Abstract
Among the bacterial symbionts harbored by the model organism Paramecium, many still lack a recent investigation that includes a molecular characterization. The genus Lyticum consists of two species of large-sized bacteria displaying numerous flagella, despite their inability to move inside their hosts' cytoplasm. We present a multidisciplinary redescription of both species, using the deposited type strains as well as newly collected material. On the basis of 16S rRNA gene sequences, we assigned Lyticum to the order Rickettsiales, that is intensely studied because of its pathogenic representatives and its position as the extant group most closely related to the mitochondrial ancestor. We provide conclusive proofs that at least some Rickettsiales possess actual flagella, a feature that has been recently predicted from genomic data but never confirmed. We give support to the hypothesis that the mitochondrial ancestor could have been flagellated, and provide the basis for further studies on these ciliate endosymbionts.
In the mid-twentieth century, T. Sonneborn revealed two features of Paramecium that had a lasting impact beyond the field of protozoology. First, he identified many morphologically identical strains of “Paramecium aurelia” that were not sexually compatible1. This observation provided one of the first and most extreme cases of a “sibling species” complex – fifteen different species have been described within the P. aurelia species complex until now2,3. Another important discovery was that of “cytoplasmic particles” of various kinds, found many times in several Paramecium species and often able to confer non-genetically inherited traits4. Years later, all these particles were identified as bacterial endosymbionts5.
Many of these symbionts have peculiar biological properties, and sometimes remarkably distinctive morphologies. Examples include the infectious Holospora with its specialized nucleus-invading form6, and the “killer” symbionts, that confer to infected paramecia the ability to kill uninfected “sensitive” strains present in the same culture medium5,7. Much interest was directed to the unusual bacteria belonging to genus Caedibacter (formerly “kappa particles”) and their complex cytoplasmic inclusions, the “R-bodies”8,9,10,11,12,13. Other equally intriguing killer symbionts were characterized, and among them were those belonging to the genus Lyticum4,5.
Lyticum bacteria appear as large rods (2.0–10.0 μm long) harbored in the hundreds in the cytoplasm of three different species of the P. aurelia complex4,5,14,15. They are non-motile, despite being covered by numerous flagella16. The two species were formally described as Lyticum flagellatum (formerly “lambda particle”, type species of the genus) and Lyticum sinuosum (“sigma particle”)17. They differ in shape (respectively, straight vs. curved rods) and host specificity (respectively, Paramecium tetraurelia or Paramecium octaurelia vs. Paramecium biaurelia)5,18.
The original descriptions of Lyticum and many other symbionts detected in the last century left many questions unanswered. One of the most important issues from an evolutionary point of view concerns the phylogenetic relationships of these bacteria.
The study of prokaryotic symbionts of protozoa is currently attracting a renewed interest, and is performed with the aid of molecular tools complementing ultrastructural methods like electron microscopy [e.g.19,20,21,22,23,24,25]. In recent years, the focus has shifted to the remarkable biodiversity of these organisms and their close relationships with human pathogens, e.g. Rickettsia26,27,28,29 and Francisella30,31.
In this work, we have characterized the symbionts of P. octaurelia strain 299 and P. biaurelia strain 114 following a multidisciplinary approach. They represent the type strains of L. flagellatum and L. sinuosum, respectively. We also reported a recently sampled environmental isolate (P. biaurelia USBL-36I1) infected by L. sinuosum, for which only one host strain was known so far. Morphology, ultrastructure and killer capabilities of the two bacterial species were investigated and molecular tools for their identification developed and tested. Moreover, we established their phylogenetic relationships, placing them inside the order Rickettsiales (Alphaproteobacteria) together with other obligate intracellular symbionts. This discovery not only clarifies the Lyticum affiliation, but also provides evidence supporting the hypothesis that Rickettsiales, the extant bacteria most closely related to the mitochondrial ancestor32,33,34,35, were ancestrally flagellated36. This finding provides a relevant contribution in inferring the features of the free-living ancestor of both Rickettsiales and mitochondria, supporting the view that it was motile.
