Abstract
Background
Rifampicin may decrease efavirenz concentrations by inducing expression of cytochrome P450 isoenzyme 2B6 (CYP2B6), which metabolises efavirenz. The CYP2B6 516G>T polymorphism impairs efavirenz metabolism and occurs more commonly in Africans than Caucasians. We explored the effect of concomitant rifampicin-based antitubercular therapy and the 516G>T polymorphism on efavirenz mid-dosing interval plasma concentrations, and investigated risk factors for efavirenz concentrations outside 1-4mg/L, in a South African population.
Methods
Mid-dosing interval efavirenz plasma concentrations were measured in adults on antiretroviral therapy (efavirenz 600 mg daily throughout) with and without antitubercular therapy, using validated LC/MS/MS methods. Between patient and within patient comparisons were made.
Results
There were 142 participants: 40 on antitubercular therapy and 102 controls; mean weight 66 kg. Median efavirenz concentration was 2.4mg/L (IQR 1.3, 3.1) and 1.8mg/L (IQR 1.4, 4.4) in participants on antitubercular therapy and controls, respectively (p=0.734). Paired efavirenz concentrations during and after antitubercular therapy in 17 participants were also similar (p=0.113). In 122 participants genotyped, 60 (49%) had G/G genotype, 46 (38%) were G/T heterozygotes and 16 (13%) were T/T homozygotes. In a multivariate logistic regression model, adjusted for sex, weight and concomitant antitubercular therapy, the 516G>T polymorphism was strongly associated with high efavirenz concentrations (>4 mg/L): odds ratios 4.4 (95%CI 1.3-14.9) for G/T versus G/G and 31.1 (95%CI 6.6-146.6) for T/T versus G/G. High efavirenz concentrations were associated with severe sleep disturbance (p=0.048). Low efavirenz concentrations (<1mg/L) were associated with virological failure (odds ratio 12.5; 95% CI 2.7-57.3).
Conclusions
Efavirenz can be used together with rifampicin-based antitubercular therapy without dose adjustment in this population. The 516G>T polymorphism occurred commonly and was associated with high efavirenz concentrations.
Introduction
HIV-associated tuberculosis is very common in resource-limited settings, and many patients require concomitant antiretroviral therapy (ART) and rifampicin-based antitubercular therapy. The non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz is widely prescribed in first-line ART. Efavirenz is metabolized primarily by the hepatic cytochrome P450 isoenzyme 2B6 (CYP2B6) [1]. Rifampicin induces hepatic enzymes [2]. In 8 Spanish patients taking efavirenz 600 mg daily, addition of rifampicin-based antitubercular therapy decreased median efavirenz peak concentration, area under the curve and trough concentration by 24%, 18% and 10% respectively [3].
Because of the decrease in efavirenz concentrations induced by rifampicin some experts recommend a 33% dose increase, particularly in patients weighing more than 60 kg [4]. World Health Organization treatment guidelines recommend an efavirenz-based ART regimen in patients requiring antitubercular therapy [5]. However, the need for dose increase of efavirenz when prescribed concomitantly with antitubercular therapy is debated, as the bulk of pharmacokinetic data comes from studies in Thai patients with low body weights [6].
Hepatic clearance of efavirenz was shown in the 2NN study to be 28% higher in Western patients than in South African patients [7]. The CYP2B6 516G>T single nucleotide polymorphism has been shown in a number of studies to be associated with increased efavirenz concentrations [8-11] and to be more common in Africans and African Americans than in Caucasian Americans [8, 11-13].
