Abstract
The studies presented in this review explore three distinct areas with potential for inhibiting HIV infection in women. Based on emerging information from the physiology, endocrinology and immunology of the female reproductive tract (FRT), we propose unique ‘works in progress’ for protecting women from HIV. Various aspects of FRT immunity are suppressed by estradiol during the menstrual cycle, making women more susceptible to HIV infection. By engineering commensal Lactobacillus to secrete the anti-HIV molecule Elafin as estradiol levels increase, women could be protected from HIV infection. Selective estrogen response modifiers enhance barrier integrity and enhance secretion of protective anti-HIV molecules. Finally, understanding the interactions and regulation of FRT endogenous antimicrobials, proteases, antiproteases, etc., all of which are under hormonal control, will open new avenues to therapeutic manipulation of the FRT mucosal microenvironment. By seeking new alternatives to preventing HIV infection in women, we may finally disrupt the HIV pandemic.
Keywords: Antimicrobials, female reproductive tract, HIV, selective estrogen response modifier
Introduction
Heterosexual transmission of HIV is the predominant driver of the growing HIV pandemic, and studies indicate that women are disproportionately affected compared to men.1 Infection rates exceed 35% in parts of sub-Saharan Africa; however, the development of anti-HIV vaccines and topical microbicides has been unsuccessful in stemming the flow of the HIV pandemic. Recently, hope has risen with initial results using Tenofovir gel, a vaginal microbicide that has shown promise for preventing HIV through vaginal sex in a clinical trial,2 but this possible one exception requires further study. Scientists are challenged to explore and develop new and innovative ways to protect women from this dreadful pandemic. In this review, we provide information on three interventions that we believe have the potential for preventing infection in women. These ‘works in progress’ are built on solid analysis and understanding of the innate and adaptive immune systems in the female reproductive tract (FRT) (for reviews, see 3–5).
Reproduction is essential to continuing our species, and we have evolved overlapping and layered mechanisms of innate and adaptive immunity in the FRT to maintain fertility and protect the mother from infection by pathogenic microorganisms. In addition, immunity in the FRT must be tempered to accept a semi-allogeneic fetus. By looking at the physiology, endocrinology and immunity of the FRT, we have hypothesized that there are three areas that have been under-explored for their potential to manipulate FRT immune mechanisms. These include engineering Lactobacilli to secrete an anti-HIV molecule at a time when immunity is suppressed in the FRT, using selective estrogen response modifiers (SERMs) to enhance immunity locally in the FRT (barrier function, endogenous microbicide secretion, etc.) and understanding the advantages and disadvantages of the interactions among FRT antimicrobials, enzymes, hormones, etc. in the FRT environment to selectively regulate protection from HIV infection. These innovative yet unproven approaches are designed to regulate immunity in the FRT in ways that will reduce HIV infection in women.
Development of commensal bacteria that secrete a natural anti-HIV peptide during the window of vulnerability
Commensal bacteria such as Lactobacillus inhibit HIV infection in the FRT owing to the secretion of acids that create a low-pH environment (pH = 4)6,7 and the secretion of molecules like H2O2.8,9 However, when deposited in the vagina, semen neutralizes the pH and inactivates the H2O2,10 thereby abrogating much of the protective effects produced by commensal bacteria against HIV during heterosexual sex, possibly making it easier for women to become infected. It is becoming increasingly clear that cells from the upper and lower FRT constitutively produce molecules that prevent HIV-1 infection (see Wira, et al. in this issue,4,5,11,12). Cervical vaginal lavage (CVL) from healthy women contains many molecules (e.g., Elafin, SLPI, HDB2, CCL20/MIP3α) that have anti-HIV activity.11 The concentration and/or the biologic activity of these anti-HIV molecules in CVL vary considerably during the menstrual cycle.
We have previously suggested that there is a time during the menstrual cycle when women are most susceptible to infection by HIV.1 This ‘window of vulnerability’ occurs because of sex hormone depression of immunity at the time of ovulation and during the secretory phase of the menstrual cycle. Antibody induction, immunoglobulin transport, cytotoxic T cell activity, natural antimicrobial secretion including anti-HIV molecules, etc. are selectively suppressed because of the increase in estradiol that accompanies hormone-induced maturation, release and transport of the ovum to the endometrium. The immunosuppression at this time in the FRT has evolved to reduce and/or prevent immune reaction against sperm and very early development of the semi-allogeneic fetal unit, thus optimizing conditions for the start of a successful pregnancy. However, the natural immune depression during mid-cycle, combined with the introduction of immunosuppressive HIV(+) semen, could establish FRT conditions that are conducive to HIV infection.
