Abstract
The BRAVO-II study was a randomized controlled study of endovascular radiation therapy as compared to sham radiation therapy, following angioplasty of a thrombosed PRFE graft. The results did not show a benefit of endovascular radiation therapy, albeit in the context of an early termination of the study at less than 50% enrollment due to business reasons. Emphasis is laid on the fact that there may still be a role for radiation therapy in specific clinical settings associated with dialysis vascular access dysfunction.
Hemodialysis vascular access dysfunction is an important cause of morbidity and hospitalization in the hemodialysis population at a cost of over one billion dollars per annum (1–3). A very large component of this problem is due to polytetrafluroethylene (PTFE) dialysis access graft stenosis and thrombosis. Despite their widespread use as a form of permanent dialysis access, PTFE dialysis access grafts have 1 and 2 year primary patency rates of 50% and 25%, respectively (4,5). More recently a large randomized clinical trial of Aggrenox in patients with new PTFE grafts documented a 1 year primary patency rate of only 23% (6). Once PTFE grafts develop a stenosis, their response to angioplasty is even worse (with post-intervention primary patencies of 50% at 6 months for nonthrombosed grafts which drops down to 40% at 3 months for thrombosed grafts) (5).
Dialysis access graft stenosis occurs primarily at the graft-vein anastomosis and is due to venous neointimal hyperplasia (VNH) (7). We and others have previously demonstrated that VNH is characterized by the proliferation and migration of smooth muscle cells, myofibroblasts and endothelial cells (angiogenesis), together with the presence of a prominent layer of peri-graft macrophages (7–9).
Since radiation therapy is known to block the proliferation of these cell types in-vitro, it could be an effective therapy for dialysis access dysfunction (10–12). Proof for this hypothesis comes from a large number of experimental and clinical studies on the use of radiation therapy (both external beam and endovascular) for the prevention and treatment of coronary and ileofemoral stenosis (13–17). In the specific setting of dialysis access dysfunction there are a number of small anecdotal studies with equivocal results (18,19). More recently, however, a pilot study of endovascular radiation therapy in nonthrombosed grafts dialysis access grafts was able to demonstrate an improvement in target lesion primary patency (BRAVO I) (20). Following on from this previous study, the BRAVO II study is a double-blind randomized controlled study of endovascular beta radiation following angioplasty in thrombosed PTFE dialysis access grafts.
Subjects and Methods
General Description
The Beta Radiation for Arterio-Venous Graft Outflow Stenosis II (BRAVO II) study (sponsored by the Novoste Corporation) was a large multicenter, doubleblind, randomized, placebo-controlled study to assess the efficacy and safety of endovascular radiation therapy (also known as vascular brachytherapy [VBT]) in he-modialysis patients with thrombosed grafts, due to a stenosis at the graft-vein anastomosis. The original plan was for the enrollment of 215 patients (see statistical considerations). Unfortunately, due to business and recruit- ment issues, the study was halted after the recruitment of 95 patients only. We will therefore describe the results for these 95 patients only.
Primary Endpoints
The Primary Efficacy endpoint for BRAVO II was a significant improvement in 3-month postintervention primary patency, which was defined as freedom from repeat intervention and freedom from thrombosis requiring intervention. The Primary Safety endpoint was a composite of Serious Adverse Events at 3 months including death, venous rupture, and aneurysm formation.
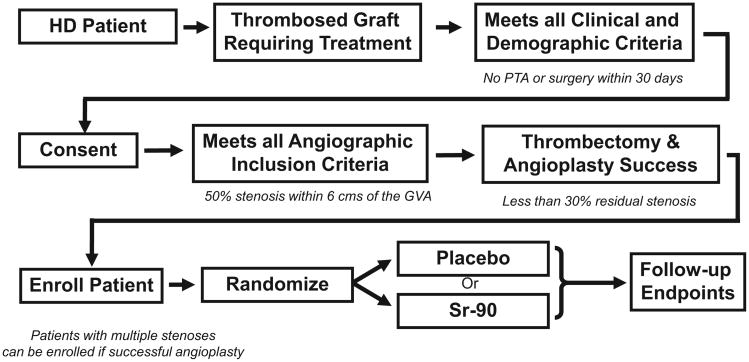
Study Protocol (see overview in Fig. 1)
Fig. 1.
