high level of neutralisation (19/19 <80%), whereas 12/20 anti-E showed >90% and 6/20 showed 100% neutralisation. All the anti-prM antibodies showed ADE of 10-800 fold, while the anti-E antibodies showed more variable ADE.
The failure of anti-prM antibodies to fully neutralise dengue viruses with a clear plateau in the response was puzzling and suggested the virus may exist in two populations, one susceptible to neutralisation and another not. Cleavage of prM during viral maturation is believed to be a prerequisite for viral replication, which is exemplified by the very low infectivity of DENV and tick borne encephalitis viral particles with wholly uncleaved prM (11-13). In many virus preparations prM cleavage is incomplete and cyro-electron microscopy yields particles that contain both full length prM and processed M protein, suggesting that a distribution of virus maturation may be present in virus cultures (14, 15). To our knowledge, it has not been formally demonstrated whether only fully processed virus is infectious or whether the virus can contain small numbers of prM molecules at its surface and still remain infectious. The demonstration here that the human anti-prM antibodies can show partial neutralisation implies that some prM containing particles remain infectious.
The propensity towards incomplete cleavage of prM in DENV leads to two interesting predictions, first that the density of prM at the surface of the virus may not be high enough to allow full neutralisation with most anti-prM antibodies. Instead, viruses with low levels of prM may be susceptible to ADE. Second, viruses which are inherently non-infectious by virtue of displaying a high density of prM may be rendered infectious by ADE.
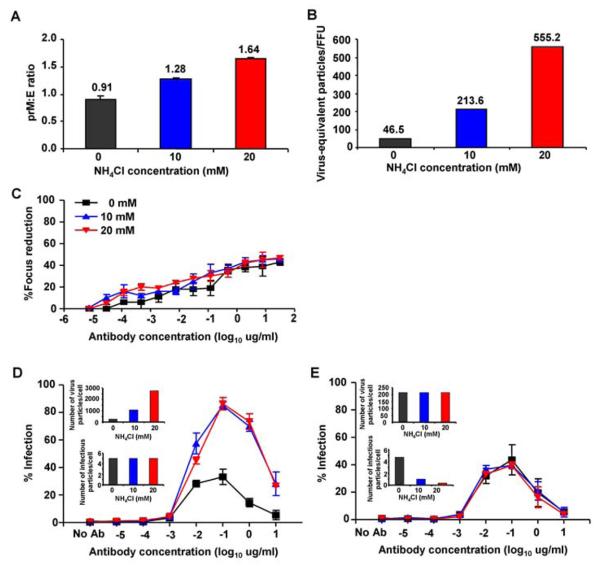
To investigate the effect of prM cleavage on neutralisation and enhancement, virus was produced in cells cultured in the presence of ammonium chloride to raise intracellular pH and reduce the efficiency of furin cleavage (12). ELISA assays were performed to measure E and prM in the virus preparation; the E assay was calibrated by plotting a standard curve using recombinant E protein produced in Sf9 cells. A measure of the number of potential virus particles (virus equivalent particles) was derived assuming each particle contained 180 copies of E and the relative density of uncleaved prM, calculated by the ratio of prM:E. The prM:E ratio was increased by roughly 40% and 80% when virus was cultured in 10 mM and 20 mM ammonium chloride respectively (Fig. 3A). As expected, the infectivity of virus produced in the presence of NH4Cl was markedly reduced from 46 to 555 virus-equivalent particles/FFU (Fig. 3B). Although infectivity was reduced, infectious virus produced under each condition remained partially susceptible to neutralisation as before, and the titration curves for virus produced in 0, 10 and 20 mM NH4Cl were similar (Fig. 3C).
Figure 3. Anti-prM can rescue infectivity in virus containing high densities of prM.
DENV produced in the presence of NH4Cl. The density of uncleaved prM and E were measured by ELISA and expressed as the prM:E ratio (A). Infectivity was determined in Vero cells, expressed as FFU, and the amount of total virus-equivalent particles was calculated based upon the concentration of E protein measured by a sensitive sandwich ELISA. Data is presented as virus-equivalent particles/FFU ratio (B). Neutralisation assays with purified human anti-prM mAb (3-147) (C). Enhancement assays of U937 cells read out by FACS based intracellular staining for DENV antigens (4G2) using either a constant amount of infectious virus (D) or constant number of virus particles (E); mean±SE from three independent experiments.