One innovative strategy for preventing HIV infection is to engineer commensal bacteria to produce one or more known anti-HIV molecules to counter the reduction in secretions of endogenous anti-HIV molecules during this susceptible period. In this way, bacterial secretions would nullify the mid-cycle hormone-dependent suppression of immune function that places women at risk for HIV infection. There have been successes in developing commensals such as Lactobacillus that produce anti-HIV molecules. Lactobacilli have been engineered to secrete therapeutic proteins, antibodies, antigens and coreceptors with the capability of interfering with viral infectivity.13 An important proof-of-concept study has shown that it is possible to engineer lactobacilli to secrete a two-domain CD4 protein that could bind to HIV and reduce infectivity.14 A recently completed Phase I trial using vaginally inoculated L. crispatus to control recurrent urinary tract infections demonstrated that L. crispatus can effectively colonize the lower human FRT and persist at high levels for the duration of the trial (4 weeks).15
We set out to develop a bacterial system that would (i) colonize the lower human FRT, (ii) accept an engineered gene expressing a potent endogenous anti-HIV molecule and (iii) adapt the bacteria to secrete anti-HIV peptides in response to an increase in estradiol. For a model anti-HIV peptide, we chose Elafin. Using in vitro studies, we had previously shown in cell culture that recombinant Elafin inhibits both CXCR4 and CCR5 tropic HIV infection.16 In other studies, we have found that CVL from HIV(−) women had higher concentrations of Elafin compared to HIV(+) women.11 Iqbal and colleagues demonstrated that Elafin levels in CVL correlated with protection against HIV infection.17 Elafin contains a whey acidic protein motif [WAP, also known as (whey) four disulfide core (W)FDC], a distinct domain of approximately 50 amino acids in which 8 conserved cysteines form 4 disulfide bonds.18 Trap-pin-2/Elafin (pre-Elafin) and Elafin are prototypical WAP proteins of the innate immune system with antiprotease, antibacterial and antiviral properties. Other anti-HIV molecules of the innate immune system use a threefold disulfide motif and are represented by the defensin family.19 Trappin-2/Elafin is translated with a signal peptide that is cleaved during secretion. Trappin-2/Elafin is later proteolytically processed to form Elafin.
Following preliminary studies with different Lactobacilli to identify the ideal commensal bacteria, we selected Lactobacillus jensenii and Lactobacillus crispatus, which gave us the best preliminary results for secretion of Elafin. These bacteria have good colonization properties, are constitutive to the human vagina and have been shown to be amenable to genetic manipulation.14 In addition, Phase I clinical trials with L. crispatus have demonstrated an effective vaginal delivery system permitting long-term colonization.15 While WAP proteins are effective against pathogenic bacteria such as P. aeruginosa (Gram negative) and S. aureus (Gram positive), they are benign with respect to Lactobacillus.18 Human epithelial cells isolated from either the lower or upper FRT secrete antimicrobial factors that were effective in inhibiting Neisseria gonorrhea, Candida albicans and HIV, but had no effect on the growth of L. crispatus.12
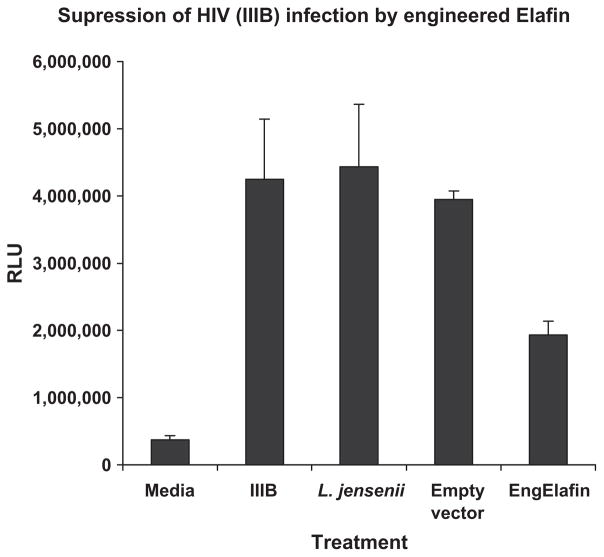
We adopted a vector platform that directs secretion through the Lactobacillus SecA export pathway, as suggested by Dr Bharat Ramratnam of Brown Medical School. He and his colleagues have used this vector to successfully secrete a number of protein/peptide products such as fusion inhibitors cyanovirin and FL-1, FL-2 and FL-3.20,21 Seen in Fig. 1 is the preliminary data indicating that L. jensenii, engineered to express and secrete Elafin, produce Elafin in sufficient concentrations to inhibit infection of target cells by HIV IIIB. Studies to show the efficacy of the engineered Elafin against other HIV reference strains, as well as transmitted/founder HIV clones, are currently underway.
Fig. 1.
Elafin suppresses HIV infection. Supernates (20-fold dilution) from 24-hr cultures of L. jensenii, which contained either no vector, the empty APU vector or APU encoding for engineered Elafin, were incubated with the IIIB HIV virus for 1 hr. The mixture was transferred to TZM-bl cells and cultured for an additional 48 hr. Viability and beta galactosidase activity were determined. Increased beta galactosidase activity expressed as relative light units (RLU) is a measure of HIV infection. Data are presented as mean ± SD of replicate samples.
Studies are underway to utilize changes in estrogen levels during the menstrual cycle to regulate Elafin secretion by Lactobacillus. Our approach is to utilize the conformational changes associated with estradiol binding to the ligand-binding domain of the estrogen receptor (ER) to form the basis of an estrogen sensor. The sensor would direct an increase in Elafin secretion at ovulation and during the secretory phase of the menstrual cycle when FRT and blood estrogen levels are the highest.