Study Algorithm: This describes the sequence of events for the BRAVO II study. Note the presence of both clinical and angio-graphic inclusion and exclusion criteria.
Patients with thrombosed grafts were assessed for clinical and demographic inclusion and exclusion criteria (see Figs. 2 and 3). Eligible patients underwent a standard thrombectomy and angiogram. If the patient had a greater than 50% stenosis at the graft-vein anastomosis and fulfilled the angiographic inclusion and exclusion criteria (see Figs. 4 and 5), the patient was randomized to receive either vascular brachytherapy or a sham radiation procedure. Of note, the residual stenosis at the target lesion had to be less than 30% to enroll a patient in this study. Patients with stenotic lesions upstream of the graft-vein stenosis could be randomized following a successful angioplasty of these lesions (less than 30% residual stenosis).
Fig. 2.
Clinical and demographic inclusion criteria.
Fig. 3.
Clinical and demographic inclusion criteria.
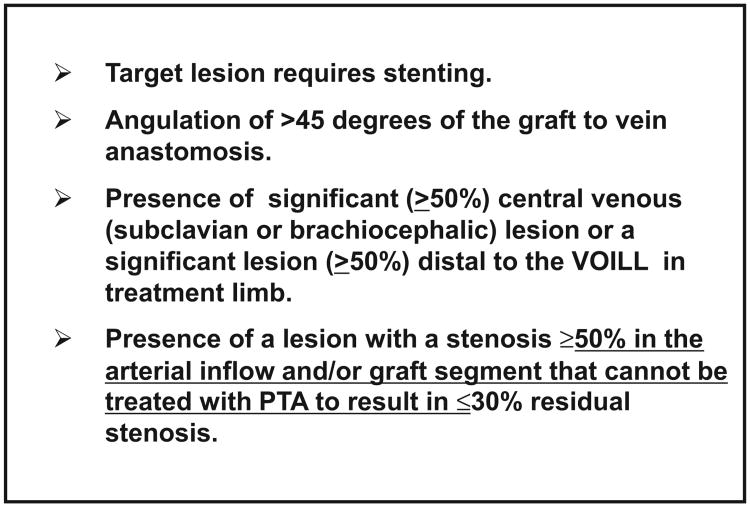
Fig. 4.
Angiographic inclusion criteria.
Fig. 5.
Angiographic exclusion criteria.
Vascular Brachytherapy
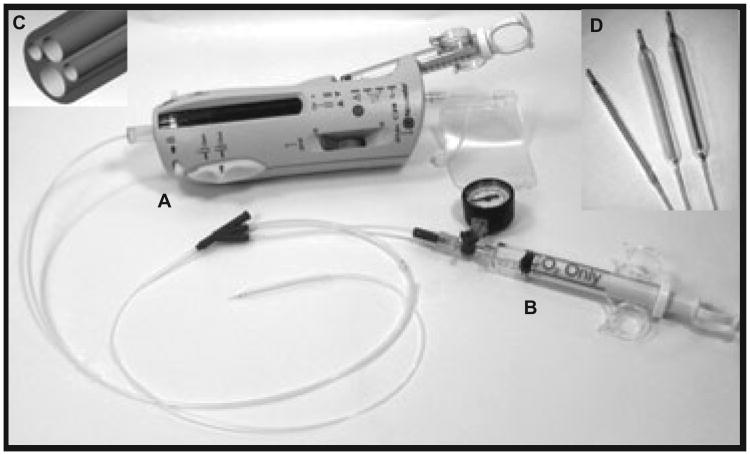
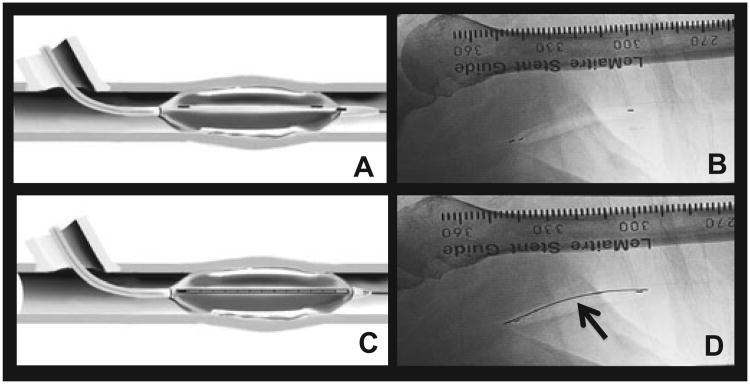
From a procedural viewpoint, the entire area of angioplasty injury was exposed to beta radiation after a standard angioplasty. The BRAVO study used the fourlumen Corona delivery system (Fig. 6), which has been successfully used in the coronary setting. Following angioplasty a carbon dioxide filled balloon was inflated to 1–2 atmospheres over the entire area of angioplasty injury (Fig. 7A,B). Fluorescent tape markers were used to ensure exact placement of the CO2-filled balloon (Fig. 7A,B). The radioactive source train, which contained 24 miniature radioactive seeds was then delivered to cover the entire length of balloon injury (Fig. 7C,D). A dose of 18.4 Gy was delivered at a distance of 0.5 mm from the surface of the balloon. Building upon the experience acquired from previous studies of endovascular radiation therapy and current FDA recommendations (21), the BRAVO protocol incorporated strict guidelines to ensure that the entire area of injury was irradiated with adequate margins to avoid the “candy wrapper” effect described in earlier studies of en-dovascular radiation in the coronary circulation. All patients were also started onto Aspirin for the duration of the study to avoid potential problems with late thrombosis (21). If the lesion length was more than 3 cm, two sequential balloons were used with a pullback methodology.
Fig. 6.
Corona endovascular radiation device: Note the main device (A) with the attached radiation balloon, the CO2 syringe (B) to inflate the radiation balloon, the blown up representation of the four lumen tip (C) and high power views of the differentially sized catheters (D) that were used in this study.