Results
Morphology and ultrastructure
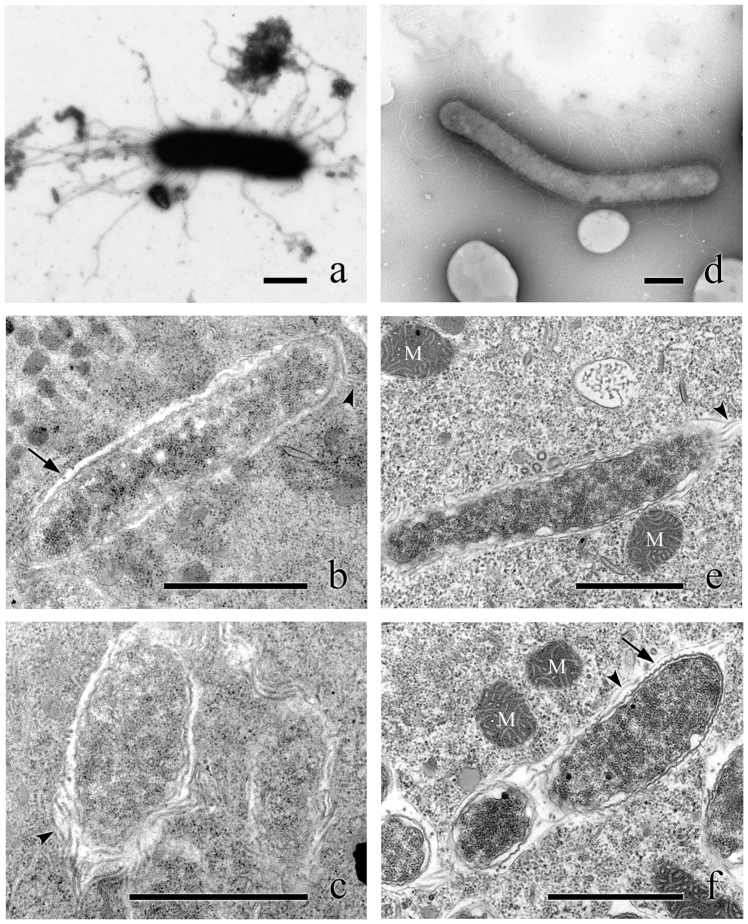
The cytoplasmic symbionts of P. octaurelia 299 (L. flagellatum) are straight rod-shaped bacteria 0.6–0.9 × 2.0–4.0 μm in size (Fig. 1a–c), while those harbored by P. biaurelia USBL-36I1 are bigger – up to 1.1 × 7.8 μm – and curved (Fig. 1d–f), perfectly fitting the description of L. sinuosum. Both are covered by numerous thick, peritrichous flagella about 4 μm long, clearly visible in TEM sections and negative staining. Nevertheless, in vivo observations did not show any sign of motility. The cytoplasm of both kinds of bacteria is homogeneous, with no visible inclusion. They both feature a Gram-negative type cell organization, with two membranes, and the symbionts are enclosed in a membrane-bound vesicle, often with several bacteria inside the same vesicle (Fig. 1b, f). These results are in good accordance with previous descriptions5.
Figure 1. Morphology and ultrastructure of Lyticum species.
Negative staining (a) and ultrathin sections (b, c) of L. flagellatum harbored by P. octaurelia strain 299. Negative staining (d) and ultrathin sections (e, f) of L. sinuosum harbored by P. biaurelia strain USBL-36I1. Bars stand for 1 μm. Arrowheads highlight some of the flagella, arrows point at symbiosomal membranes. M, mitochondria.
Molecular characterization
The 16S rRNA gene sequences of the symbionts harbored by strains 114 and USBL-36I1 are identical. They differ by 6 out of 1331 (0.5%) sites from the homologous sequence of the 299 symbiont. The most similar sequences available according to NCBI blastn are those of the Acanthamoeba spp. UWC8 and UWC36 symbionts (87.1–88.0% similarity), which belong to the “Candidatus Midichloriaceae” family within Rickettsiales37.
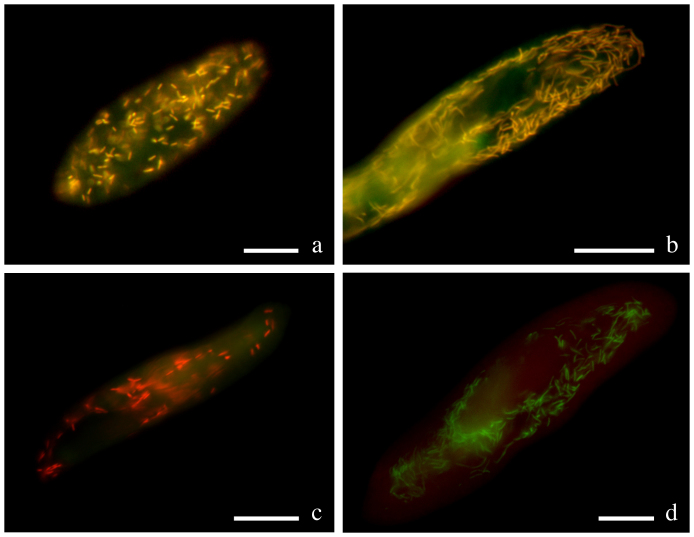
Hybridizations with the genus-specific oligonucleotide probe LytiProb_433 (that provides no match on RDP) gave clear signals deriving from bacteria localized in both 299 and USBL-36I1 cells at formamide concentrations in the range of 0–50% (with an optimum at 30%). Lyticum bacteria were always present in all examined paramecia, usually numbering in the hundreds, but sometimes far fewer – especially in the case of 299. L. flagellatum bacteria in 299 were often found concatenated in groups of 2 or more cells. Double hybridizations with the eubacterial probe EUB338 demonstrated that there are no other intracellular bacteria hosted by the Paramecium strains studied (Fig. 2a, b). The species-specific probes Lflag_268 (providing only 2 matches on RDP, both corresponding to uncultured Proteobacteria) and Lsinu_268 (providing 546 matches on RDP, but only 5 inside the order Rickettsiales) used in competition were able to discriminate between 299 and USBL-36I1 symbionts at formamide concentrations in the range of 10–20% (with an optimum at 20%; Fig. 2c, d).