In conclusion, under these conditions, Elafin will be secreted by engineered Lactobacillus in response to the increase in estradiol that occurs during the window of vulnerability.1 The uniqueness of this system is that it combines the identification of a potentially susceptible time for HIV infection to deliver a natural anti-HIV molecule using the very signal (increase in estradiol) that limits its expression by immune cells in the lower FRT. This approach is innovative and integrates the biology of the lower FRT with the use of naturally occurring delivery system that delivers naturally occurring antimicrobials to the FRT in synchrony with the normal hormone cycle.
The effect of SERMs on innate immunity and HIV infection in the FRT
Estradiol can influence both the innate and adaptive immune system locally and systemically to protect women, including pregnant women and a developing fetus, from potential pathogens. Estradiol binds to two main forms of the ER, ERα and ERβ, which mediate transcription and translation of hundreds of genes.22 Within the upper and lower FRT, ERα and ERβ, as well as progesterone receptors (PR), are expressed in a cell-specific manner locally by stromal and epithelial cells (Table I). Levels of sex hormone receptor expression change throughout the FRT during the reproductive cycle in pre-menopausal women with the highest expression observed during the proliferative phase and declining expression during the secretory phase.23 Immunohistochemical analysis of ER and PR indicate scattered staining throughout the stroma of reproductive tissues. This staining is most likely due to immune cells that are present throughout the FRT. As seen in Table II, when analyzed for ER and PR receptors, peripherally derived naive immune cells tend to show low levels or no staining for steroid receptors, while activated immune cells show increased expression of ERα and ERβ with limited expression of PR. These findings suggest that most immune cells as well as epithelial cells and stromal cells throughout the FRT are responsive to sex hormones.
Table I.
Estrogen receptor α (ERα), estrogen receptor β (ERβ) and progesterone receptor (PR) expression in the human pre-menopausal female reproductive tract
| Tissue | Cell type | ERα | ERβ | PR | References |
|---|---|---|---|---|---|
| Ovary | Germinal epithelium | +++ | +++ | ++ | 61,62 |
| Theca | +++ | + | ++ | 61–64 | |
| Corpora leuteal cells | − | − | ++ | 62,64,65 | |
| Granulosa | − | ++++ | +++ | 62,63,65,66 | |
| Fallopian Tube | Ciliated epithelium | ± | ± | − | 67 |
| Secretory epithelium | +++ | +++ | +++ | 67 | |
| Stroma | + | ++ | + | 67,68 | |
| Uterusa | Luminal epithelium | ++++ | ± | +++ | 23,61,62,69 |
| Stroma | ++++ | ± | +++ | 23,61,62,66,69–71 | |
| Glandular epithelium | ++++ | ± | +++ | 23,61,69–71 | |
| Cervix | Epithelium | ++ | ± | − | 62,72 |
| Stroma | ++ | ++ | + | 62,72 | |
| Vagina | Basalis epithelium | ++++ | ± | +++ | 61,73,74 |
| Stroma | ++ | − | +++ | 61,73,74 |
Receptor expression represented as strong (++++), moderate (+++), low (++), weak (+), varying low and negative results (±) and undetected (−).
Uterine expression in the uterus deceases over the menstrual cycle with high expression observed during the proliferative stage and low expression or no detection during secretory stage.
Table II.
Estrogen receptor α (ERα), estrogen receptor β (ERβ) and progesterone receptor (PR) expression in human activated immune cells
| Cell type | ERα | ERβ | PR | References |
|---|---|---|---|---|
| Monocyte | +++ | ++ | − | 75–77 |
| Macrophage | ++++ | + | ++ | 75,78 |
| Dendritic cell | ++ | ++ | + | 79,80 |
| Neutrophil | +++ | ++ | − | 81,82 |
| Uterine NK cell | − | +++ | uNK−/mature ++ | 83,84 |
| CD4+ T cell | +++ | ++ | + | 76,85 |
| CD8+ T cell | + | +++ | + | 76,85 |
| B cell | + | ++++ | − | 67,76,80 |
In most instances, naïve immune cells only express hormone receptor upon activation. Furthermore, the level of expression is regulated by the presence of steroid hormones. Receptor expression represented as strong (++++), moderate (+++), low (++), weak (+) and undetected (−).
In addition to estradiol, a number of pharmacological compounds known as selective estrogen receptor modulators (SERMs) bind to ERα and ERβ, inducing cell-specific agonist or antagonist effects. Individual responses vary with the cellular complement of coactivators or corepressors present in target cells.22,24 Selective estrogen receptor modulators in clinical use include Tamoxifen and Raloxifene, which are administered for treatment of breast cancer and osteoporosis, respectively. Tamoxifen and Raloxifene bind to both ERα and ERβ; however, they show tissue-specific ER antagonist/partial agonist activity that is different from that seen with estradiol.25,26 Another SERM, ICI 182780 has high affinity to both ERα and ERβ and acts primarily as an estrogen antagonist.27,28 In contrast, Y134 and PHTPP are highly selective for ERα and ERβ, respectively.29,30
The focus of our work is the understanding of SERM treatment on innate immune mechanisms that protect the FRT from infection by pathogens, particularly HIV. Using primary human and mouse epithelial cells in in vitro and in vivo studies with mice, we have focused on the role of SERMs in regulating the barriers that protect the FRT from infectious pathogens. Previously, we reported that estradiol lowers human and mouse uterine columnar epithelial cell barrier integrity as measured by trans-epithelial resistance (TER).31 More recently, we found that SERMs ICI 182780, Raloxifene and Tamoxifen significantly increased TER in human and mouse uterine epithelial cells relative to controls and estradiol, while Y134 and PHTPP had minimal effect (unpublished observations). Our work has also shown that SERM treatment has a direct effect on the secretion of antimicrobial chemokines by mouse uterine epithelial cells. CCL20/MIP3α, a potent anti-HIV molecule,32 is significantly enhanced when mouse uterine epithelial cells are treated in vitro with ICI 182780 or Y134 (Fig. 2). In in vivo studies using an ovariectomized mouse model, estradiol and SER-Ms given via either subcutaneous injection or locally deposited into the vaginal vault altered production of CCL20/MIP3α in the upper and lower FRT (Hickey and Wira, manuscript in preparation). Enhanced production of CCL20/MIP3α potentially protects uterine mucosa from pathogens, in particular HIV. Studies are underway to examine the extent to which SERMs enhance the expression and/or biologic activity of other antimicrobials including α defensins, β defensins and SLPI without recruiting and/or activating immune cells capable of being infected by HIV. The identification and development of specific SERMs that enhance the production of antimicrobials and increase barrier integrity throughout the reproductive cycle has the potential of protecting the FRT against STI without compromising reproductive potential.
