Abstract
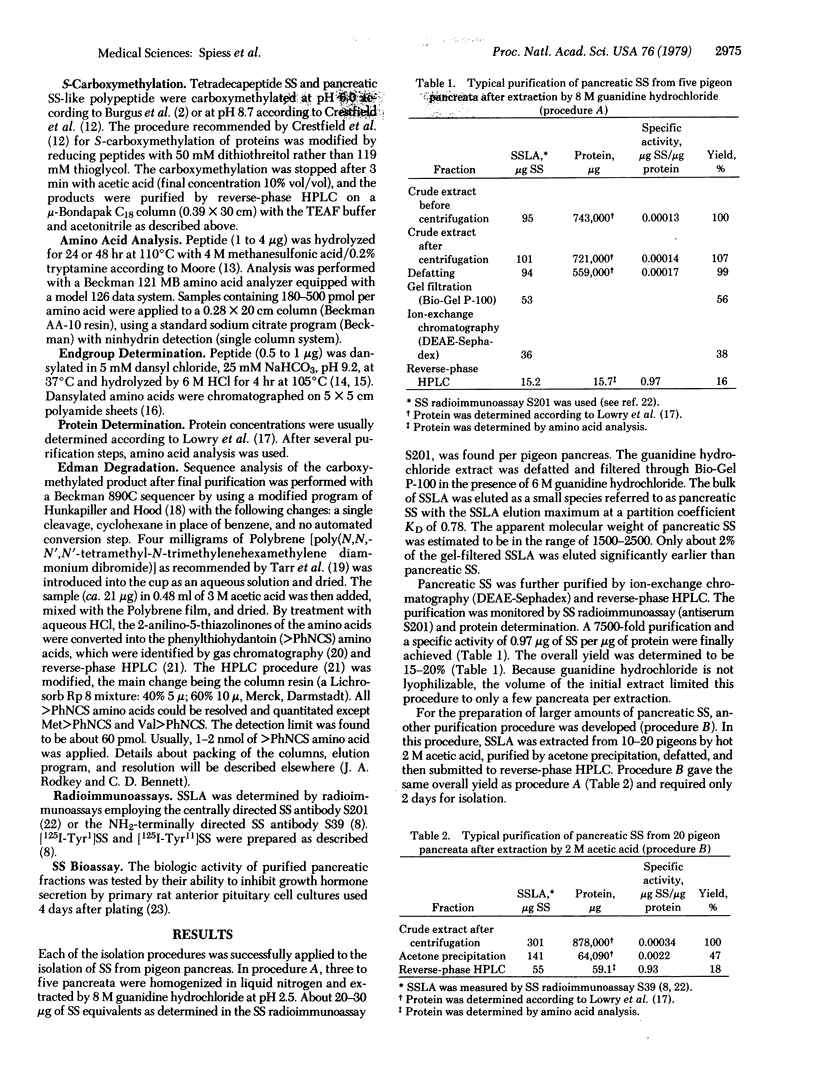
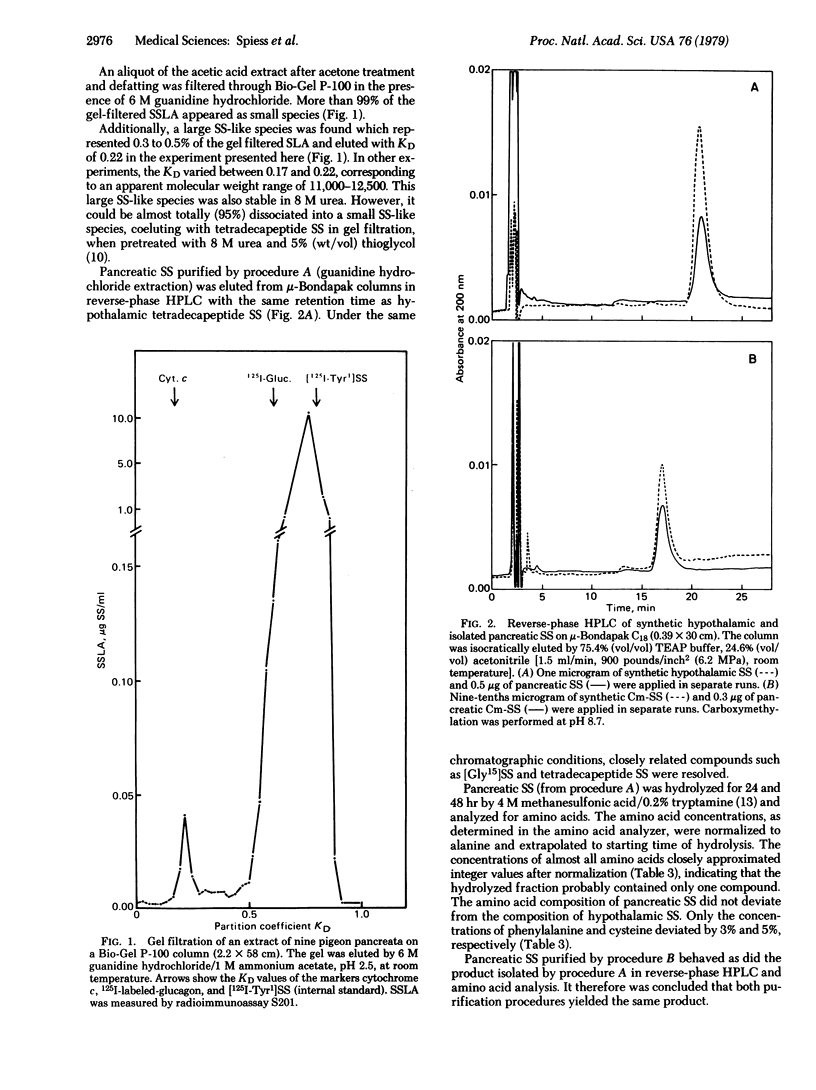
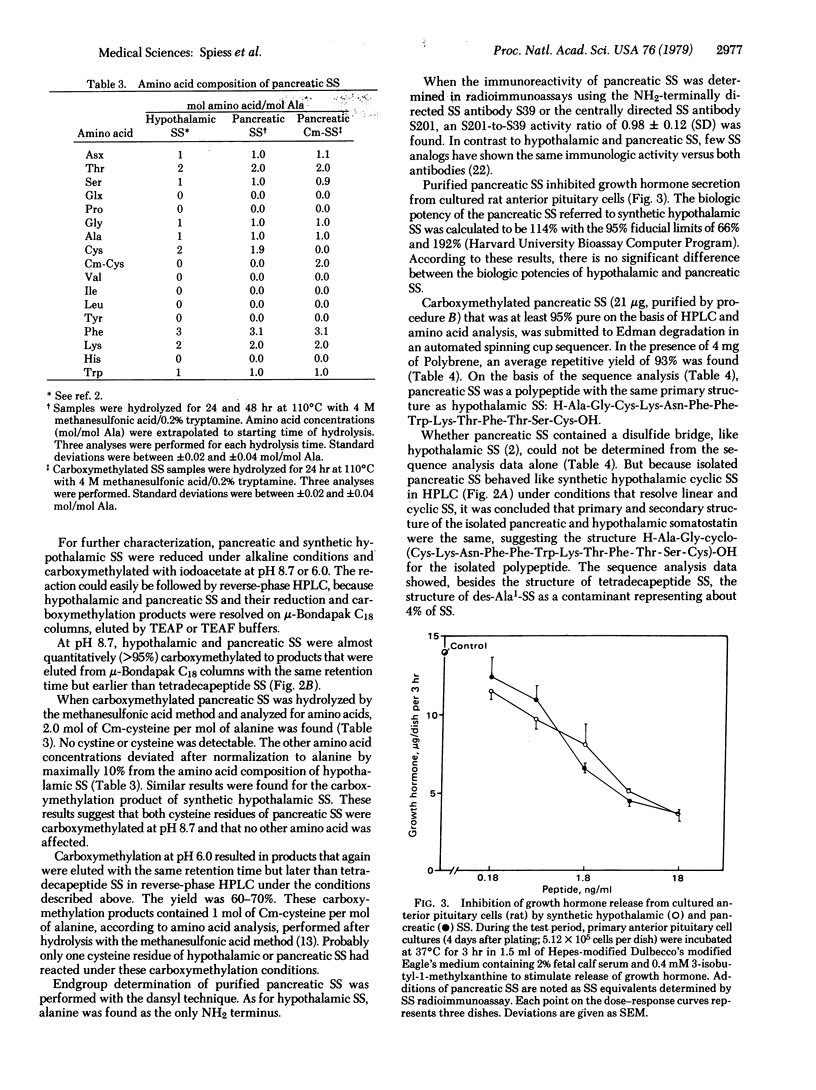
Most of the somatostatin-like activity from pigeon pancreas was found to correspond to small species with an apparent molecular weight of 1500--2500. This species was isolated under conditions minimizing intermolecular interactions and protease activities. The isolated product was characterized by two somatostatin radioimmunoassays, a bioassay, endgroup determination, and amino acid analysis. The structure of the isolated compound was determined to be H-Ala-Gly-cyclo-(Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys)-OH. Additionally, small amounts of des-Ala1-somatostatin, a possible degradation product of pancreatic somatostatin, and a large somatostatin-like species with an apparent molecular weight of 11,000--12,500 were detected. It is concluded that the main somatostatin-like polypeptide isolated from pigeon pancreas is identical to the mammalian hypothalamic tetradecapeptide somatostatin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Sato H., Dupont A., Nishi N., Schally A. V. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975 Sep 19;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- Bennett C. D., Rodkey J. A., Sondey J. M., Hirschmann R. Dihydrofolate reductase: the amino acid sequence of the enzyme from a methotrexate-resistant mutant of Escherichia coli. Biochemistry. 1978 Apr 4;17(7):1328–1337. doi: 10.1021/bi00600a030. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brownstein M., Arimura A., Sato H., Schally A. V., Kizer J. S. The regional distribution of somatostatin in the rat brain. Endocrinology. 1975 Jun;96(6):1456–1461. doi: 10.1210/endo-96-6-1456. [DOI] [PubMed] [Google Scholar]
- Burgus R., Ling N., Butcher M., Guillemin R. Primary structure of somatostatin, a hypothalamic peptide that inhibits the secretion of pituitary growth hormone. Proc Natl Acad Sci U S A. 1973 Mar;70(3):684–688. doi: 10.1073/pnas.70.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Dubois M. P. Immunoreactive somatostatin is present in discrete cells of the endocrine pancreas. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1340–1343. doi: 10.1073/pnas.72.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont A., Alvarado-Urbina G. Conversion of big pancreatic somatostatin without peptide bond cleavage into somatostatin tetradecapeptide. Life Sci. 1976 Nov 1;19(9):1431–1433. doi: 10.1016/0024-3205(76)90444-6. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rivier J., Brazeau P., Vale W., Guillemin R. Somatostatin analogs. Relative importance of the disulfide bridge and of the Ala-Gly side chain for biological activity. J Med Chem. 1975 Feb;18(2):123–126. doi: 10.1021/jm00236a001. [DOI] [PubMed] [Google Scholar]
- Rodkey J. A., Bennett C. D. Micro-Edman degradation: the use of high pressure liquid chromatography and gas chromatography in the amino terminal sequence determination of 8 nanomoles of dihydrofolate reductase from a mouse sarcoma. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1407–1413. doi: 10.1016/s0006-291x(76)80170-2. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Dupont A., Arimura A., Redding T. W., Nishi N., Linthicum G. L., Schlesinger D. H. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976 Feb 10;15(3):509–514. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- Spiess J., Vale W. Investigation of larger forms of somatostatin in pigeon pancreas and rat brain. Metabolism. 1978 Sep;27(9 Suppl 1):1175–1178. doi: 10.1016/0026-0495(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Vale W., Brazeau P., Rivier C., Brown M., Boss B., Rivier J., Burgus R., Ling N., Guillemin R. Somatostatin. Recent Prog Horm Res. 1975;31:365–397. doi: 10.1016/b978-0-12-571131-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Vale W., Brazeau P., Rivier C., Brown M., Boss B., Rivier J., Burgus R., Ling N., Guillemin R. Somatostatin. Recent Prog Horm Res. 1975;31:365–397. doi: 10.1016/b978-0-12-571131-9.50014-1. [DOI] [PubMed] [Google Scholar]
- Vale W., Ling N., Rivier J., Villarreal J., Rivier C., Douglas C., Brown M. Anatomic and phylogenetic distribution of somatostatin. Metabolism. 1976 Nov;25(11 Suppl 1):1491–1494. doi: 10.1016/s0026-0495(76)80175-8. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Ling N., Brown M. Biologic and immunologic activities and applications of somatostatin analogs. Metabolism. 1978 Sep;27(9 Suppl 1):1391–1401. doi: 10.1016/0026-0495(78)90081-1. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