Figure 2. Genus- and species-specific in situ detection of Lyticum flagellatum and Lyticum sinuosum.
Merge of the signals from probes EUB338 marked with fluorescein (green) and LytiProb_433 marked with Cy3 (red) on P. octaurelia strain 299 (a) and P. biaurelia strain USBL-36I1 (b). The signals coincide, and Lyticum bacteria appear yellowish. Merge of the signals from probes Lflag_268 marked with Cy3 (red) and Lsinu_268 marked with fluorescein (green) on P. octaurelia strain 299 (c) and P. biaurelia strain USBL-36I1 (d). At 20% formamide concentration, the probes used in competition are able to discriminate between the species. Bars stand for 20 μm.
Molecular phylogeny
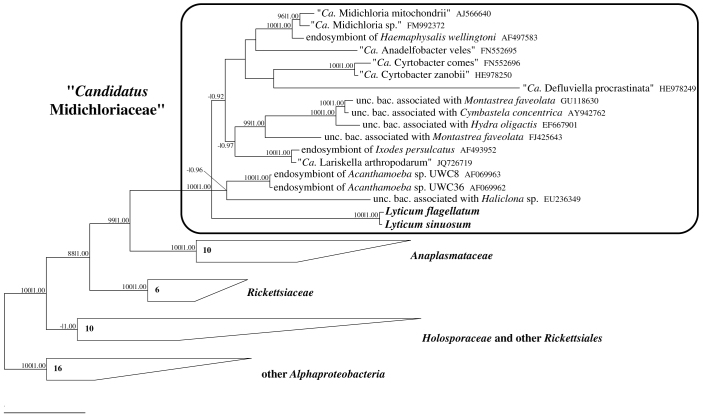
Details of tree topology differ, according to the method and the character matrix employed, especially within the families “Ca. Midichloriaceae” and Holosporaceae sensu lato18. Nevertheless, all trees recover the monophyly of the Rickettsiales families, including Holosporaceae sensu stricto18,25, and their relative positions, confirming other recent 16S rRNA analyses37,38. All trees confirm the monophyly of the Lyticum genus, as suggested by high similarity values between the strains, and its association to the candidate family Midichloriaceae within Rickettsiales (Fig. 3). The exact relationships within this family are not clearly resolved; however, the four genera of ciliate symbionts affiliated to this clade (“Ca. Anadelfobacter”, “Ca. Cyrtobacter”, “Ca. Defluviella” and Lyticum) do not form a monophyletic group.
Figure 3. Phylogenetic position of Lyticum species.
Bayesian tree built on the unmodified character matrix (60 sequences, 1331 characters) employing the GTR + I + G model (with the continuous gamma distribution approximated by 4 discrete categories). Numbers associated to each node correspond to ML bootstrap values and posterior probability values (values below 70|0.85 are omitted); numbers inside trapezoids show the number of sequences used to represent that clade. The bar stands for an estimated sequence divergence of 10%. Ca., Candidatus; unc. bac., uncultured bacterium.
Killer effect
No killer effect was detected in any of the performed experiments. The number of living cells did not decrease in the treatments nor in the controls, and the pre-lethal symptoms described by Jurand and colleagues39 were never observed.
Discussion
The infected Paramecium strains 299 and 114 were sampled almost a century ago4. Nevertheless, cultures of these ciliates still retain their original symbionts, although those of strain 114 are almost instantly lost after adaptation to standard cultivation conditions. On the other hand, the stability of the L. flagellatum-P. octaurelia 299 relationship supports the hypothesis that the symbiosis is obligate for the host, which possibly depends on metabolites provided by the bacterium40.