We next tested enhancement of these viruses using either a constant amount of infectious virus i.e. fixed focus forming units (Fig. 3D) or a constant amount of virus equivalent particles (Fig 3E). These results show that the relatively poorly infectious virus cultured in the presence of NH4Cl can be rendered much more infectious in the presence of enhancing anti-prM antibodies and indeed can be restored nearly to the level of control virus (Fig. 3E). These results were further exemplified using virus produced in LoVo cells that lack functional furin and therefore produce virus with very low levels of cleaved prM (Fig. S4)(13). Virus produced in LoVo cells as expected had a high prM:E ratio and very low infectivity (< 10×10-5 ffu/virus equivalent particle) that could be enhanced in the presence of anti-prM antibody.
Three populations of dengue virus appear to be produced: first, a population containing relatively high levels of prM that are inherently non-infectious but that can be made infectious in the presence of enhancing anti-prM antibodies; second, a population with an intermediate density of prM at the surface that can infect, but are susceptible to neutralisation at high antibody titre; third, a population with low or absent prM at the surface that would not normally be susceptible to neutralisation.
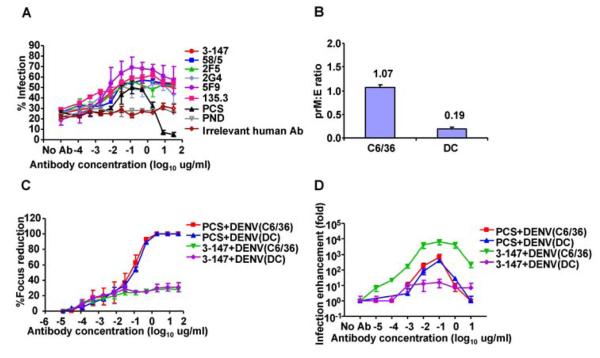
To test the relative roles of the anti-prM antibodies in neutralisation and enhancement of primary cells we looked at human monocytes, which are thought to be a major site of virus replication in vivo. Monocytes can be infected in the absence of antibody and as they express Fc receptors infection can be increased by ADE. To our surprise, human anti-prM mAbs failed to show any neutralisation activity on primary monocytes and instead, even at concentrations of antibody as high as 30 μg/ml, enhanced infection from 20% to 70% (Fig. 4A) over a large range of antibody concentration.
Figure 4. The roles of anti-prM on neutralisation and enhancement of DENV infection of PBMC.
PBMC were infected with DENV2 in the presence of human anti-prM mAbs, at 24hrs DENV Ag was stained intracellularly (4G2) and detected by flow cytometry in gated monocytes (A). PCS, PND and irrelevant human mAb were used as control. The density of prM on DENV from C6/36 cells and DC were detected by ELISA and presented as prM:E ratio (B). Neutralisation (C) and antibody-dependent enhancement of infection (D), performed on Vero and U937 cells respectively, of DENV generated from either C6/36 cells or DC in the presence of PCS or anti-prM mAb (3-147); mean±SE from three independent experiments.
Virus was generated in the insect cell line C6/36, which is known to cleave prM inefficiently, and the results we have obtained are therefore analagous to the first encounter with DENV i.e., insect-produced virus following a bite from an infected mosquito. Finally, we set out to determine whether virus produced in primary mammalian cells contained non-cleaved prM and whether anti-prM had any enhancing capacity on such virus. Virus was produced in immature dendritic cells (DC) where cleavage of prM was more efficient than in the insect cell culture, but still not complete (Fig. 4B). As with insect-produced virus, the anti-prM antibodies were unable to fully neutralise DC-produced virus with a clear plateau in efficacy (Fig. 4C), but were still able to enhance infection, although to a lesser degree (Fig. 4D).
This is the first description, using human monoclonal antibodies, of the serological response in DENV infection. Anti-prM is a dominant component of the response and most of these antibodies display limited virus neutralisation capacity. The combination of partial cleavage of prM, together with substantial crossreaction between serotypes, makes the anti-prM response particularly susceptible to enhancement. Promotion of such an antibody response towards anti-prM could thus be interpreted as immune evasion or even as an immune enhancement strategy of the virus.