Fig. 7.
Delivery of Endovascular Radiation to the site of Angioplasty Injury: (A) and (B) describe the placement of the Corona balloon over the entire site of angioplasty induced vascular injury. (C) and (D) document the placement of a ribbon of Strontium-90 seeds into the catheter (arrow in (D)) to irradiate the entire region of injury.
Follow-Up
Patients were assessed clinically immediately after the procedure and at the 3-month endpoint. Patients were also assessed at the 6-month and 1-year intervals for the safety endpoints (until August 2005).
Statistical Considerations
The current literature suggests that the primary patency of thrombosed PTFE dialysis access grafts following angioplasty is 40% at 3 months (22). The BRAVO II protocol was powered to be able to identify a 20% absolute increase in primary patency (from 40% in the Control group to 60% in the Radiation group) at 3 months, with a power of 80% and a two-sided alpha error of 5%, through the recruitment of 215 patients. The recruitment of only 95 patients clearly changes the initial power calculations. However, despite the reduction in patient numbers, the BRAVO II study remains by far the largest randomized study of radiation therapy in the field of dialysis access. We therefore feel that the presented results below are an important addition to the literature in this area (see Discussion also).
Results
One hundred and fifty-four patients were screened for the BRAVO II study, of which 95 patients (59 screen failures) were enrolled from 21 sites in the United States. Fifty patients received radiation therapy while 45 patients were randomized to the placebo group. Of these 95 patients, 69 patients (29 in the radiation group and 40 in the placebo group) successfully completed the 3-month follow-up visit. The main reason for not completing the 3-month visit was termination of the study due to business and financial reasons (23).
Demographics and Vascular Access History
Table 1 shows the demographics of the Radiation and Control groups. In keeping with the current demographics of hemodialysis patients in the United States, the mean age for the Radiation and Control groups was 61.1 and 62 years, respectively. There were, however, significantly more females in the placebo group as compared with the radiation group. African Americans constituted 56% of the total study population and were equally distributed between the two groups. The major cause of ESRD in both groups was diabetes mellitus. With the exception of gender (described above), there were no other demographic differences between the Radiation and Control groups. Table 2 describes the location (forearm versus upper arm) and configuration (straight versus loop) of the current hemodialysis graft. There were no differences in location or configuration between the two groups.