L. sinuosum has been reported so far only in P. biaurelia 114. We obtained a new environmental isolate of P. biaurelia which is infected by the same bacterial species, as can be inferred by morphology and the identity of 16S rRNA gene sequences. Interestingly, the monoclonal strain P. biaurelia USBL-36I1 was established from a water sample collected in the surroundings of the Indiana University, where T. Sonneborn was working at the time of his Lyticum description.
The morphological difference between the two Lyticum species corresponds to a difference in their 16S rRNA gene sequences, albeit small. Due to the diagnostic characters separating the two bacteria and the species-specific probes herein developed we recommend maintaining their status of separate but closely related species.
Although the identification of the described symbionts is sound, we could not repeat previous results concerning the killer trait. This was not entirely unexpected: the original literature describes the death of non-infected paramecia induced by Lyticum as extremely rapid (10–40 minutes), but triggered only in some Paramecium strains belonging to P. triaurelia, P. pentaurelia and P. novaurelia. Those strains were not available for the killer tests performed in this study. Therefore, our results suggest that those sensitive strains were the exception, and not the rule. The common adaptive explanation of the killer trait as a competitive advantage for the hosts11,41 would not apply to Lyticum, which apparently has no effect on most strains of the P. aurelia complex, including those belonging to the same species as their hosts (P. biaurelia, P. tetraurelia and P. octaurelia). It is also possible, of course, that the Lyticum killer effect requires specific physiological conditions in the sensitive, the killer and/or its symbiotic bacteria, and that those requirements were not met in our experiments. However, also the recently sampled strain USBL-36I1 did not act as a killer. This result makes it highly unlikely that an “ageing” effect of the cultures is responsible for the loss of killer activity.
Lyticum clearly belongs to the recently established candidate family “Ca. Midichloriaceae”37 within Rickettsiales, like several other symbionts of ciliates24,38, amoebas42 and metazoa [e.g.43,44]; a member of this group was also associated to fish disease45. The present study enables, for the first time, the assignment of a valid genus to this clade. Like other cytoplasmic bacteria belonging to “Ca. Midichloriaceae”24,44 and Anaplasmataceae46, but in contrast to members of Rickettsiaceae26,27,46, Lyticum symbionts are enclosed with an additional membrane, likely of host origin.
On the basis of genome annotations and phylogenomic analyses recently performed on “Candidatus Midichloria mitochondrii”, a hypothesis concerning the presence of flagella and motility in the Rickettsiales-mitochondria ancestor was proposed36, even though none of the so far characterized Rickettsiales bacteria actually possesses flagellar structures. Additionally to genome-derived evidences, further support is provided by the expression of flagellar genes on RNA and in one case also on protein level by “Ca. Midichloria mitochondrii”47. This hypothesis would confer an important role to motility in the establishment of the ancient symbiotic relationship that turned free-living bacteria into organelles. Our results support this view, revealing for the first time that heavily flagellated bacteria can be found among members of the order, and suggesting that the last common ancestor of Rickettsiales, or at least of “Ca. Midichloriaceae”, possessed flagella. The next step required for corroborating this scenario would be obtaining the sequence of Lyticum flagellar genes, and comparing them with those found in the “Ca. Midichloria mitochondrii” genome to test the alternative hypothesis that they were acquired independently.
“Ca. Midichloria mitochondrii” displays no flagella and is non-motile. Curiously enough, the Lyticum species do not use their flagella for movement. The question arises whether flagella or single flagellar proteins can also serve other than locomotion related functions. In a syntrophic symbiosis between a fermentative bacterium and a methanogenic archaeon, the significant role of the flagellar cap protein FliD to synchronize their metabolism was described48. One might speculate about an involvement of the numerous Lyticum flagella in establishment or maintenance of the symbiosis with Paramecium, hence this question awaits future analyses.
Methods
Hosts identification and culture
The P. octaurelia strain 299 and the P. biaurelia strain 114 were kindly provided by T. G. Doak and M. Lynch (Indiana University). The P. biaurelia strain USBL-36I1 was collected in 2011 from a small pond near Spencer (IN, USA, 39°17′45″N, 86°48′1″W). In order to confirm the identity of the host strains, morphological diagnostic features were checked49 and the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) was sequenced according to Barth and colleagues50; sequences are available at EMBL database with the accession numbers HF969031-3. The cultures were maintained at 19°C on a 12:12 h light/dark cycle and fed with Raoultella planticola inoculated in modified Cerophyl medium according to Boscaro and colleagues25 or, alternatively, with Enterobacter aerogenes inoculated in lettuce medium at room temperature. Strain 114 was obtained several times, but the symbionts were always lost shortly after the paramecia started to propagate. Thus, Transmission Electron Microscopy (TEM), fluorescence in situ hybridizations (FISH) and killer tests could not be performed on this strain.