Fig. 2.
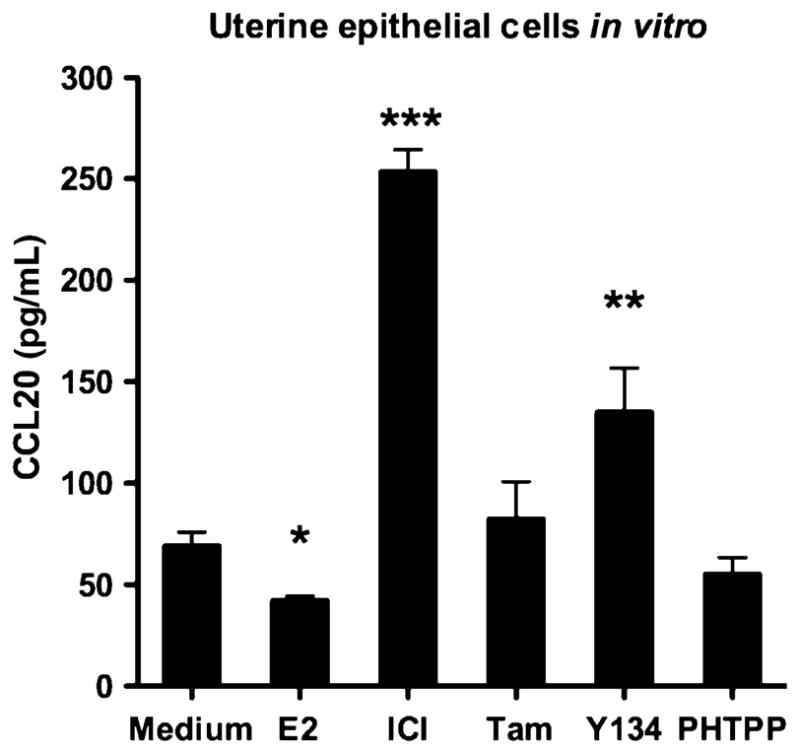
CCL20 is secreted from isolated uterine epithelial cells in vitro following 24-hr stimulation with 10−7 M estradiol, 10−7 M ICI 182780, 10−7 M Tamoxifen, 10−7 M Y134 or 10−7 M PHTPP. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA, Bonferroni’s post hoc.
Estradiol can have indirect as well as direct effects on regulation of FRT immunity. For example, uterine epithelial cell proliferation and endometrial development is dependent on estradiol stimulation of underlying ERα-positive stromal cells to produce growth factors.33 We have found that estradiol stimulates human uterine stromal fibroblasts to secrete hepatocyte growth factor.34 Furthermore, keratinocyte growth factor, an important pluripotential molecule, enhances the secretion of the anti-HIV molecule CCL20/MIP3α from mouse uterine epithelial cells.35 Growth factors mediate their effects on epithelial cells through binding directly to their surface receptor but also via ER activation (for reviews, see 36,37). The effect of SERMs on the paracrine interactions of growth factors and antimicrobial factors between stromal and epithelial cells is under investigation.
Regulation and interactions in the microenvironment regarding HIV infection: complexity of the FRT mucosal milieu
There are several immune mechanisms in the FRT that have the potential to inhibit HIV infection. These include barrier integrity by epithelial cells, constitutive secretion of natural antiviral peptides, production by epithelial cells of chemokines and cytokines to recruit, retain and elicit protective responses by immune cells, presence of enzymes that regulate activation of other molecules, intracellular anti-HIV factors, antibodies produced in response to HIV infection, alterations in HIV coreceptors, etc. (for reviews, see 4,5). Regulation of immunity in the FRT evolved to enhance success of reproduction and virtually all these molecules, cells and mechanisms are regulated by the sex hormones estradiol and/or progesterone.4 To withstand the assault of a diverse and increasing pool of pathogens, the FRT has developed a complex integrative prevention strategy of layered protection that is regulated by multiple mechanisms.