Table 1. Demographics.
| Radiation (n = 45) | Placebo (n = 50) | p-value | |
|---|---|---|---|
| Median age | 61.1 | 62.0 | 0.363 |
| Gender | 15M | 29M | 0.0232 |
| 30F | 21F | ||
| Ethnicity | |||
| White | 18 | 19 | 1.00 |
| Black | 25 | 28 | |
| Other | 2 | 3 | |
| Median years on hemodialysis | 2.0 | 2.0 | 0.200 |
| ESRD cause | |||
| Diabetes | 21 | 21 | 0.9704 |
| Hypertension | 14 | 16 | |
| Glomerulonephritis | 3 | 5 | |
| Others | 7 | 8 |
Table 2. Hemodialysis vascular access history.
| Radiation (n = 45) | Placebo | p-value (n = 50) | |
|---|---|---|---|
| Location of current access | |||
| Forearm | 20 | 23 | 0.2383 |
| Upper arm | 25 | 27 | |
| Configuration of current access | |||
| Loop | 26 | 30 | 0.8341 |
| Straight | 19 | 19 | |
Efficacy Outcomes
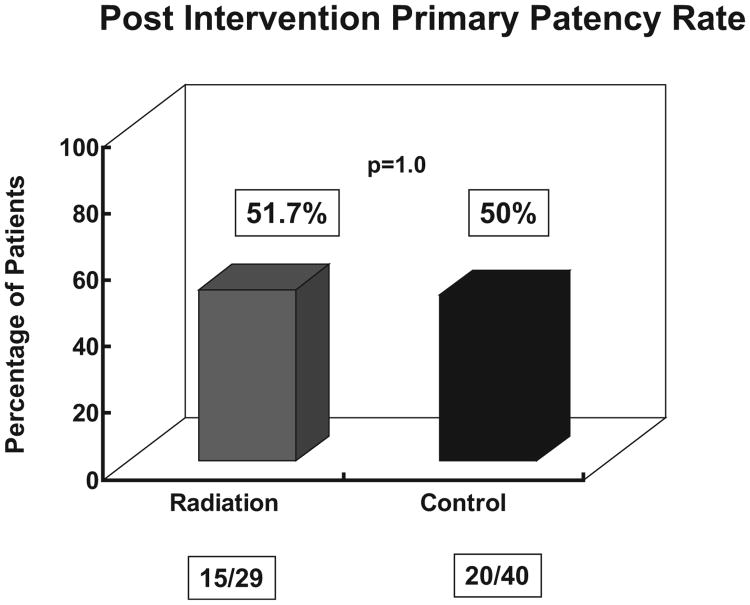
There was no difference in the primary efficacy endpoint between the two groups. Of the 29 patients in the radiation group that completed the 3-month assessment, 15 patients (51.7%) had not had a thrombosis or intervention as compared to 20/40 (50%) of patients in the placebo arm (p = 1.00, Chi-square analysis) (Fig. 8).
Fig. 8.
Post-Intervention primary patency rate: There is no difference between the control and radiated groups. Note that data are available for only 69 patients at the 3-month follow-up.
Safety Outcomes
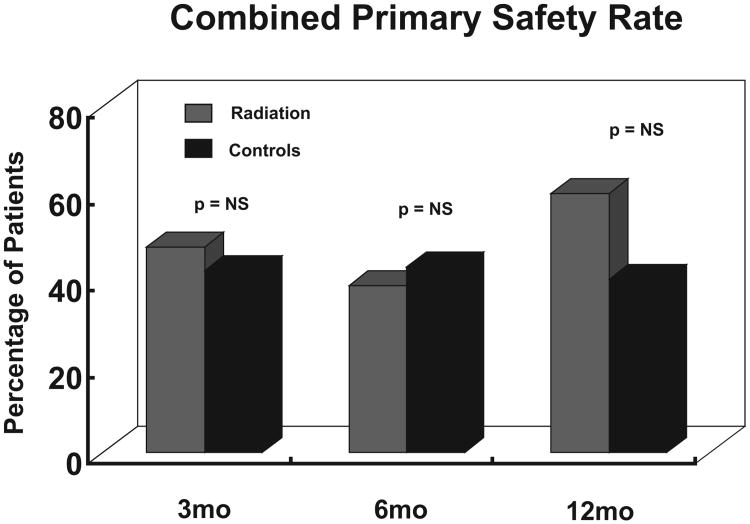
There were no significant differences in the number of patients who had device related events, serious adverse events or the composite of death, venous rupture or aneurysm formation. Figure 9 shows the combined primary safety rate (which includes all the above) between the two groups. It is important to note, however, that the 12-month time point comprised five patients in each group only.