Transmission electron microscopy
Ciliate cells were harvested by gentle centrifugation and fixed with 2.5% glutardialdehyde in 0.1 M Cacodylate buffer (pH 7.4) for 1 hour at room temperature. After washing in buffer, cells were post-fixed in 1% OsO4 in 0.1 M Cacodylate buffer (1 hour at room temperature). Three washing steps in this buffer were performed prior to dehydration in an acetone series and consecutive infiltration into Spurrs resin51. After ultrathin sectioning, sections were post-stained with 1% aqueous uranyl acetate and lead citrate52. Images were taken with a Zeiss EM 10 electron microscope at 60 kV. Alternatively, the cells were fixed in a mixture containing 2.5% glutaraldehyde and 1.6% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 2 hours at room temperature followed by a wash in the same buffer containing 12.5% sucrose and post-fixation in 1.6% OsO4 (1 hour at 4°C). The cells were dehydrated through a graded series of alcohol and acetone and embedded in Epoxy embedding medium (Fluka, BioChemika). Polymerization was carried out according to the manufacturer's protocol. Ultrathin sections were cut using a Reichert-Jung Ultracut E or Leica UC6, and stained with aqueous 1% uranyl acetate and 1% lead citrate. The samples were visualized using a Jeol JEM-1400 at 89 kV.
For negative staining of bacteria, several Paramecium cells were briefly washed in distilled water, squashed with a thin glass capillary in a drop of water, and a drop of the resulting suspension was placed on a Pioloform coated grid. Bacteria were allowed to precipitate for 2–3 min, then a drop of 1% uranyl acetate in distilled water was added for no longer than 1 min. The liquid was then absorbed with filter paper and the grid was air-dried.
16S rRNA gene sequencing
The almost complete 16S rRNA gene sequences were obtained through several PCR amplifications of overlapping regions and direct sequencing of the products (299 symbiont), or through cloning of PCR products, RFLP analyses and sequencing of 5 clones showing the most represented pattern to produce a consensus (for details of primers and PCR reactions, see Supplementary Methods online). The sequences are available at EMBL with the accession numbers HF969034-44.
FISH
Hybridizations were performed according to the protocol of Manz and colleagues53 on individually collected Paramecium cells fixed with 2% paraformaldehyde (w/v). Preliminary FISH experiments were performed with the eubacterial probe EUB33854 and the alphaproteobacterial probe ALF1b53. Oligonucleotide probes specific for the obtained 16S rRNA gene sequences were developed [LytiProb_433 5′-TATCTTCCCCACCAAAAGAAC-3′, genus Lyticum specific; Lflag_268 5′-GCTAAAGATCGAAGCCTTGGTAA-3′, L. flagellatum specific; Lsinu_268 5′-GCTAAAGATCGTAGCCTTGGTAA-3′, L. sinuosum specific]. These novel probes were tested with a wide range (0–50%) of formamide concentrations in the hybridization buffer. Paramecium strains containing different alphaproteobacterial symbionts were employed as negative controls. Probe specificities were checked also in silico with the ProbeMatch tool of the Ribosomal Database Project (RDP) website55 and probe data were deposited at probeBase56.
Phylogenetic analyses
Non-identical 16S rRNA gene sequences obtained were aligned with 42 homologous sequences of Rickettsiales bacteria and 16 of non-Rickettsiales alphaproteobacteria (as outgroup) using the ARB software package57. Sequence lengths were reduced to that of the shortest one, then multiple character matrices were produced according to Boscaro and colleagues38; unless otherwise stated, similarity values were calculated on the unmodified dataset. Maximum likelihood (ML) phylogenetic analyses were performed with PhyML58, employing bootstrap analysis (1,000 pseudoreplicates) to evaluate the reliability of nodes. Bayesian inference (BI) analyses were performed with MrBayes59, using three different runs with three heated and one cold chain each, iterating for 1,000,000 generations. The evolutionary model was selected according to the Akaike information criterion calculated by jModelTest60.
Killer tests
5 cells of the putative killer strains (299 or USBL-36I1) and 5 cells of putative sensitive Paramecium strains (see Supplementary Table S1 online) were put together in a depression slide containing 50 μL of sterile Cerophyl or lettuce medium. Numbers of motile cells were checked after 30 and 60 minutes. 10 cells of putative sensitives were employed as controls in each experiment, which was independently repeated three times. Attempts with sterile water instead of medium and/or extended observation periods were also performed.