In this study our primary objective was to assess the effect of rifampicin-based antitubercular therapy on efavirenz mid-dosing interval plasma concentrations in a group of South African patients. Secondary objectives were to assess the effect of the CYP2B6 516G>T polymorphism on efavirenz concentrations and to identify risk factors in participants for efavirenz concentrations outside the therapeutic range of 1-4mg/L, as per current antiretroviral therapeutic drug monitoring guidelines [14]
Methods
Study design and setting
A cross sectional study, with a repeated component, was conducted in HIV infected South African adults.. To evaluate the effect of rifampicin based antitubercular therapy on efavirenz mid-dosing interval (12-20 hours after the last dose) plasma concentrations, two comparisons were made: first, between those receiving antitubercular therapy and those who did not receive it; second, between samples taken during antitubercular therapy and subsequent samples from the same participants at least one month after the completion of antitubercular therapy. Participants were recruited from a workplace (gold miners) ART programme in Carletonville and public sector ART clinics in Cape Town, South Africa. All participants were HIV-infected patients taking ART consisting of 2 NRTIs and efavirenz for at least one month. Efavirenz was taken as a single daily dose, at night, by all participants. Efavirenz plasma concentrations were measured in participants in the continuation phase of rifampicin-based antitubercular therapy (TB group) and a comparison group of participants not on antitubercular therapy (control group). Efavirenz mid-dosing interval concentrations were repeated a month or more after completion of antitubercular therapy in the TB group, to allow for induction due to rifampicin to wane.. Participants in the TB group were taking the standard dose of 600 mg efavirenz once daily. Continuation phase antitubercular therapy consisted of rifampicin (600mg in those weighing ≥50 kg and 450 mg for those <50 kg) and isoniazid 300 mg, given 5 days a week as directly observed therapy as per national guidelines. Exclusion criteria were poor venous access, Karnofsky score <70, known severe renal, hepatic or gastrointestinal disease, malabsorption or severe diarrhoea, weight <40 kg, or exposure to rifampicin in the month prior to recruitment in those patients not currently on antitubercular therapy. Blood was sampled 12-20 hours after the last dose of efavirenz to obtain a mid-dosing interval plasma concentration, and a cheek swab was used to collect a sample for genotyping. A structured questionnaire, based on the AIDS Clinical Trial Group Questionnaire [15], was used. This questionnaire recorded self-reported ethnicity of maternal and paternal grandparents, date and time of last dose of efavirenz and of antitubercular therapy (where applicable), all concomitant medications, a 4 day recall of missed doses of all antiretroviral and antitubercular medicines, and when the participant last missed a dose of antiretroviral or antitubercular medication. To assess presence of side effects, participants were asked whether on not they had experienced any of a list of 22 symptoms in the past 4 weeks. Each symptom was recorded as absent, or graded on scale of 1(“it doesn’t bother me”) to 4 (“it bothers me terribly”). There was also space to capture additional symptoms outside the list of 22. The questionnaire also queried smoking and alcohol and recreational drug use.“ CD4+ lymphocyte counts, quantitative HIV-1 RNA (viral loads) and alanine transaminase (ALT) results were obtained from the participants’ 6 monthly routine monitoring results. TB group participants were interviewed and sampled again one month or more after completion of antitubercular therapy
Assessment of efavirenz concentrations
Efavirenz concentrations were determined by validated liquid chromatography tandem mass spectrometry (LC/MS/MS), as previously described [16, 17], in the Division of Clinical Pharmacology laboratory, University of Cape Town. The calibration curve was linear over the range of 0.1 to 15 mg/L. Efavirenz concentrations above 15 mg/L were recorded as16 mg/L in calculation of summary statistics, and those less than 0.1 were entered as 0 mg/L.
Genotyping
DNA was extracted from the swabs using either the DNA Micro Kit (Qiagen) or the ZR Genomic DNA II kit (Zymo Research) according to the manufacturers’ protocols. The CYP2B6 516G>T single nucleotide polymorphism was determined by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis. The change from G516 to T516 results in the loss of a restriction site for BsrI. The primers 5′-CTT GAC CTG CTG CTT CTT CC-3′ and 5′-TCC CTC TCC GTC TCC CTG-3′ were used to amplify a 204 bp fragment using the following conditions; denaturation at 94°C for 4 min, followed by 30 cycles of denaturation at 94°C for 30s, annealing at 58°C for 30s and extension at 72°C for 30s. A final extension step at 72°C for 7 minutes completed the reaction. Each PCR reaction contained 100 ng of genomic DNA in 1× PCR buffer, 1.5mM MgCl2, 0.2mM dNTP mixture, 0.2μM of each primer and 1U of GoTaq Flexi DNA polymerase (Promega, USA) in a final volume of 50 μl. The amplified products were digested with BsrI restriction enzyme (New England Biolabs,Inc., Beverley, MA) at 65°C for 3 h. The digested PCR products were electrophoresed on 2.5% agarose gel. The homozygous G/G genotype resulted in two fragments that were 152 and 52 base pairs, the heterozygous G/T genotype resulted in three fragments 204, 152 and 52 base pairs while the homozygous T/T genotype resulted in a single undigested fragment of 204 base pairs.
Statistical analysis
Statistical analysis was performed using STATA version 10 (Stata Corp. College Station, TX). The results of a Spanish pharmacokinetic study with mean efavirenz trough concentration 0.63 mg/L (SD0.32) with and 0.84mg/L (SD 0.99) without rifampicin-based antitubercular therapy [3] were used to calculate the sample size. An estimated sample size of 40 participants on efavirenz and antitubercular therapy, and 100 participants on efavirenz alone, had 80% power at a 5% level of significance to detect a 21% drop in efavirenz mid-dosing interval plasma concentrations due to concomitant rifampicin-based antitubercular therapy, calculated based upon the use of an unpaired t test.
Continuous variables were summarised using means and standard deviations if normally distributed, and medians and ranges if not normally distributed. Within-group comparisons of continuous variables were made using a paired t test if parametrically distributed and the Wilcoxon signed rank test for paired observations if non-parametrically distributed. Between-group comparisons were made using a t test if parametrically distributed and the Wilcoxon/Mann-Whitney rank sum or Kruskal-Wallis test if non-parametrically distributed.