Fig. 9.
Combined primary safety rate: There is no difference in the combined primary safety rate at between 3 and 12 months post intervention. The data for each time point is for the patients who were followed till that time point (only 5 at 12 months).
Discussion
The BRAVO II study is by far the largest randomized study of radiation therapy (external beam or endovascular) in the setting of dialysis access stenosis (PTFE graft or AV fistula). Although this study has clearly demonstrated both the feasibility and safety of endovascular radiation therapy in the setting of thrombosed dialysis access grafts, it has unfortunately not been able to deliver a final answer on the efficacy of this procedure. While the described results do not demonstrate a beneficial effect of radiation therapy on primary patency, it needs to be emphasized that this analysis is based on data from 95 patients only, as opposed to the proposed 215; and of these 95 patients, only 69 had a full follow-up at the 3-month time point.
This unfortunate situation arose because of the early termination of the study. This was not done because of any safety concerns, but rather because of a confluence of business issues (23), related primarily to the concurrent rapid clinical penetration of drug eluting stents for the prevention of coronary restenosis (24,25). This resulted in a dramatic decrease in coronary endovascular radiation therapy and since this was the main revenue source for the Novoste Corporation, it became impossible to continue to support the ongoing dialysis access study. We also recognize that this early termination of the study has resulted in an imbalance within the 69 subjects who reached the 3-month endpoint, with 40 of these subjects having randomized to the placebo arm.
So is there still a need to identify whether endovascular radiation therapy could still be a useful therapeutic intervention for dialysis access graft dysfunction. We believe that there is such a need.
Thus, while the drug eluting stents have completely changed the management of coronary artery restenosis in a very positive fashion, it needs to be emphasized that they are not without their own problems, especially with regard to concerns about late thrombotic events and the requirement for prolonged clopidogrel therapy with its attendant clinical morbidity and financial costs (26–29).
In addition, it needs to be emphasized, that there are significant anatomical (vein versus artery), physiological (continuous high flow system with regions of turbulence in dialysis access versus a low flow system with predominantly laminar flow in coronary stenosis), and metabolic (increased uremia, inflammation and oxidative stress in dialysis vascular access) differences between dialysis access and coronary restenosis. The lesser efficacy of endovascular radiation therapy in the coronaries as compared with the drug eluting stents does not necessarily mean that it would be ineffective in the setting of dialysis access stenosis. The current lack of effective therapies for dialysis vascular access (there are no drug eluting stents for dialysis vascular stenosis and a recent study of stent grafts documented a significant improvement in primary patency but not cumulative patency (30,31)), further shifts the risk-benefit ratio in favor of additional studies of endovascular radiation therapy in this area.
We would therefore like to suggest the following potential areas for future research in the context of endovascular radiation therapy in the setting of dialysis access stenosis:
A study of endovascular radiation therapy in combination with a second antistenotic agent (stent grafts or local drug/cell/gene therapy) for patients with PTFE graft stenosis.
The use of endovascular radiation therapy to potentially enhance arteriovenous fistula maturation in view of the current epidemic of arteriovenous fistula (AVF) maturation failure (due to a combination of neointimal hyperplasia and perhaps a lack of adequate dilation). Note that small anecdotal studies have documented the successful use of endovascular radiation therapy in the setting of AVF stenosis (R Bonan; personal communication).
The use of endovascular radiation therapy in patients with intractable dialysis access stenosis (AVF or PTFE graft) who have already had multiple ineffective procedures to try and maintain patency.
The use of endovascular radiation therapy for the current epidemic of central vein stenosis, which often precludes the creation of a new dialysis access on that side.