Author Contributions
V.B., M.S., K.A.B., S.K., F.S., M.S. and E.V.S. participated in experimental procedures and data analysis. V.B. wrote the main manuscript text. T.U.B., M.S., F.V., E.V.S. and G.P. coordinated the work. All authors reviewed the manuscript.
Supplementary Material
Supplementary Methods
Acknowledgments
The authors wish to thank Thomas G. Doak and Michael Lynch for providing Paramecium strains 114 and 299, Alexey Potekhin for supporting most of the strains used in killer tests, and Yana Eglit for her help during sampling. Sergei I. Fokin and Anna Steyer are acknowledged for their help in TEM fixations. Simone Gabrielli is gratefully acknowledged for his help with graphic artwork. This work was supported by European Commission FP7-PEOPLE-2009-IRSES project CINAR PATHOBACTER (project number 247658), by Cooperlink action (protocol CII11DH8DZ), by PRIN fellowship (protocol 2008R9WRTB) from the Italian Research Ministry (MIUR), the COST action BM1102 and the Volkswagen foundation (project number 84816).
References
- Sonneborn T. M. Mating types in P. aurelia: diverse conditions for mating in different stocks; occurrence, number and interrelations of the types. Proc. Amer. Phil. Soc. 79, 411–434 (1938). [Google Scholar]
- Sonneborn T. M. The Paramecium aurelia complex of fourteen sibling species. Trans. Amer. Micros. Soc. 94, 155–178 (1975). [Google Scholar]
- Aufderheide K. J., Daggett P.-M. & Nerad T. A. Paramecium sonneborni n. sp., a new member of the Paramecium aurelia species-complex. J. Protozool. 30, 128–131 (1983). [Google Scholar]
- Sonneborn T. M. Kappa and related particles in Paramecium. Advan. Virus Res. 6, 229–356 (1959). [Google Scholar]
- Preer J. R., Preer L. B. & Jurand A. Kappa and other endosymbionts in Paramecium aurelia. Bacteriol. Rev. 38, 113–163 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokin S. I. & Görtz H.-D. [Diversity of Holospora bacteria in Paramecium and their characterization]. Endosymbionts In Paramecium [Fujishima, M. (ed.)] (Microbiology Monograph, Münster, 2009). [Google Scholar]
- Schrallhammer M. & Schweikert M. [The killer effect of Paramecium and its causative agents]. Endosymbionts In Paramecium [Fujishima, M. (ed.)] (Microbiology Monograph, Münster, 2009). [Google Scholar]
- Pond F. R., Gibson I., Lalucat J. & Quackenbush R. L. R-body producing bacteria. Microbiol. Rev. 53, 25–67 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer N. et al. Occurrence of fragmented 16S rRNA in an obligate bacterial endosymbiont of Paramecium caudatum. Proc. Natl. Acad. Sci. USA 90, 9892–9895 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier C. L. et al. The genus Caedibacter comprises endosymbionts of Paramecium spp. related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria). Appl. Environ. Microbiol. 68, 6043–6050 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch J. et al. Competitive advantages of Caedibacter-infected paramecia. Protist 153, 47–58 (2002). [DOI] [PubMed] [Google Scholar]
- Schrallhammer M., Fokin S. I., Schleifer K.-H. & Petroni G. Molecular characterization of the obligate endosymbiont “Caedibacter macronucleorum” Fokin and Görtz, 1993 and of its host Paramecium duboscqui strain Ku4–8. J. Eukaryot. Microbiol. 53, 499–506 (2006). [DOI] [PubMed] [Google Scholar]
- Schrallhammer M. et al. Tracing the role of R-bodies in the killer trait: absence of toxicity of R-body producing recombinant E. coli on paramecia. Eur. J. Protistol. 48, 290–296 (2012). [DOI] [PubMed] [Google Scholar]
- van Wagtendonk W. J., Clark J. A. D. & Godoy G. A. The biological status of lambda and related particles in Paramecium aurelia. Proc. Natl. Acad. Sci. USA 50, 835–838 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldo A. T., van Wagtendonk W. J. & Godoy G. A. Nucleic acid and protein content of purified endosymbiont particles of Paramecium aurelia. Biochim. Biophys. Acta 204, 325 (1970). [DOI] [PubMed] [Google Scholar]
- Jurand A. & Preer L. B. Ultrastructure of flagellated lambda symbionts in Paramecium aurelia. J. Gen. Microbiol. 54, 359–364 (1969). [DOI] [PubMed] [Google Scholar]
- Preer J. R. & Preer L. B. Revival of names of protozoan endosymbionts and proposal of Holospora caryophila nom. nov. Int. J. Syst. Bacteriol. 32, 140–141 (1982). [Google Scholar]