Biologic Activity of Anti-HIV Molecules
Within the FRT, many anti-HIV molecules, such as defensins and Elafin, are expressed as precursor proteins that must be cleaved for activation and/or release in secretions. For example, Trappin-2/Elafin attaches to the cell surface where activation by tryptase releases Elafin into secretions.38 Also present in FRT secretions are the cathepsins, a family of matrix metalloproteases, which are responsible for activating/deactivating innate immune factors including the anti-HIV molecules CXCL12 and HNP1.39–42 Cathepsins are also responsible for directly regulating anti-HIV innate factors.43–45 For example, cathepsin D, a cysteine protease present in vaginal secretions,46 has been shown to enhance HIV replication,47,48 possibly because of the inhibition of CCL20/MIP3α,43 a known anti-HIV factor in CVL.32 Kallikreins (KLK) are another family of serine proteases present in the genital mucosa that can activate/deactivate multiple immune factors in the FRT.49,50 For example, KLK5 has been shown to regulate the antimicrobial activity of LL37, a potent anti-HIV molecule.51,52 These protease families are regulated throughout the menstrual cycle by protease inhibitors present in the secretions. A particular CVL molecule may be quantitatively detected in an ELISA, but it may not be biologically active depending on proteases/antiproteases present at that time and the hormonal condition of the FRT. Therefore, studies using correlations of concentrations of an antimicrobial factor with anti-HIV activity may not always be correct.
A unique study by Burgener et al.53 looked at cervical mucosa of HIV-1-resistant women for bio-markers of HIV-1 resistance using two-dimensional gel electrophoresis. They found that, relative to controls, HIV-1-resistant women over-expressed several proteases from the serpin B family and cystatin A, a cysteine antiprotease and inhibitor of HIV infection. Several serpins inhibit cathepsin G, which is found in CVL, and is known to enhance HIV infectivity in culture,54 possibly by cleaving RANTES.44 This is another example where the relative concentrations and biologic activity of anti-HIV factors, proteases, antiproteases, etc. will in turn determine whether the local FRT environment is conducive to HIV infection.
Many Mucosal Anti-HIV Factors, Many Mechanisms for Inhibition
There are many peptide/protein molecules, such as chemokines, cytokines, proteases, protease inhibitors, immune regulators, immunoglobulins, matrix metalloproteases, growth factors, etc., in genital tract secretions that can potentially modulate HIV infection either directly or indirectly. Indeed, it is estimated by proteomic array analysis that there are 685 proteins in cervical vaginal fluid (CVL),55 and it is likely that several of these will have either direct or indirect inhibitory or enhancing effects on HIV infection. However, neutralizing one suspected factor that has passed the aforementioned tests may still leave the CVL with anti-HIV activity, because there are so many endogenous anti-HIV factors. Why are there so many factors? Singh et al.56 reached relevant conclusions 10 years ago in a distinctly different mucosal system, human airway surface liquid (ASL), which contains many of the same antimicrobial factors that are found in CVL, namely lysozyme, lactoferrin, SLPI, HBD, etc. They hypothesized that there could be several reasons for having multiple antimicrobial factors in ASL, including redundancy in maintaining sterility in the lower respiratory tract, broadening the spectrum of antimicrobial activity and that some factors could interact in an additive or synergistic fashion. Their results showed that ASL antibacterial activity was significantly increased by synergistic and additive interactions between antimicrobial factors. In fact, the triple combination of lysozyme, lactoferrin and SLPI was very synergistic, showing enhanced effects better than double combinations. None of the known antimicrobial combinations they tested were antagonistic. We had hypothesized similar additive and synergistic properties for the multiple antimicrobial molecules in CVL/genital tract secretions.5 An additional beneficial effect of multiple anti-HIV factors is that it might prevent emergence of HIV strains resistant to one or two endogenous anti-HIV factors, potentially giving rise to a new mutations.
Because many mucosal secretions, including genital tract fluids, tears, saliva, breast milk, etc. contain many of the same antimicrobial factors, it is likely that they have evolved to prevent infection at mucosal surfaces throughout the body. Also, because individual antimicrobials are effective against a variety of pathogens (bacteria, fungi and viruses; that is, non-specific but biologically multi-faceted), it is possible that each has evolved this broad-spectrum capacity to be maximally effective in protecting against a variety of pathogens. In addition to antimicrobial activity, some have evolved to have multiple functions. For example, SLPI, which inhibits HIV, bacterial and fungal pathogens at multiple mucosal surfaces, is also a potent antiprotease and an inhibitor of NFkB, both of which result in a reduced secretion of local proinflammatory mediators. SLPI secretion by uterine epithelial cells is enhanced by treatment with estradiol, and estradiol inhibits the secretion of induced proinflammatory mediators IL-6 and MIF by blocking expression of NFkB57; reduced local inflammation will result in decreasing susceptibility to HIV infection. So, when SLPI inhibits HIV infection, possibilities include a direct effect on the virus, an effect on the target cell, an effect on a proteolytic enzyme necessary for or antagonistic to HIV infection or some other possible mechanism(s) with additive and/or synergistic potential. Parenthetically, when the antimicrobial SLPI is purchased from commercial sources as a recombinant, the activity of the molecule is expressed in terms of antiprotease activity on a particular enzyme, not on anti-HIV activity of a reference virus in an HIV infection assay. In addition, how a recombinant is made can determine its bioactivity, which can lead to controversial results. For example, although SLPI is now generally regarded as an anti-HIV molecule, 15 years ago there was compelling doubt shed on this premise.58 Possible explanations for the different conclusions may have been in the quality (incomplete linkage of eight disulfide bridges) of different recombinant SLPIs that were used, variability in the expression of a cell surface receptor for SLPI on HIV target cells59,60 or the selection of assay for HIV activity.
Overall, these findings indicate several levels of complexity present in the FRT, which needs to be kept in mind as anti-HIV molecules are identified in mucosal secretions. Fig. 3 shows some of the interactions between known anti-HIV factors and enzymes in genital tract secretions. The challenge in determining exact mechanisms by which HIV infection is inhibited or enhanced in a given CVL sample lies in the sheer number of innate immune factors, their regulators present in CVL and the extent to which they are all bioactive throughout the menstrual cycle.
Fig. 3.
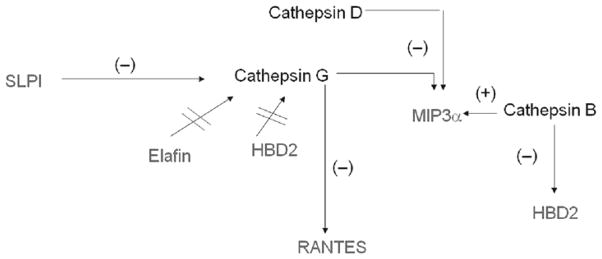
An example of antimicrobial complexity in the FRT: regulation of anti-HIV molecules by cathepsin proteases. Other regulators of innate immune factors in the FRT include proteases, antiproteases, Kallikreins, matrix metalloproteases, etc. The majority of proteases and their substrates are regulated by sex hormones. The antimicrobials SLPI and Elafin are protease inhibitors and anti-HIV factors.
In conclusion, this manuscript presents three distinct novel approaches to address inhibition of HIV infection in women. Engineered Lactobacillus has the potential to secrete anti-HIV molecules during the menstrual cycle when women are most susceptible to infection. SERMs that stimulate secretion of anti-HIV molecules have shown initial promise in in vitro studies that they may protect women from infection. Finally, to understand anti-HIV immunity in the FRT, we should consider the complexity associated with these anti-HIV-secreted factors to ensure that bioactivity is preserved for best efficacy to protect women. What these approaches have in common is that they are using mucosal immunity, in particular natural antimicrobial factors, in the FRT in conjunction with the dynamic changes associated with the sex steroid hormones to enhance protection against HIV infection. A corollary to the direct effects on HIV, these secreted factors have broad-spectrum antimicrobial activity and will protect women from other STI in addition to HIV. By exploring innovative technology and understanding the natural immunity in the FRT, we will improve the chances of protecting women from HIV infection.
Acknowledgments
This work was funded by a National Institutes of Health Grants AI-51877, AI-13541, AI-071761 (CRW), AI084121 (JEB) and by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative (JVF/CRW).
References
- 1.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh M, Fahey J, Wira C. Differential susceptibility of HIV strains to innate immune factors in human cervical-vaginal secretions. Virus Adapt Treat. 2010;2010:63–71. [Google Scholar]
- 4.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 6.Connor RI. Sensitivity of non-clade B primary HIV-1 isolates to mildly acidic pH. J Acquir Immune Defic Syndr. 2006;43:499–501. doi: 10.1097/01.qai.0000243048.50451.18. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher PS, Harman SJ, Boothe AR, Doncel GF, Shattock RJ. Preclinical evaluation of lime juice as a topical microbicide candidate. Retrovirology. 2008;5:3. doi: 10.1186/1742-4690-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baeten JM, Hassan WM, Chohan V, Richardson BA, Mandaliya K, Ndinya-Achola JO, Jaoko W, McClelland RS. Prospective study of correlates of vaginal Lactobacillus colonisation among high-risk HIV-1 seronegative women. Sex Transm Infect. 2009;85:348–353. doi: 10.1136/sti.2008.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkin SS, Linhares IM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21:347–354. doi: 10.1016/j.bpobgyn.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.O’Hanlon DE, Lanier BR, Moench TR, Cone RA. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect Dis. 2010;10:1–8. doi: 10.1186/1471-2334-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS ONE. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill EM, Fahey JV. Epithelial Cell Secretions from the Human Female Reproductive Tract Inhibit Sexually Transmitted Pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2010 doi: 10.1038/mi.2010.72. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Secchi M, Xu Q, Lusso P, Vangelista L. The superior folding of a RANTES analogue expressed in lactobacilli as compared to mammalian cells reveals a promising system to screen new RANTES mutants. Protein Expr Purif. 2009;68:34–41. doi: 10.1016/j.pep.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci U S A. 2003;100:11672–11677. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infect Dis Obstet Gynecol. 2007;2007:35387. doi: 10.1155/2007/35387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2009;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal SM, Ball TB, Levinson P, Maranan L, Jaoko W, Wachihi C, Pak BJ, Podust VN, Broliden K, Hirbod T, Kaul R, Plummer FA. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23:1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 18.Bingle CD, Vyakarnam A. Novel innate immune functions of the whey acidic protein family. Trends Immunol. 2008;29:444–453. doi: 10.1016/j.it.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Bauer F, Schweimer K, Kluver E, Conejo-Garcia JR, Forssmann WG, Rosch P, Adermann K, Sticht H. Structure determination of human and murine beta-defensins reveals structural conservation in the absence of significant sequence similarity. Protein Sci. 2001;10:2470–2479. doi: 10.1110/ps.24401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusch O, Boden D, Hannify S, Lee F, Tucker LD, Boyd MR, Wells JM, Ramratnam B. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. J Acquir Immune Defic Syndr. 2005;40:512–520. doi: 10.1097/01.qai.0000187446.76579.d3. [DOI] [PubMed] [Google Scholar]
- 21.Pusch O, Kalyanaraman R, Tucker LD, Wells JM, Ramratnam B, Boden D. An anti-HIV microbicide engineered in commensal bacteria: secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS. 2006;20:1917–1922. doi: 10.1097/01.aids.0000247112.36091.f8. [DOI] [PubMed] [Google Scholar]
- 22.Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999;5:559–564. doi: 10.1093/molehr/5.6.559. [DOI] [PubMed] [Google Scholar]
- 24.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 25.Wakeling AE, Valcaccia B, Newboult E, Green LR. Non-steroidal antioestrogens – receptor binding and biological response in rat uterus, rat mammary carcinoma and human breast cancer cells. J Steroid Biochem. 1984;20:111–120. doi: 10.1016/0022-4731(84)90197-3. [DOI] [PubMed] [Google Scholar]
- 26.Weatherman RV, Clegg NJ, Scanlan TS. Differential SERM activation of the estrogen receptors (ERalpha and ERbeta) at AP-1 sites. Chem Biol. 2001;8:427–436. doi: 10.1016/s1074-5521(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 27.de Cupis A, Noonan D, Pirani P, Ferrera A, Clerico L, Favoni RE. Comparison between novel steroid-like and conventional nonsteroidal antioestrogens in inhibiting oestradiol- and IGF-I-induced proliferation of human breast cancer-derived cells. Br J Pharmacol. 1995;116:2391–2400. doi: 10.1111/j.1476-5381.1995.tb15085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 29.Compton DR, Sheng S, Carlson KE, Rebacz NA, Lee IY, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazolo[1,5-a]pyrimidines: estrogen receptor ligands possessing estrogen receptor beta antagonist activity. J Med Chem. 2004;47:5872–5893. doi: 10.1021/jm049631k. [DOI] [PubMed] [Google Scholar]
- 30.Ning M, Zhou C, Weng J, Zhang S, Chen D, Yang C, Wang H, Ren J, Zhou L, Jin C, Wang M-W. Biological activities of a novel selective oestrogen receptor modulator derived from raloxifene (Y134) Br J Pharmacol. 2007;150:19–28. doi: 10.1038/sj.bjp.0706960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman KD, Wright JA, Ghosh M, Wira CR, Fahey JV. Estradiol modulation of hepatocyte growth factor by stromal fibroblasts in the female reproductive tract. Fertil Steril. 2009;92:1107–1109. doi: 10.1016/j.fertnstert.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad SN, Wira CR. Keratinocyte Growth Factor Stimulates Macrophage Inflammatory Protein 3alpha and Keratinocyte-derived Chemokine Secretion by Mouse Uterine Epithelial Cells. Am J Reprod Immunol. 2010;64:197–211. doi: 10.1111/j.1600-0897.2010.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. Mechanism of estrogen action: lessons from the estrogen receptor-alpha knockout mouse. Biol Reprod. 1998;59:470–475. doi: 10.1095/biolreprod59.3.470. [DOI] [PubMed] [Google Scholar]
- 37.Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 38.Guyot N, Zani ML, Berger P, Dallet-Choisy S, Moreau T. Proteolytic susceptibility of the serine protease inhibitor trappin-2 (pre-elafin): evidence for tryptase-mediated generation of elafin. Biol Chem. 2005;386:391–399. doi: 10.1515/BC.2005.047. [DOI] [PubMed] [Google Scholar]
- 39.Jokimaa V, Oksjoki S, Kujari H, Vuorio E, Anttila L. Expression patterns of cathepsins B, H, K, L and S in the human endometrium. Mol Hum Reprod. 2001;7:73–78. doi: 10.1093/molehr/7.1.73. [DOI] [PubMed] [Google Scholar]
- 40.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 41.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 42.Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- 43.Hasan L, Mazzucchelli L, Liebi M, Lis M, Hunger RE, Tester A, Overall CM, Wolf M. Function of liver activation-regulated chemokine/CC chemokine ligand 20 is differently affected by cathepsin B and cathepsin D processing. J Immunol. 2006;176:6512–6522. doi: 10.4049/jimmunol.176.11.6512. [DOI] [PubMed] [Google Scholar]
- 44.Lim JK, Lu W, Hartley O, DeVico AL. N-terminal proteolytic processing by cathepsin G converts RANTES/CCL5 and related analogs into a truncated 4–68 variant. J Leukoc Biol. 2006;80:1395–1404. doi: 10.1189/jlb.0406290. [DOI] [PubMed] [Google Scholar]