In conclusion, therefore, we believe that there is still significant potential for further investigation into the role of endovascular radiation therapy in the setting of dialysis vascular access stenosis. Specifically, the current lack of effective therapies, for this difficult clinical problem (huge unmet clinical need), makes this a fertile area for future high quality clinical investigation.
Acknowledgments
Sponsored by the Novoste Corporation, Norcross, GA (now a part of Best Vascular).
With thanks to all the BRAVO II Study Centers and Investigators. Special thanks to Wendy Wiley and Andrew Green from Novoste.
Dr. Roy-Chaudhury is supported by NIH 5U01-DK82218, NIH 5R01-EB004527, NIH 1R21-DK089280-01, NIH 1R01DK088777 (MPI), a VA Merit Review, a University of Cincinnati NIH/NCCR UL1RR026314 CTSA grant, and industry grants from WL Gore, Shire and BioConnect Systems.
Dr Roy-Chaudhury was a Consultant for Novoste (now a part of Best Vascular) during the period of the study.
Parts of this work have been presented at the American Society of Nephrology Annual Meeting 2006 in San Diego.
References
- 1.Egger P. Trends in medicare expenditure for vascular access. Cincinnati Hemodialysis Vascular Access Symposium 2004; Cincinnati, OH. 2004. [Google Scholar]
- 2.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity. J Am Soc Nephrol. 1996;7:523–535. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 3.McCarley P, Wingard RL, Shyr Y, Pettus W, Hakim RM, Ikizler TA. Vascular access blood flow monitoring reduces access morbidity and costs. Kidney Int. 2001;60:1164–1172. doi: 10.1046/j.1523-1755.2001.0600031164.x. [DOI] [PubMed] [Google Scholar]
- 4.Roy-Chaudhury P, Kelly BS, Melhem M, Zhang J, Li J, Desai P, Munda R, Heffelfinger SC. Vascular access in hemodialysis: issues, management, and emerging concepts. Cardiol Clin. 2005;23:249–273. doi: 10.1016/j.ccl.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.DOQI III NKF-K/DOQI. Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001;37:S137–S181. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 6.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr, Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–2201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 8.Rekhter M, Nicholls S, Ferguson M, Gordon D. Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb. 1993;13:609–617. doi: 10.1161/01.atv.13.4.609. [DOI] [PubMed] [Google Scholar]
- 9.Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC. Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients Clinical, immunocytochemical, light and electron microscopic assessment. Circulation. 1989;80:1726–1736. doi: 10.1161/01.cir.80.6.1726. [DOI] [PubMed] [Google Scholar]
- 10.Fareh J, Martel R, Kermani P, Leclerc G. Cellular effects of beta-particle delivery on vascular smooth muscle cells and endothelial cells: a dose-response study. Circulation. 1999;99:1477–1484. doi: 10.1161/01.cir.99.11.1477. [DOI] [PubMed] [Google Scholar]
- 11.Haimovitz-Friedman A, Fuks Z. Signaling in the radiation response of endothelial cells. In: Rubin DB, editor. The Radiation Biology of the Vascular Endothelium. Boston: CRC Press; 1998. pp. 101–127. [Google Scholar]
- 12.Rubin P, Williams JP, Riggs PN, Bartos S, Sarac T, Pomerantz R, Castano J, Schell M, Green RM. Cellular and molecular mechanisms of radiation inhibition of restenosis Part I: role of the macrophage and platelet-derived growth factor. Int J Radiat Oncol Biol Phys. 1998;40:929–941. doi: 10.1016/s0360-3016(97)00937-1. [DOI] [PubMed] [Google Scholar]
- 13.Waksman R, Robinson KA, Crocker IR, Wang C, Gravanis MB, Cipolla GD, Hillstead RA, King SB., 3rd Intracoronary low-dose beta-irradiation inhibits neointima formation after coronary artery balloon injury in the swine restenosis model. Circulation. 1995;92:3025–3031. doi: 10.1161/01.cir.92.10.3025. [DOI] [PubMed] [Google Scholar]