- Görtz H.-D. & Schmidt H. J. [Holosporaceae fam. nov.]. Bergey's Manual Of Systematic Bacteriology, 2nd edn. [Garrity, G. M., Brenner, D. J., Krieg, N. R. & Staley, J. T. (eds.)] (Springer, New York, 2005). [Google Scholar]
- Petroni G., Spring S., Schleifer K.-H., Verni F. & Rosati G. Defensive extrusive ectosymbionts of Euplotidium (Ciliophora) that contain microtubule-like structures are bacteria related to Verrucomicrobia. Proc. Natl. Acad. Sci. USA 97, 1813–1817 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini C., Rosati G., Verni F. & Petroni G. Identification of the bacterial endosymbionts of the marine ciliate Euplotes magnicirratus (Ciliophora, Hypotrichia) and proposal of “Candidatus Devosia euplotis”. Int. J. Syst. Evol. Microbiol. 54, 1151–1156 (2004). [DOI] [PubMed] [Google Scholar]
- Rinke C. et al. “Candidatus Thiobios zoothamnicoli”, an ectosymbiotic bacterium covering the giant marine ciliate Zoothamnium niveum. Appl. Environ. Microbiol. 72, 2014–2021 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinzato N. et al. Phylogenetic analysis and fluorescence in situ hybridization detection of archaeal and bacterial endosymbionts in the anaerobic ciliate Trimyema compressum. Microb. Ecol. 54, 627–636 (2007). [DOI] [PubMed] [Google Scholar]
- Eschbach E. et al. “Candidatus Paraholospora nucleivisitans”, an intracellular bacterium in Paramecium sexaurelia shuttles between the cytoplasm and the nucleus of its host. Syst. Appl. Microbiol. 32, 490–500 (2009). [DOI] [PubMed] [Google Scholar]
- Vannini C. et al. “Candidatus Anadelfobacter veles” and “Candidatus Cyrtobacter comes”, two new Rickettsiales species hosted by the protist ciliate Euplotes harpa (Ciliophora, Spirotrichea). Appl. Environ. Microbiol. 76, 4047–4054 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscaro V., Fokin S. I., Schrallhammer M., Schweikert M. & Petroni G. Revised systematics of Holospora-like bacteria and characterization of “Candidatus Gortzia infectiva”, a novel macronuclear symbiont of Paramecium jenningsi. Microb. Ecol. 65, 255–267 (2013). [DOI] [PubMed] [Google Scholar]
- Vannini C., Petroni G., Verni F. & Rosati G. A bacterium belonging to the Rickettsiaceae family inhabits the cytoplasm of the marine ciliate Diophrys appendiculata (Ciliophora, Hypotrichia). Microb. Ecol. 49, 434–442 (2005). [DOI] [PubMed] [Google Scholar]
- Ferrantini F. et al. “Candidatus Cryptoprodotis polytropus”, a novel Rickettsia-like organism in the ciliated protist Pseudomicrothorax dubius (Ciliophora, Nassophorea). J. Eukaryot. Microbiol. 56, 119–129 (2009). [DOI] [PubMed] [Google Scholar]
- Sun H. Y. et al. Endosymbiotic bacteria in the parasitic ciliate Ichthyophthirius multifiliis. Appl. Environ. Microbiol. 75, 7445–7452 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrallhammer M. et al. “Candidatus Megaira polyxenophila” gen. nov., sp. nov.: considerations on evolutionary history, host range and shift of early divergent rickettsiae. PLoS ONE 8, e72581 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrallhammer M., Schweikert M., Vallesi A., Verni F. & Petroni G. Detection of a novel subspecies of Francisella noatunensis as endosymbiont of the ciliate Euplotes raikovi. Microb. Ecol. 61, 455–464 (2011). [DOI] [PubMed] [Google Scholar]
- Boscaro V., Vannini C., Fokin S. I., Verni F. & Petroni G. Characterization of “Candidatus Nebulobacter yamunensis” from the cytoplasm of Euplotes aediculatus (Ciliophora, Spirotrichea) and emended description of the family Francisellaceae. Syst. Appl. Microbiol. 35, 432–440 (2012). [DOI] [PubMed] [Google Scholar]
- Andersson S. G. E. et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–143 (1998). [DOI] [PubMed] [Google Scholar]
- Gray M. W., Burger G. & Lang B. F. Mitochondrial evolution. Science 283, 1476–1481 (1999). [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. A., Creevey C. J. & McInerney J. O. Genome phylogenies indicate a meaningful α-proteobacterial phylogeny and support a grouping of the mitochondria with the Rickettsiales. Mol. Biol. Evol. 23, 74–85 (2006). [DOI] [PubMed] [Google Scholar]
- Derelle R. & Lang B. F. Rooting the eukaryotic tree with mitochondrial and bacterial proteins. Mol. Biol. Evol. 29, 1277–1289 (2012). [DOI] [PubMed] [Google Scholar]