Most current DENV vaccine candidates, whether naturally attenuated, recombinantly attenuated, chemically inactivated virus or DENV/yellow fever chimeras contain native dengue prM sequences (16). It may be advisable to design DENV vaccines that minimise the anti-prM response. There is relatively low sequence conservation between DENV prM and sequences from other flaviviruses and the majority of anti-dengue prM antibodies do not crossreact with JEV. Chimeric attenuated viruses containing heterologous flaviviral prM sequences may therefore not lead to such cross-reactive anti-prM responses as seen on infection with viruses containing native dengue prM sequences, although the feasibility of making such chimeras has to our knowledge not yet been tested.
Material and Methods
Samples
Blood samples were taken from patients after consent and approvals from the ethical committee of Siriraj, Khon Kaen and Songkhla hospitals, Thailand. The study was also approved by the Riverside Ethics Committee in the UK. Acute DENV infection was identified by RT-PCR-based DENV gene identification or DENV-specific IgG and IgM capture ELISA (17, 18). Disease severity was classified according to the World Health Organization criteria (19).
Of the patients enrolled in the study, 1 patient was classified as DF, 6 as DHF. Blood samples were collected in heparin (BD). PBMC were isolated from whole blood by Ficoll-Hypaque density gradient centrifugation and cryopreserved until tested. Characteristics of DENV-infected cases enrolled in the study are summarised in table S1.
Cells and Antibodies
C6/36, a cell line derived from the mosquito Aedes albopictus was cultured in Leibovitz L-15 medium supplemented with 10% heat-inactivated foetal bovine serum (FBS), 0.26% tryptose phosphate broth (TPB), 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM L-Glutamine at 28°C. For endotoxin-free conditions, cells were grown in the absence of TPB. Vero, a cell line derived from the kidney of African green monkeys and U937, a human monocytic cell line, were grown in MEM and RPMI1640, respectively. The media were supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mM L-Glutamine in a 37°C humidified 5% CO2 incubator. Monocyte-derived dendritic cells (DC) were prepared as previously described (20). LoVo cells were cultured in Nutrient mixture (Ham) F12 medium containing 20% FBS.
Conjugated antibodies against human or mouse Ig (DAKO) were used. Pooled convalescent dengue hyperimmune human serum (PCS) (hemagglutination titre ≥ 1/25600), pooled non-dengue immune serum (PND) (hemaggutination inhibition titre and anti-dengue Ab ELISA negative) and mouse anti-DENV envelope, 4G2, were kindly provided by AFRIMS, Thailand. NS1-F3, 2G6 and 1H10 are anti-NS1 and anti-prM mAb, respectively (21, 22).
Virus stock
DENV serotype 1 (Hawaii), serotype 2 (16681), serotype 3 (H87) and serotype 4 (H241) were propagated in C6/36 cells and virus supernatant was collected and stored at −80°C. The DENV stock propagated from C6/36 and MDDC's were free from endotoxin and mycoplasma detected by Limulus amebocyte lysate assay (Whittaker M.A.) and PCR using the mycoplasma detection set (TAKARA BIO INC), respectively. For poorly infectious DENV, C6/36 cells were infected with DENV2. Four days after infection, culture medium was replaced by fresh L-15 containing 1.5% FBS and 0.26% TPB with 10 or 20 mM NH4Cl for 2 hrs and the medium was then replaced again. At 24 hrs after the medium containing NH4Cl was added, virus particles were harvested and precipitated by 10% PEG 8000. Fully immature virus was produced on LoVo cells as previously described (13). Briefly, virus was produced by infecting LoVo cells with DENV2 strain 16681 at MOI of 10 and virus was harvested at 2 days.
Focus forming assay
The titres of virus were determined by a focus forming assay on Vero cells and expressed as focus-forming units (FFU) per ml. Briefly, virus was serially diluted and incubated with Vero cells for 2 hrs at 37°C. The monolayers were then overlaid with 1.5% carboxymethylcellulose and incubated at 37°C for 3 days. Virus foci were stained with anti-E antibody (4G2) followed by peroxidase-conjugated anti-mouse Ig and visualized by the addition of DAB substrate.