Virological suppression was defined as a viral load below 400 copies/mL, and virological failure as a viral load above 400 copies/mL six months or more after initiation of therapy. Participants were categorised as black or white if they described all 4 grandparents as such.
A Chi-squared test was performed to test the null hypothesis of Hardy-Weinberg equilibrium of the distribution of CYP2B6 516 genotypes.
For the secondary objective of exploring risk factors for high efavirenz concentrations, efavirenz concentrations were classified as either high (>4mg/L) or normal/low (<= 4mg/L). To explore risk factors for low concentrations, efavirenz concentrations were categorized as either low (<1mg/L) or normal/high (>= 1mg/L). The association between high and low efavirenz concentration and several risk factors was explored in univariate analysis by cross-tabulation, unadjusted odds ratios and 95% confidence intervals (CI). Multivariate logistic regression models were constructed using a forward-fitting approach, adding first those variables that appeared most strongly associated with high or low efavirenz concentrations on univariate analysis to the model; sex, weight and concomitant rifampicin were included in the models based on an a priori decision.
Ethics
The study was approved by the research ethics committees of the University of Cape Town and the London School of Hygiene and Tropical Medicine. Written informed consent was taken from all participants.
Results
Participant characteristics
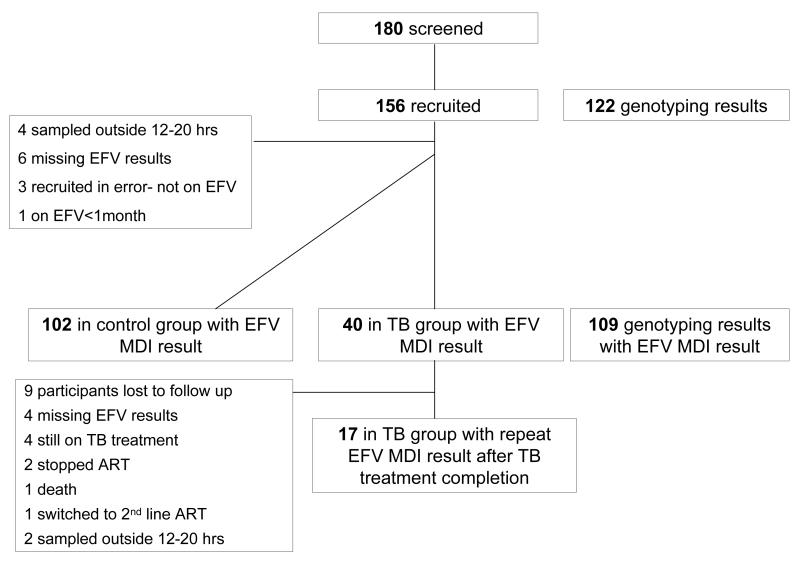
One hundred and eighty patients were screened for inclusion, of whom 156 were recruited (figure 1). After exclusions, a cross-sectional sample of 142 participants with efavirenz results remained: 40 in the TB group and 102 in the control group. Seventeen of the 42 participants in the TB group were sampled again a month or more after completing antitubercular therapy. Reasons for exclusions and reasons for failure to obtain a second specimen in 23 TB group participants are outlined in figure 1. Characteristics of participants are described in table 1.
Figure 1.
Flow of participants through study.
EFV: efavirenz
MDI: Mid dosing interval
Table 1.
Participant characteristics at time of first sampling
| Characteristic | No antitubercular therapy n=102 |
Antitu bercular therapy n=40 |
|---|---|---|
| Male | 76 (75%) | 27 (68%) |
| Age years mean (SD) |
41.0 (8.0) | 39.6(8.7) |
| WHO stage 4 | 55(55%)* | 24 (60%) |
| Weight kg mean (SD) |
67.6(10.8) | 62.8(8.8) |
| Black | 86 (84%) | 39 (100%)** |
| Mixed race | 15 (15%) | - |
| White | 1 (1%) | - |
| Duration ART days median (IQR) |
219(95-468) | 84(42-167) |
| CD4 count cells/mm3 median (IQR) |
234(140-383) | 104(46-196) |
| ALT U/L median (IQR) |
27(20-36) | 31(18-58) |
100 participants with data
39 participants with data.
Self-reported adherence data were available from 139/142 participants at 155 sampling occasions. One participant in the control group and one in the TB group reported non-adherence to efavirenz within the 4 days before sampling; these patients were included in the analysis. Non-adherence to ART at any point within the month preceding sampling was reported at 11% of sampling occasions: by 10 participants in the control group, 4 in the TB group at the first sampling occasion, and 3 in the TB group sampled after completing antitubercular therapy.
Effect of rifampicin-based antitubercular therapy on efavirenz concentrations
After exclusions, there were 159 mid-dosing interval concentrations in 142 participants. There was large interpatient variability in efavirenz concentrations. Two efavirenz concentrations were above 15mg/L, the upper limit of quantification of the assay, and 10 were below 0.1mg/L, the lower limit of quantification.
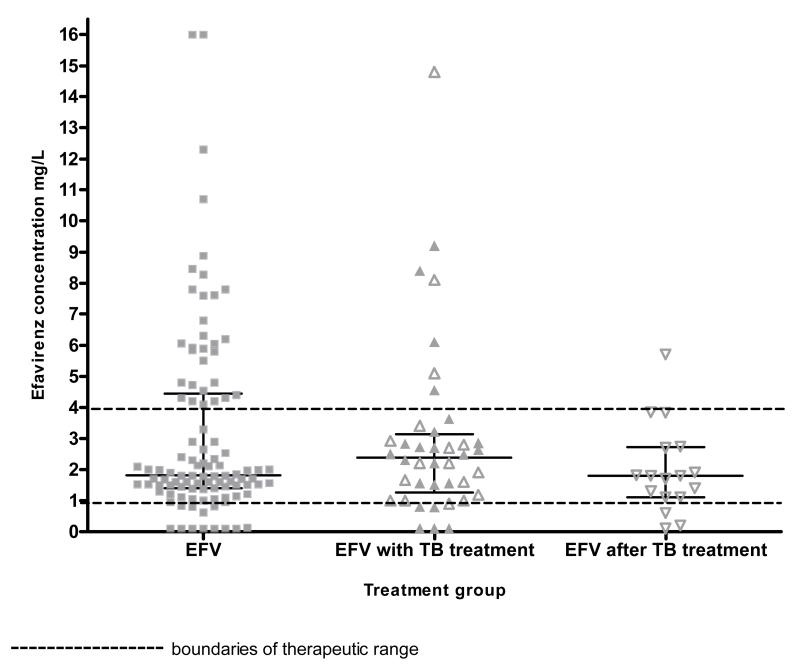
There was no significant difference between the median efavirenz concentrations of the 40 participants in the TB group at time of first sampling and the 102 control participants not taking antitubercular therapy(2.4 mg/L (IQR 1.3-3.1) and 1.8 mg/L (IQR 1.4-4.4) respectively; Mann Whitney p= 0.734) (figure 2). There was no difference in the sampling time after the last efavirenz dose between the non-TB group and the TB group (15.8 hours (95% CI: 15.5-16.1 hrs) versus 16.0 hours (95% CI: 15.5-16.6 hrs); t test p=0.472).
Figure 2.
Dot plot of efavirenz concentrations by rifampicin-based antitubercular therapy exposure group, with median and interquartile ranges (142 participants)
open symbols denote participants who had efavirenz mid dosing interval concentrations measured twice; on TB treatment and after TB treatment completion.
In the TB group, median efavirenz concentration was 1.6 mg/L (IQR 1.0-3.2) in 19 participants weighing 60 kg or less, and 2.6 (1.9-2.9) in 21 participants weighing more than 60kg (Mann-Whitney p=0.228).
In the 17 participants in the TB group with repeated efavirenz concentrations, the median concentration was 2.2 mg/L (IQR 1.3-3.1) on rifampicin-based antitubercular therapy and 1.8 mg/L (IQR 1.1-2.7) a month or more after completion of antitubercular therapy (figure 2), (Wilcoxon matched pairs signed rank p=0.113). The median change in efavirenz concentrations between sampling occasions was −0.3 mg/L (IQR −1.1, 0.2), which corresponds to a median percentage change of −18% (IQR −50%, 11%). Participants’ weight changed significantly between sampling occasions (paired t test p=0.002) - 13/17 participants gained weight, with a mean change between sampling occasions of 5.5 kg (SD 6.0). There was no significant difference in the time between last efavirenz dose and the time of sampling between the two occasions (paired t test p= 0.265).
CYP2B6 516G>T genotype
Genotyping results were available from 122 participants: 60/122(49%) had the G/G genotype, 46/122 (38%) were G/T heterozygotes and 16/122 (13%) T/T homozygotes, with an allele frequency for T of 32%. We were unable to obtain data on the remaining 20 participants due to poor specimen quality. There were 104 black participants with genotyping data, 50/104 (48%) had the G/G genotype, 40/104(39%) were G/T heterozygotes, and 14/104(13%) were T/T homozygotes. These proportions are consistent with Hardy-Weinberg equilibrium (Chi squared p=0.51).. There was no difference in genotype distribution between the TB and the control group (Chi squared p=0.94)
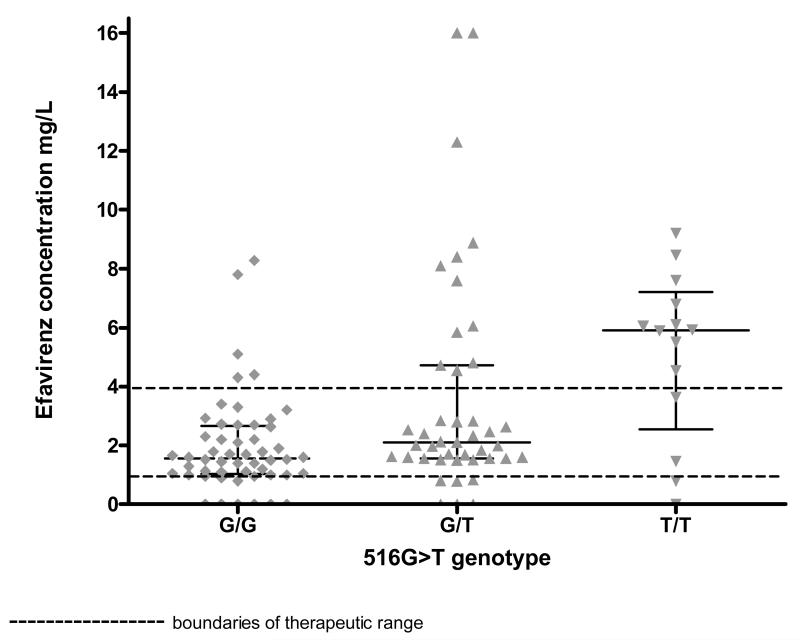
In the 109 participants with both efavirenz and genotyping results, median efavirenz concentration differed by genotype (figure 3): G/G 1.6mg/L (IQR 1.0 to 2.7), G/T 2.1mg/L (IQR 1.6 to 4.7) and T/T 5.9mg/L (IQR 3.6 to 6.8) on the first sampling occasion (Kruskal-Wallace p= 0.0008). Median efavirenz concentration was 2.1mg/L (IQR 1.6 to 4.2) in the 33 participants without genotyping results at time of first sampling , and there was no evidence of a difference in efavirenz concentration between those with and without missing genotyping results (Mann Whitney p=0.3327)
Figure 3.
Dot plot of efavirenz concentrations by CYP2B6 516G>T genotype group at first sampling occasion, with medians and interquartile ranges (109 participants with both efavirenz and genotyping results)
Associations with efavirenz concentrations outside the therapeutic range
On univariate analysis, there was no association between efavirenz concentrations above 4 mg/L and sex, age, disease stage, weight, ethnicity, self reported adherence and concomitant rifampicin-based antitubercular therapy (table 2). There was an association between efavirenz concentrations and the presence of severe sleep disturbance, where 5/9(56%) compared with 33/131(25%) had high concentrations in those with and without sleep disturbance respectively (Chi squared p=0.048), but there were no significant associations between efavirenz concentrations and other neuropsychiatric symptoms.
Table 2.
Univariate analysis of association between exposures and efavirenz plasma concentration above 4mg/L at first sampling occasion
| Characteristic | Category | Number with high efavirenz concentration/total (percentage) |
OR (95% confidence interval) for high efavirenz concentration |
|---|---|---|---|
| Sex | male | 27/103 (26) | 1 |
| female | 11/39 (28) | 1.11 (0.48-2.53) | |
| Age | <30 | 3/13 (23) | 1 |
| 30-40 | 14/63 (22) | 0.95 (0.23-3.98) | |
| 40-50 | 12/46(26) | 1.18 (0.27-5.07) | |
| >50 | 9/19 (47) | 3.00 (0.58-15.61) | |
| WHO clinical stage | Stage 4 | 24/79 (30) | 1 |
| Stage 3 | 11/51(22) | 0.63 (0.28-1.44) | |
| Stage 1 & 2 | 1/10 (10) | 0.25 (0.03-2.20) | |
| On ART greater than 6 months |
ART<6 months & viral load<400 copies/mL |
19/52(37) | 1 |
| ART>6 months and viral load>400 copies/mL |
1/11(9) | 0.17 (0.02-1.58) | |
| Taking concomitant antitubercular therapy |
No antitubercular therapy |
31/102 (30) | 1 |
| Antitubercular therapy | 7/40 (18) | 0.49 (0.19-1.23) | |
| Ethnicity | Black | 31/125 (25) | 1 |
| Other | 7/16 (44) | 2.35 (0.81 -6.86) | |
| Weight | <60kg | 13/44 (30) | 1 |
| 60-80kg | 22/87(25) | 0.81 (0.36-1.82) | |
| >80kg | 3/11(27) | 0.97 (0.21-3.97) | |
| Genotype | G/G | 4/42 (10) | 1 |
| G/T | 13/33 (39) | 3.64 (1.17-11.35) | |
| G/T | 13/41(32) | 23.50 (5.34-103.38) | |
| CNS symptoms | No symptoms | 22/88 (25) | 1 |
| symptoms | 15/49 (31) | 1.34 (0.62-2.93) | |
| Sleep disturbance | No severe sleep disturbance |
33/131(25) | 1 |
| Severe sleep disturbance |
5/9 (56) | 3.71 (0.92-15.01) | |
| Adherence | Adherent | 29/108 (27) | 1 |
| Non-adherent within preceding month |
8/31 (26) | 0.95 (0.39-2.36) |
After adjustment for sex, weight and concomitant rifampicin-based antitubercular therapy in a multivariate logistic regression model, the 516G>T polymorphism was strongly associated with efavirenz concentrations above 4mg/L (table 3), with odds ratios 4.44 (95% CI 1.33 to 14.88) for G/T versus G/G and 31.05 (95% CI 6.58 to 146.56) for T/T versus G/G.
Table 3.
Multivariate logistic regression model for the association between risk factors and efavirenz concentration above 4mg/L (109 participants at first sampling occasion included in the model)
| Variable | Number (percentage) |
Crude odds ratio (95% confidence interval) |
Adjusted odds ratio (95% confidence interval) |
Wald test p value |
|---|---|---|---|---|
|
| ||||
| CYP2B6 G/G | 52 (48) | 1 | 1 | - |
| CYP2B6 G/T | 43 (39) | 3.64(1.17-11.34) | 4.44(1.32-14.88) | 0.016 |
| CYP2B6 T/T | 14 (13) | 23.50(5.34-103.38) | 31.05(6.58-146.56) | <0.0001 |
|
| ||||
| No antitubercular therapy |
82 (75) | 1 | 1 | - |
| Concomitant antitubercular therapy |
27 (25) | 0.55(0.20-1.52) | 0.41(0.12-1.37) | 0.146 |
|
| ||||
| Male | 84 (77) | 1 | 1 | - |
| Female | 25 (23) | 1.61(0.60-4.30) | 2.32(0.70-7.62) | 0.168 |
|
| ||||
| Weight** | 109 | 0.98(0.94-1.03) | 0.96(0.91-1.02) | 0.233 |
calculated on the 109 participants in the multivariate model.
weight is included in the model as a continuous variable. The odds ratio is therefore for a 1 unit i.e. 1 kg increase in weight.
Efavirenz concentrations below 1 mg/L were strongly associated with virological failure (Chi squared p<0.0001). Efavirenz concentrations were available in 66 participants who were on ART for more than 6 months: 6/10 (60%) failing virologically and 6/56 (11%) who were virologically suppressed had an efavirenz concentration of less than 1mg/L, odds ratio 12.5 (95% CI 2.7 to 57.3). There was no association between efavirenz concentrations less than 1mg/L and sex, age, ethnicity, weight, self-reported adherence, clinical stage, concomitant rifampicin-based antitubercular therapy or CYP2B6 516G>T genotype (analysis results not shown).
Discussion
We found no evidence of a decrease in efavirenz mid-dosing interval plasma concentrations in the presence of concomitant rifampicin-based antitubercular therapy in African patients on standard doses of efavirenz, even in patients weighing >60 kg. Thirteen percent of participants were T/T homozygotes for the CYP2B6 516G>T polymorphism, with an allele frequency of 32%. The CYP2B6 516G>T polymorphism was strongly associated with high efavirenz plasma concentrations. Low efavirenz concentrations were associated with a 12.5-fold increased risk of virological failure, and high efavirenz concentrations were associated with severe sleep disturbance.
Current World Health Organization and Centers for Disease Control guidelines for ART with rifampicin-based antitubercular therapy suggest that an efavirenz dose increase to 800 mg may be necessary in patients weighing more than 60 kg [4, 5]. This recommendation is based on a pharmacokinetic study in 8 Spanish patients which reported decreases in median efavirenz Cmax, Cmin and AUC of 24%, 18% and 10% respectively after 7 days of concomitant rifampicin, however none of these differences reached statistical significance [3]. A Thai study randomized patients on rifampicin-based antitubercular therapy to receive either efavirenz 600 mg or 800 mg and found no difference in efavirenz mid-dosing interval concentrations or time to virologic suppression. However, this finding may not be generalisable because Thai patients have lower efavirenz clearance than South African patients [7], and lower body weight than our patients [18], which resulted in higher median efavirenz concentrations than we found. A large South African antiretroviral cohort study reported that patients on standard doses of efavirenz with concomitant rifampicin-based antitubercular therapy had similar rates of virologic suppression to patients who were not treated for tuberculosis [19]. We found no evidence of an effect of rifampicin-based antitubercular therapy on mid-dosing interval efavirenz concentrations, the pharmacokinetic parameter used for therapeutic monitoring of efavirenz. Taken together, these data do not support the need for higher efavirenz doses with rifampicin in Thai and Southern African patients.
A therapeutic range of 1-4mg/L has been suggested for efavirenz mid dosing interval concentrations, as efavirenz concentrations below 1mg/L are associated with an increased risk of virological failure [20, 21] and concentrations above 4mg/L are associated with central nervous system side effects [20, 22]. Although these cutoffs are based upon limited data, they form the basis for currently used therapeutic drug monitoring guidelines for efavirenz [14]. There is no clear consensus on the role of therapeutic drug monitoring for efavirenz. Efavirenz concentrations above 4 mg/L where associated with severe sleep disturbance in our study. This is in keeping with previous studies showing impaired sleep and persistent insomnia in patients with high efavirenz concentrations [22, 23]. Night time dosing of efavirenz, which occurred in all our participants, may have contributed to sleep disturbance. Our failure to find an association between high concentrations and other central nervous system side effects is not surprising, as most central nervous system adverse effects occur early in treatment, and rapidly wane and our inclusion criteria required participants to have been on ART for at least 1 month, so that they would be at steady state. We found strong evidence of an association between efavirenz concentrations of less than 1mg/L and virological failure, consistent with findings from previous studies [20, 21].
We found 13% of Southern African patients to be T/T homozygotes, with an allele frequency of 32%, and a strong association between 516G>T genotype and efavirenz mid-dosing interval concentrations above 4 mg/L. The cytochrome P4502B6 516G>T polymorphism, in the *6 allele, is known to be associated with high efavirenz concentrations [8, 9, 24, 25]. The *6 allele occurs more commonly in African Americans than Caucasian Americans [8], and also occurs commonly in patients from Zimbabwe [11], Botswana[13] and Ghana [12] (*6 allele frequencies of 49%, 37%, and 47% respectively). There are a number of other polymorphisms which may play a role and which we have not evaluated for this study, such as 983T>C and the other polymorphism found in the *6 allele, 785A>G [26].
Our study has several limitations. The cross sectional design allows us to identify associations but not to prove causality. In most participants we only have efavirenz concentrations on a single sampling occasion, thus we cannot explore intra-individual variability, although this has been previously shown to be far less than inter-individual variability [27, 28]. The effect of concomitant antitubercular therapy on pharmacokinetic parameters other than mid-dosing interval concentration was not assessed. However, mid-dosing interval concentrations are the standard pharmacokinetic parameter used for therapeutic drug monitoring of efavirenz. Time of sampling within the mid-dosing interval was not standardised (12-20 hours) and therefore could potentially confound the comparisons of concentrations with vs without concomitant rifampicin-based antitubercular therapy. However, we found no evidence of a difference in sampling time for either the within patient or between patient comparisons. Dosing times and adherence were determined by self report, which is not a sensitive measure of adherence. We excluded patients who had been on ART for less than a month, which limited the ability to detect an association between central nervous system side effects and high efavirenz concentrations. Patients with severe side effects may discontinue or switch therapy by one month, which may have resulted in a biased sample. In addition symptoms tend to occur within the first month of therapy and then wane [29]. Genotype data were missing on 23% of participants with efavirenz concentrations due to poor specimen quality, but this is unlikely to have resulted in bias. Our viral load assay is sensitive down to 400 copies/ml and we were therefore unable to explore associations with virological suppression below 400copies/ml.
In conclusion, our study findings support the use of the standard 600 mg efavirenz dose in African patients being treated concomitantly with rifampicin-based antitubercular therapy, irrespective of weight. A high proportion of Southern Africans have the CYP2B6 516G>T polymorphism, which impairs the metabolism of efavirenz.
Acknowledgements
Funding for this study was provided by the Aurum Institute for Health Research and an unrestricted educational grant from Merck Sharp and Dohme (MSD). Alison Grant was supported by a Public Health Career Scientist Award from the UK Department of Health. Daphne Pholeli, Rene Goliath and Washiefa Isaacs screened and recruited participants.
Footnotes
Conflict of interest declaration: Helen McIlleron has received an honorarium from Abbott Laboratories, South Africa. The other authors have none to declare.
Presented in part: 8th international HIV workshop on Clinical pharmacology of HIV therapy, Budapest, 2007.
References
- 1.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 2.Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–857. [PubMed] [Google Scholar]
- 3.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, Sarasanacenta M, Lopez-Pua Y, Pachon J. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 4.CDC Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis. [updated 18 May 2008, cited 18 September 2008]. Available from http://www.cdc.gov/tb/TB_HIV_Drugs/default.htm.