- Sassera D. et al. Phylogenomic evidence for the presence of a flagellum and cbb3 oxidase in the free-living mitochondrial ancestor. Mol. Biol. Evol. 28, 3285–3296 (2011). [DOI] [PubMed] [Google Scholar]
- Montagna M. et al. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphapreoteobacteria. Appl. Environ. Microbiol. 79, 3241–3248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscaro V., Petroni G., Ristori A., Verni F. & Vannini C. “Candidatus Defluviella procrastinata” and “Candidatus Cyrtobacter zanobii”, two novel ciliate endosymbionts belonging to the “Midichloria clade”. Microb. Ecol. 65, 302–310 (2013). [DOI] [PubMed] [Google Scholar]
- Jurand A., Rudman B. M. & Preer J. R. Prelethal symptoms of the killing action of some non-kappa killers in the Paramecium aurelia complex. J. Exp. Zool. 220, 135–145 (1982). [Google Scholar]
- Soldo A. T. & Godoy G. A. Observations on the production of folic acid by symbiont lambda particles of Paramecium aurelia stock 299. J. Protozool. 20, 502 (1973). [Google Scholar]
- Landis W. G. Factors determining the frequency of the killer trait within populations of the Paramecium aurelia complex. Genetics 115, 197–205 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche T. R. et al. In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl. Environ. Microbiol. 65, 206–212 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassera D. et al. “Candidatus Midichloria mitochondrii”, an endosymbiont of the tick Ixodes ricinus with a unique intramitochondrial lifestyle. Int. J. Syst. Evol. Microbiol. 56, 2535–2540 (2006). [DOI] [PubMed] [Google Scholar]
- Matsuura Y., Kikuchi Y., Meng X. Y., Koga R. & Fukatsu T. Novel clade of alphaproteobacterial endosymbionts associated with stinkbugs and other arthropods. Appl. Environ. Microbiol. 78, 4149–4156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S. J. et al. Strawberry disease lesions in rainbow trout from southern Idaho are associated with DNA from a Rickettsia-like organism. Dis. Aquat. Org. 82, 111–118 (2008). [DOI] [PubMed] [Google Scholar]
- Dumler J. S. & Walker D. H. [Rickettsiales Gieszczykiewicz 1939, 25AL emend. Dumler, Barbet, Bekker, Dasch, Palmer, Ray, Rikihisa and Rurangirwa 2001, 2156]. Bergey's Manual Of Systematic Bacteriology, 2nd edn. [Garrity, G. M., Brenner, D. J., Krieg, N. R. & Staley, J. T. (eds.)] (Springer, New York, 2005). [Google Scholar]
- Mariconti M. et al. A study on the presence of flagella in the order Rickettsiales: the case of “Candidatus Midichloria mitochondrii”. Microbiology 158, 1677–1683 (2012). [DOI] [PubMed] [Google Scholar]
- Shimoyama T., Kato S., Ishii S. & Watanabe K. Flagellum mediates symbiosis. Science 323, 1574 (2009). [DOI] [PubMed] [Google Scholar]
- Fokin S. I. Paramecium genus: biodiversity, some morphological features and the key to the main morphospecies discrimination. Protistology 6, 227–235 (2010/11). [Google Scholar]
- Barth D., Krenek S., Fokin S. I. & Berendonk T. U. Intraspecific genetic variation in Paramecium revealed by mitochondrial cytochrome c oxidase I sequences. J. Eukaryot. Microbiol. 53, 20–25 (2006). [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43 (1969). [DOI] [PubMed] [Google Scholar]
- Venable J. H. & Coggeshall R. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 25, 407–408 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W., Amann R., Ludwig W., Wagner M. & Schleifer K.-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15, 593–600 (1992). [Google Scholar]
- Amann R. I. et al. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56, 1919–1925 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. R. et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy A., Maixner F., Wagner M. & Horn M. probeBase – an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res. 35, D800–D804 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S. & Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704 (2003). [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J. P. & Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17, 754–755 (2001). [DOI] [PubMed] [Google Scholar]
- Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 25, 1253–1256 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods