Abstract
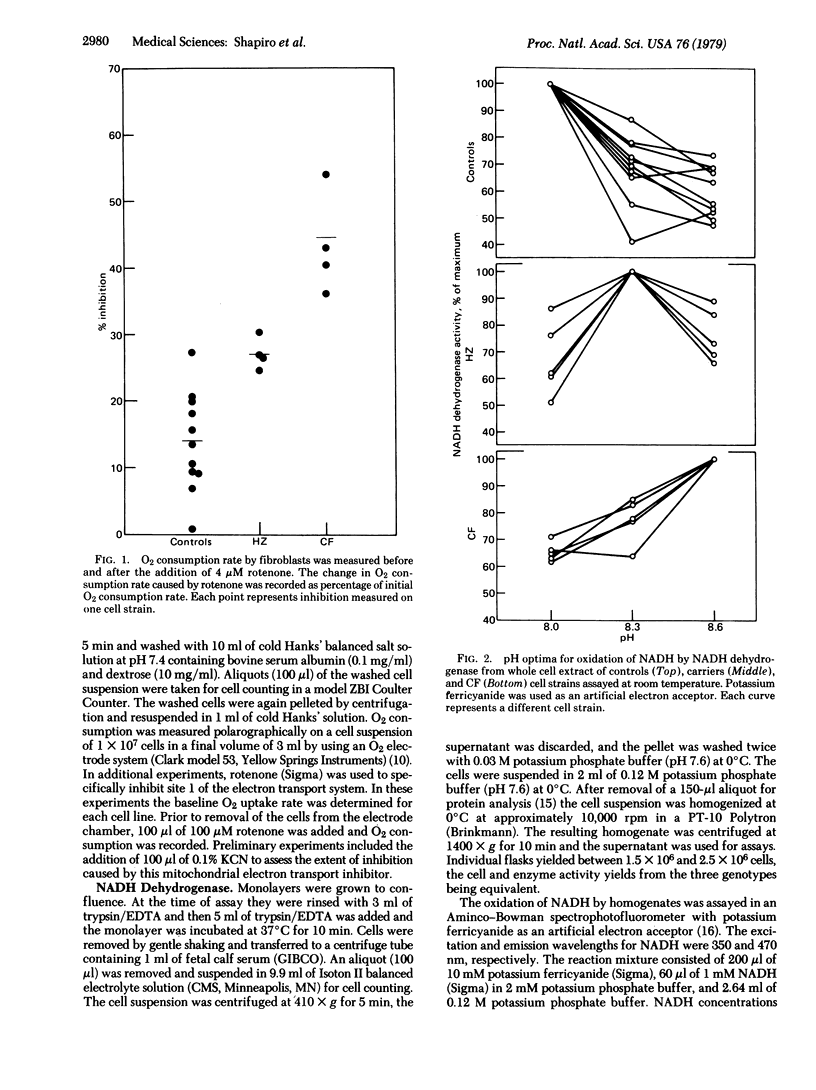
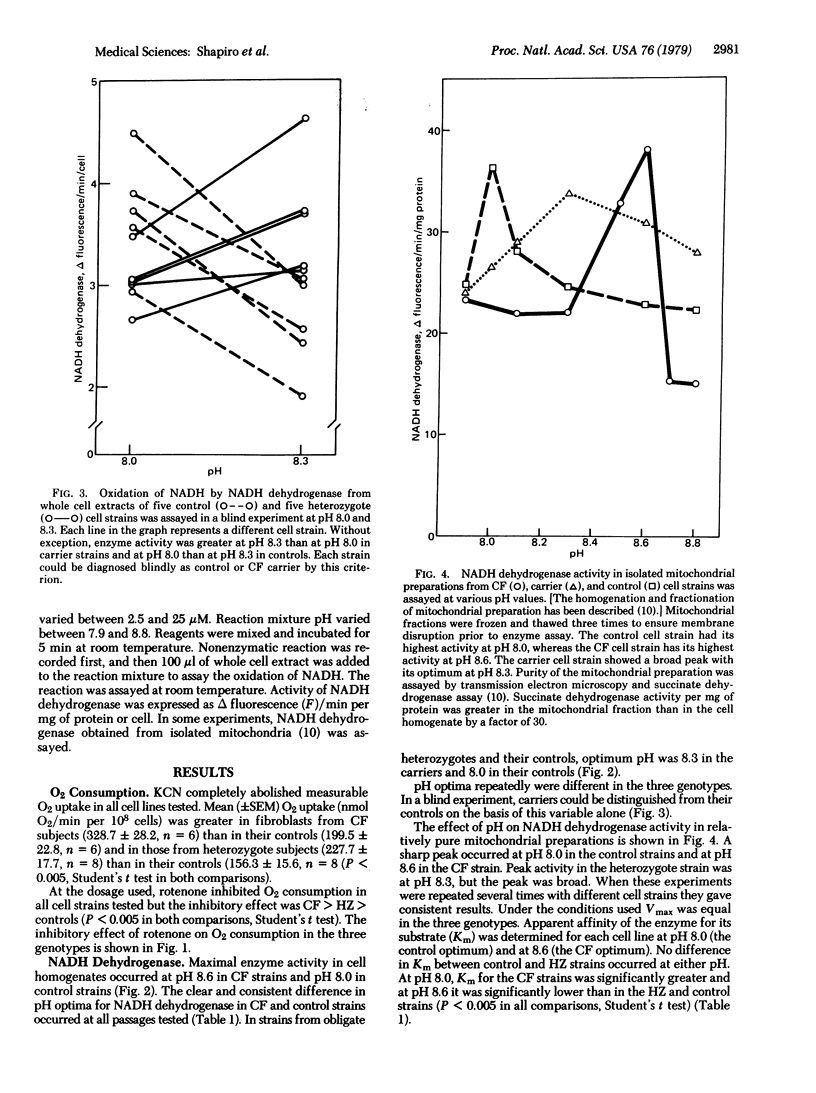
We have shown that skin fibroblast from patients with cystic fibrosis (CF) and from carriers for CF [heterozygotes (HZ)] consume more O2 than do their controls. When the mitochondrial electron transport inhibitor rotenone was added to the cells, the relative inhibition of O2 consumption was CF greater than HZ greater than controls (P less than 0.005 in both comparisons). Because rotenone specifically inhibits NADH dehydrogenase, [NADH: (acceptor) oxidoreductase, EC 1.6.99.3], which is the enzyme of energy-conserving site 1 of the mitochondrial electron transport system, activity and kinetics of this enzyme system were studied in fibroblast homogenates. NADH dehydrogenase activity was equal in cells from the three genotypes. At pH 8.0, affinity of the enzyme for its substrate was CF greater than HZ = controls; at pH 8.6, affinity was CF greater than HZ = controls (P less than 0.005 for the differences). pH optima for the genotypes were without exception 8.6 (CF), 8.3 (HZ), and 8.0 (control). HZ and control lines were distinguished unequivocally in a blind test on the basis of differences in pH optima. Purified mitochondrial preparations revealed pH optima identical to those found in whole cell homogenates. These data suggest that the mutant gene responsible for CF is expressed in the complex mitochondrial NADH dehydrogenase system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argent B. E., Case R. M., Scratcherd T. Amylase secretion by the perfused cat pancreas in relation to the secretion of calcium and other electrolytes and as influenced by the external ionic environment. J Physiol. 1973 May;230(3):575–593. doi: 10.1113/jphysiol.1973.sp010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygrave F. L. Mitochondria and the control of intracellular calcium. Biol Rev Camb Philos Soc. 1978 Feb;53(1):43–79. doi: 10.1111/j.1469-185x.1978.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- Di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (first of three parts). N Engl J Med. 1976 Aug 26;295(9):481–485. doi: 10.1056/NEJM197608262950905. [DOI] [PubMed] [Google Scholar]
- Feigal R. J., Shapiro B. L. Altered intracellular calcium in fibroblasts from patients with cystic fibrosis and heterozygotes. Pediatr Res. 1979 Jun;13(6):764–768. doi: 10.1203/00006450-197906000-00009. [DOI] [PubMed] [Google Scholar]