- 14.Weinberger J, Amols H, Ennis RD, Schwartz A, Wiedermann JG, Marboe C. Intracoronary irradiation: dose response for the prevention of restenosis in swine. Int J Radiat Oncol Biol Phys. 1996;36:767–775. doi: 10.1016/s0360-3016(96)00294-5. [DOI] [PubMed] [Google Scholar]
- 15.Verin V, Popowski Y, Urban P, Belenger J, Redard M, Costa M, Widmer MC, Rouzaud M, Nouet P, Grob E, et al. Intra-arterial beta irradiation prevents neointimal hyperplasia in a hypercholesterolemic rabbit restenosis model. Circulation. 1995;92:2284–2290. doi: 10.1161/01.cir.92.8.2284. [DOI] [PubMed] [Google Scholar]
- 16.Teirstein P. Beta-radiation to reduce restenosis. Too little, too soon? Circulation. 1997;95:1095–1097. doi: 10.1161/01.cir.95.5.1095. [DOI] [PubMed] [Google Scholar]
- 17.Raizner AE, Oesterle SN, Waksman R, Serruys PW, Colombo A, Lim YL, Yeung AC, van der Giessen WJ, Vandertie L, Chiu JK, White LR, Fitzgerald PJ, Kaluza GL, Ali NM. Inhibition of restenosis with beta-emitting radiotherapy: report of the Proliferation Reduction with Vascular Energy Trial (PREVENT) Circulation. 2000;102:951–958. doi: 10.1161/01.cir.102.9.951. [DOI] [PubMed] [Google Scholar]
- 18.Parikh SND, Rogers D, Charytan C, Osian A, Al-saloum M, Cavallo G. External beam radiation therapy to prevent postangioplasty dialysis access restenosis: a feasibility study. Cardiovasc Radiat Med. 1999;1:36–41. doi: 10.1016/s1522-1865(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 19.Cohen GSFH, Ringold MA. External beam irradiation as an adjuctive treatment in failing dialysis shunts. J Vasc Interv Radiol. 2000;11:321–326. doi: 10.1016/s1051-0443(07)61424-9. [DOI] [PubMed] [Google Scholar]
- 20.Misra S, Bonan R, Pflederer T, Roy-Chaudhury P. BRAVO I: a pilot study of vascular brachytherapy in polytetrafluoroethylene dialysis access grafts. Kidney Int. 2006;70(11):2006–2013. doi: 10.1038/sj.ki.5001869. [DOI] [PubMed] [Google Scholar]
- 21.Sapirstein W, Zuckerman B, Dillard J. FDA approval of coronary-artery brachytherapy. N Engl J Med. 2001;344:297–299. doi: 10.1056/NEJM200101253440410. [DOI] [PubMed] [Google Scholar]
- 22.Schwab SJ, Oliver MJ, Suhocki P, McCann R. Hemodialysis arterio-venous access: detection of stenosis and response to treatment by vascular access blood flow. Kidney Int. 2001;59:358–362. doi: 10.1046/j.1523-1755.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 23.Novoste. Vascular Brachytherapy Assets Acquired by Best Medical International. Atlanta: NewsRx; 2004. [Google Scholar]
- 24.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, Colombo A, Schampaert E, Grube E, Kirtane AJ, Cutlip DE, Fahy M, Pocock SJ, Mehran R, Leon MB. Safety and efficacy of si-rolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 25.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R, RAVEL Study Group A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 26.Curfman GD, Morrissey S, Jarcho JA, Drazen JM. Drug-eluting coronary stents – promise and uncertainty. N Engl J Med. 2007;356:1059–1060. doi: 10.1056/NEJMe068306. [DOI] [PubMed] [Google Scholar]
- 27.Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 28.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 29.Maisel WH. Unanswered questions – drug-eluting stents and the risk of late thrombosis. N Engl J Med. 2007;356:981–984. doi: 10.1056/NEJMp068305. [DOI] [PubMed] [Google Scholar]
- 30.Haskal ZJ, Trerotola S, Dolmatch B, Schuman E, Altman S, Mietling S, Berman S, McLennan G, Trimmer C, Ross J, Vesely T. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010;362:494–503. doi: 10.1056/NEJMoa0902045. [DOI] [PubMed] [Google Scholar]
- 31.Salman L, Asif A. Stent graft for nephrologists: concerns and consensus. Clin J Am Soc Nephrol. 2010;5:1347–1352. doi: 10.2215/CJN.02380310. [DOI] [PubMed] [Google Scholar]