Generation of dengue-specific human monoclonal Abs
DENV-specific human mAb's were generated as previously described (8). Briefly, IgG+ memory B cells were positively selected from PBMC through magnetic sorting using MACS CD22 microbeads (Miltenyibiotec) followed by depletion of IgA, IgD and IgM expressing cells by FACS-sorting. Isolated IgG+ memory B cells were then transformed with EBV and cultured in RPMI containing 10% FCS, 2.5 ug/ml CpG, 10 ng/ml, IL-2, 30 ug/ml holo-Transferrin and irradiated allogeneic PBMC. After 2 weeks, culture supernatants were screened for anti-DENV specific antibodies. Human EBV-transformed B cells producing anti-DENV antibodies were then cloned by limiting dilution. All human monoclonal antibodies used in this study are summarised in table S3.
Detection of dengue-specific human Abs by ELISA
Mixtures of all four DENV serotypes were captured onto a MAXISORP immunoplate (NUNC) coated with mouse anti-E antibody (4G2) or anti-NS1 antibody (NS1-F3). DENV captured wells were then incubated with B cell line (BCL) culture supernatants followed by alkaline phosphatase (AP)-conjugated anti-human IgG Abs. The reaction was visualized by the addition of PNPP substrate. The reaction was stopped with NaOH.
ELISA for prM:E ratio and the number of E molecules of poorly infectious DENV
To determine the prM:E ratio and to estimate the number of virus-equivalent particles, DENV was captured onto plates coated with anti-E antibody (4G2) and then detected with human anti-prM or E mAb. Recombinant E protein (a gift from Dr. Jonathan Grimes, University of Oxford, UK), produced in Sf9 cells was used to plot a standard curve to calculate the concentration of E. The relative concentration of prM was expressed as the OD ratio of prM:E measured by ELISA and the number of virus-equivalent particles estimated assuming each particle would contain 180 copies of the E protein.
Western blot and Dot blot analysis
For western blot analysis, lysates from dengue infected C6/36 cells treated with 1% triton X-100 in PBS were run on 12% SDS polyacryramide gels without heating under non-reducing conditions and electroblotted onto nitrocellulose membranes (Amersham). For dot enzyme immunoassay, culture supernatants from mock, DENV or JEV infected C6/36 cells were dotted onto nitrocellulose membranes. The membranes were then blocked with PBS containing 5% skimmed milk and probed with BCL supernatants or plasma samples diluted 1:100 followed by peroxidase-conjugated anti-human IgG Abs. Finally, membranes were developed with enhancement chemiluminescence substrate (Amersham).
Focus reduction neutralisation test (FRNT)
Neutralizing activity of DENV-specific Abs was determined by the focus reduction neutralisation test (FRNT) (23). Briefly, serially diluted antibody was mixed with an equal volume of virus and incubated for 1 hr at 37°C. The mixtures were then transferred to Vero cell monolayers followed by the focus forming assay described above.
Antibody-dependent infection enhancement assay
Serially diluted antibody was incubated with an equal volume of virus for 1 hr at 37°C then transferred to U937 cells and incubated at 37°C for 4 days. Supernatants were then harvested and viral titre assessed by a focus forming assay as described above. Alternatively, after 24 hrs, U937 was fixed and permeabilised in 4% paraformaldehyde and 0.5% saponin. DENV antigens were then stained intracellularly with anti-DENV (4G2) followed by phycoerythrin-conjugated anti-mouse IgG Abs and analysed by flow cytometry.
In preliminary experiments, to check that intracellular staining with anti-E antibody-4G2 represented DENV infection we compared 4G2 staining with 2G6 an antibody to NS1 (Fig. S3C). Staining of infected monocytes was similar, but as 4G2 showed a larger shift in staining (Fig. S3D) this was chosen for use in further assays.
DENV infected PBMC and FACS analysis
PBMC were infected with endotoxin-free DENV in the presence of different concentrations of antibodies for 24 hrs. Cells were then fixed, permeabilised, and stained for DENV antigen by intracellular staining as described above.