- Feigal R. J., Shapiro B. L. Mitochondrial calcium uptake and oxygen consumption in cystic fibrosis. Nature. 1979 Mar 15;278(5701):276–277. doi: 10.1038/278276a0. [DOI] [PubMed] [Google Scholar]
- Forstner J. F., Forstner G. G. Effects of calcium on intestinal mucin: implications for cystic fibrosis. Pediatr Res. 1976 Jun;10(6):609–613. doi: 10.1203/00006450-197606000-00009. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. Preparation and properties of NADH: ubiquinone oxidoreductase (complexI), EC 1.6.5.3. Methods Enzymol. 1978;53:11–14. doi: 10.1016/s0076-6879(78)53006-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L., Reynafarje B., Vercesi A., Tew W. P. Transport and accumulation of calcium in mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:160–176. doi: 10.1111/j.1749-6632.1978.tb41941.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Ragan C. I. NADH-ubiquinone oxidoreductase. Biochim Biophys Acta. 1976 Nov 30;456(3-4):249–290. doi: 10.1016/0304-4173(76)90001-x. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L., Feigal R. J., Laible N. J., Biros M. H., Warwick W. J. Doubling time alpha-aminoisobutyrate transport and calcium exchange in cultured fibroblasts from cystic fibrosis and control subjects. Clin Chim Acta. 1978 Jan 2;82(1-2):125–131. doi: 10.1016/0009-8981(78)90035-9. [DOI] [PubMed] [Google Scholar]
- Shapiro B. L., Lam L. F., Fast L. H. Premature senescence in cultured skin fibroblasts from subjects with cystic fibrosis. Science. 1979 Mar 23;203(4386):1251–1253. doi: 10.1126/science.424752. [DOI] [PubMed] [Google Scholar]
- Singer T. P., Edmondson D. E. Flavoproteins (overview). Methods Enzymol. 1978;53:397–418. doi: 10.1016/s0076-6879(78)53045-0. [DOI] [PubMed] [Google Scholar]
- Wallach D., Schramm M. Calcium and the exportable protein in rat parotid gland. Parallel subcellular distribution and concomitant secretion. Eur J Biochem. 1971 Aug 16;21(3):433–437. doi: 10.1111/j.1432-1033.1971.tb01489.x. [DOI] [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (second of three parts). N Engl J Med. 1976 Sep 2;295(10):534–541. doi: 10.1056/NEJM197609022951005. [DOI] [PubMed] [Google Scholar]
- di Sant'Agnese P. A., Davis P. B. Research in cystic fibrosis (third of three parts). N Engl J Med. 1976 Sep 9;295(11):597–602. doi: 10.1056/NEJM197609092951105. [DOI] [PubMed] [Google Scholar]