- 5.World Health Organization Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2006 revision. [Cited 18 September 2008]. Available from http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. [PubMed]
- 6.Manosuthi W, Kiertiburanakul S, Sungkanuparph S, Ruxrungtham K, Vibhagool A, Rattanasiri S, Thakkinstian A. Efavirenz 600 mg/day versus efavirenz 800 mg/day in HIV-infected patients with tuberculosis receiving rifampicin: 48 weeks results. Aids. 2006;20:131–132. doi: 10.1097/01.aids.0000196181.18916.9b. [DOI] [PubMed] [Google Scholar]
- 7.Kappelhoff BS, van Leth F, MacGregor TR, Lange J, Beijnen JH, Huitema AD. Nevirapine and efavirenz pharmacokinetics and covariate analysis in the 2NN study. Antivir Ther. 2005;10:145–155. [PubMed] [Google Scholar]
- 8.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, Clifford DB, Hulgan T, Marzolini C, Acosta EP. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. Aids. 2004;18:2391–2400. [PubMed] [Google Scholar]
- 9.Tsuchiya K, Gatanaga H, Tachikawa N, Teruya K, Kikuchi Y, Yoshino M, Kuwahara T, Shirasaka T, Kimura S, Oka S. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 10.Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, Keiser O, Biollaz J, Decosterd L, Telenti A. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, Masimirembwa C. High prevalence of the CYP2B6 516G-->T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64:357–365. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 12.Klein K, Lang T, Saussele T, Barbosa-Sicard E, Schunck WH, Eichelbaum M, Schwab M, Zanger UM. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861–873. doi: 10.1097/01213011-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gross R, Aplenc R, Tenhave T, Foulkes AS, Thakur R, Mosepele M, Barrett JS, Flexner C, Strom BL, Bisson G. Slow efavirenz metabolism genotype is common in Botswana. J Acquir Immune Defic Syndr Hum Retrovirol. 2008;49:336–337. doi: 10.1097/QAI.0b013e31817c1ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.la Porte CJLBD, Blaschke T, et al. Updated guidelines to perform therapeutic monitoring for antiretroviral agents. Reviews in antiviral therapy. 2006:4–12. [Google Scholar]
- 15.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG Adherence Instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Nuttall JJ, Egbers C, Eley BS, Meyers TM, Smith PJ, Maartens G, McIlleron HM. High prevalence of subtherapeutic plasma concentrations of efavirenz in children. J Acquir Immune Defic Syndr. 2007;45:133–136. doi: 10.1097/QAI.0b013e31805c9d52. [DOI] [PubMed] [Google Scholar]
- 17.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal. 2002;30:675–684. doi: 10.1016/s0731-7085(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 18.Manosuthi W, Sungkanuparph S, Thakkinstian A, Vibhagool A, Kiertiburanakul S, Rattanasiri S, Prasithsirikul W, Sankote J, Mahanontharit A, Ruxrungtham K. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. Aids. 2005;19:1481–1486. doi: 10.1097/01.aids.0000183630.27665.30. [DOI] [PubMed] [Google Scholar]
- 19.Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, S M, M A, Goemaere E, D C, Maartens G. Effectiveness of nevirapine and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300:530–539. doi: 10.1001/jama.300.5.530. [DOI] [PubMed] [Google Scholar]
- 20.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. Aids. 2001;15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 21.Leth FV, Kappelhoff BS, Johnson D, Losso MH, Boron-Kaczmarska A, Saag MS, Livrozet JM, Hall DB, Leith J, Huitema AD, Wit FW, Beijnen JH, Lange JM. Pharmacokinetic parameters of nevirapine and efavirenz in relation to antiretroviral efficacy. AIDS Res Hum Retroviruses. 2006;22:232–239. doi: 10.1089/aid.2006.22.232. [DOI] [PubMed] [Google Scholar]
- 22.Núñez M, González de Requena D, Gallego L, Jiménez-Nácher I, González-Lahoz J, Soriano V. Higher efavirenz plasma levels correlate with development of insomnia. J Acquir Immune Defic Syndr. 2001;28:399–400. doi: 10.1097/00126334-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 23.Gallego L, Barreiro P, Rio Rd, González de Requena D, Rodríguez-Albarino A, González-Lahoz J, Soriano V. Analyzing Sleep Abnormalities in HIV-Infected Patients Treated with Efavirenz. Clinical Infectious Diseases. 2004;38:430–432. doi: 10.1086/380791. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Nóvoa S, Barreiro P, Rendon A, Jiménez-Nácher I, González-Lahoz J, Soriano V. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40:1358–1361. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 25.Haas DW, Smeaton LM, Shafer RW, Robbins GK, Morse GD, Labbe L, Wilkinson GR, Clifford DB, D’Aquila RT, De Gruttola V, Pollard RB, Merigan TC, Hirsch MS, George AL, Jr., Donahue JP, Kim RB. Pharmacogenetics of long-term responses to antiretroviral regimens containing Efavirenz and/or Nelfinavir: an Adult Aids Clinical Trials Group Study. J Infect Dis. 2005;192:1931–1942. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 26.Rotger M, Tegude H, Colombo S, Cavassini M, Furrer H, Decosterd L, Blievernicht J, Saussele T, Gunthard HF, Schwab M, Eichelbaum M, Telenti A, Zanger UM. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81:557–566. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 27.Kappelhoff BS, Huitema AD, Yalvac Z, Prins JM, Mulder JW, Meenhorst PL, Beijnen JH. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin Pharmacokinet. 2005;44:849–861. doi: 10.2165/00003088-200544080-00006. [DOI] [PubMed] [Google Scholar]
- 28.Stahle L, Moberg L, Svensson JO, Sönnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–270. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Clifford DB, Evans S, Yang Y, Acosta EP, Goodkin K, Tashima K, Simpson D, Dorfman D, Ribaudo H, Gulick RM. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143:714–721. doi: 10.7326/0003-4819-143-10-200511150-00008. [DOI] [PubMed] [Google Scholar]