DENV immunoprecipitation
The supernatants from DENV2 infected culture were pre-cleared with protein A-agarose beads for 1 hr at 4 °C. After centrifugation, supernatant was incubated with 10 μg of 4G2 for 2 hrs at 4 °C. Protein A-agarose beads were then added and incubated for 1 hr at 4 °C. After washing, proteins were eluted with non-reducing loading buffer and run on a 15% SDS–polyacrylamide gel followed by silver staining according to the manufacture protocol (SilverQuest staining kit, Invitrogen).
Supplementary Material
Materials and Methods
Figs. S1, S2, S3, S4
Table S1, S2, S3, S4
Acknowledgments
We thank W Supanchaimat, V Jarupoonphol, S Jinathongthai, K Sriruksa, P Wongsilarat, T Suphachaiyakit, K Ratarpa, Y Sutvigit and the staff of Khon Kaen and Songkhla hospitals for sample collection; L Damrikarnlerd, P Suriyapol, C Komoltri, S Udompunturak, N Tangthawornchaikul, A Jairangsri, K Sae-Jang and S Supajitkasem for data and clinical database management, and statistic analysis. This work was supported by the Medical Research Council, U.K.; the Wellcome Trust, U.K.; the National Institute for Health Research Biomedical Research Centre funding scheme; the Thailand Tropical Disease Research Program T2; and the Thailand National Centre for Genetic Engineering and Biotechnology.
References and Notes
- 1.World Health Organization Fact sheet no 117. 2008 [Google Scholar]
- 2.Guzman MG, et al. Am J Epidemiol. 2000;152:793. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 3.Sangkawibha N, et al. Am J Epidemiol. 1984;120:653. doi: 10.1093/oxfordjournals.aje.a113932. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB, O'Rourke EJ. J Exp Med. 1977;146:201. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diamond MS, Pierson TC, Fremont DH. Immunol Rev. 2008;225:212. doi: 10.1111/j.1600-065X.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modis Y, Ogata S, Clements D, Harrison SC. Nature. 2004;427:313. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 7.Li L, et al. Science. 2008;319:1830. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 8.Traggiai E, et al. Nat Med. 2004;10:871. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai C-Y, et al. J Virol. 2008;82:6631. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang K-J, et al. J Immunol. 2006;176:2825. doi: 10.4049/jimmunol.176.5.2825. [DOI] [PubMed] [Google Scholar]
- 11.Guirakhoo F, Heinz FX, Mandl CW, Holzmann H, Kunz C. J Gen Virol. 1991;72(Pt 6):1323. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 12.Yu IM, et al. Science. 2008;319:1834. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 13.Zybert IA, van der Ende-Metselaar H, Wilschut J, Smit JM. J Gen Virol. 2008;89:3047. doi: 10.1099/vir.0.2008/002535-0. [DOI] [PubMed] [Google Scholar]
- 14.Junjhon J, et al. J Virol. 2008;82:10776. doi: 10.1128/JVI.01180-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherrier MV, et al. EMBO J. 2009 [Google Scholar]
- 16.Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Nat Rev Microbiol. 2007;5:518. doi: 10.1038/nrmicro1690. [DOI] [PubMed] [Google Scholar]
- 17.Innis BL, et al. Am J Trop Med Hyg. 1989;40:418. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 18.Yenchitsomanus PT, et al. Southeast Asian J Trop Med Public Health. 1996;27:228. [PubMed] [Google Scholar]
- 19.World Health Organization Dengue haemorrahgic fever: diagnostic, treatment, prevention and control. 1997 [Google Scholar]
- 20.Dejnirattisai W, et al. The Journal of Immunology. 2008;181:5865. doi: 10.4049/jimmunol.181.9.5865. [DOI] [PubMed] [Google Scholar]
- 21.Puttikhunt C, et al. J Virol Methods. 2003;109:55. doi: 10.1016/s0166-0934(03)00045-4. [DOI] [PubMed] [Google Scholar]
- 22.Puttikhunt C, et al. J Med Virol. 2008;80:125. doi: 10.1002/jmv.21047. [DOI] [PubMed] [Google Scholar]
- 23.Jirakanjanakit N, Sanohsomneing T, Yoksan S, Bhamarapravati N. Trans R Soc Trop Med Hyg. 1997;91:614. doi: 10.1016/s0035-9203(97)90050-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and Methods
Figs. S1, S2, S3, S4
Table S1, S2, S3, S4