- 45.Taggart CC, Greene CM, Smith SG, Levine RL, McCray PB, Jr, O’Neill S, McElvaney NG. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J Immunol. 2003;171:931–937. doi: 10.4049/jimmunol.171.2.931. [DOI] [PubMed] [Google Scholar]
- 46.Cummins JE, Christensen L, Lennox JL, Bush TJ, Wu Z, Malamud D, Evans-Strickfaden T, Siddig A, Caliendo AM, Hart CE, Dezzutti CS. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–795. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 47.El Messaoudi K, Thiry L, Van Tieghem N, Liesnard C, Englert Y, Moguilevsky N, Bollen A. HIV-1 infectivity and host range modification by cathepsin D present in human vaginal secretions. AIDS. 1999;13:333–339. doi: 10.1097/00002030-199902250-00005. [DOI] [PubMed] [Google Scholar]
- 48.El Messaoudi K, Thiry LF, Liesnard C, Van Tieghem N, Bollen A, Moguilevsky N. A human milk factor susceptible to cathepsin D inhibitors enhances human immunodeficiency virus type 1 infectivity and allows virus entry into a mammary epithelial cell line. J Virol. 2000;74:1004–1007. doi: 10.1128/jvi.74.2.1004-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw JL, Diamandis EP. Regulation of human tissue kallikrein-related peptidase expression by steroid hormones in 32 cell lines. Biol Chem. 2008;389:1409–1419. doi: 10.1515/bc.2008.158. [DOI] [PubMed] [Google Scholar]
- 50.Shaw JL, Diamandis EP. A potential role for tissue kallikrein-related peptidases in human cervico-vaginal physiology. Biol Chem. 2008;389:681–688. doi: 10.1515/BC.2008.069. [DOI] [PubMed] [Google Scholar]
- 51.Steinstraesser L, Tippler B, Mertens J, Lamme E, Homann HH, Lehnhardt M, Wildner O, Steinau HU, Uberla K. Inhibition of early steps in the lentiviral replication cycle by cathelicidin host defense peptides. Retrovirology. 2005;2:2. doi: 10.1186/1742-4690-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 53.Burgener A, Boutilier J, Wachihi C, Kimani J, Carpenter M, Westmacott G, Cheng K, Ball TB, Plummer F. Identification of differentially expressed proteins in the cervical mucosa of HIV-1-resistant sex workers. J Proteome Res. 2008;7:4446–4454. doi: 10.1021/pr800406r. [DOI] [PubMed] [Google Scholar]
- 54.Moriuchi H, Moriuchi M, Fauci AS. Cathepsin G, a neutrophil-derived serine protease, increases susceptibility of macrophages to acute human immunodeficiency virus type 1 infection. J Virol. 2000;74:6849–6855. doi: 10.1128/jvi.74.15.6849-6855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaw JL, Smith CR, Diamandis EP. Proteomic analysis of human cervico-vaginal fluid. J Proteome Res. 2007;6:2859–2865. doi: 10.1021/pr0701658. [DOI] [PubMed] [Google Scholar]
- 56.Singh PK, Tack BF, McCray PB, Jr, Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol Lung Cell Mol Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 57.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turpin JA, Schaeffer CA, Bu M, Graham L, Buckheit RW, Jr, Clanton D, Rice WG. Human immunodeficiency virus type-1 (HIV-1) replication is unaffected by human secretory leukocyte protease inhibitor. Antiviral Res. 1996;29:269–277. doi: 10.1016/0166-3542(95)00907-8. [DOI] [PubMed] [Google Scholar]
- 59.Konopka K, Shine N, Pretzer E, Duzgunes N. Secretory leukocyte protease inhibitor (SLPI): oxidation of SLPI does not explain its variable anti-HIV activity. J Dent Res. 1999;78:1773–1776. doi: 10.1177/00220345990780120201. [DOI] [PubMed] [Google Scholar]
- 60.Shine NR, Wang SC, Konopka K, Burks EA, Duzgunes N, Whitman CP. Secretory leukocyte protease inhibitor: inhibition of human immunodeficiency virus-1 infection of monocytic THP-1 cells by a newly cloned protein. Bioorg Chem. 2002;30:249–263. doi: 10.1016/s0045-2068(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 61.Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors alpha and beta in the human reproductive organs. J Clin Endocrinol Metab. 2000;85:4835–4840. doi: 10.1210/jcem.85.12.7029. [DOI] [PubMed] [Google Scholar]
- 62.Taylor A, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 63.Iwai T, Nanbu Y, Iwai M, Taii S, Fujii S, Mori T. Immunohistochemical localization of oestrogen receptors and progesterone receptors in the human ovary throughout the menstrual cycle. Virchows Arch A Pathol Anat Histopathol. 1990;417:369–375. doi: 10.1007/BF01606025. [DOI] [PubMed] [Google Scholar]
- 64.Maybin JA, Duncan WC. The human corpus luteum: which cells have progesterone receptors? Reproduction. 2004;128:423–431. doi: 10.1530/rep.1.00051. [DOI] [PubMed] [Google Scholar]
- 65.Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165:359–370. doi: 10.1677/joe.0.1650359. [DOI] [PubMed] [Google Scholar]
- 66.Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G, Nordenskjöld M, Gustafsson JA. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab. 1997;82:4258–4265. doi: 10.1210/jcem.82.12.4470. [DOI] [PubMed] [Google Scholar]
- 67.Ulziibat S, Ejima K, Shibata Y, Hishikawa Y, Kitajima M, Fujishita A, Ishimaru T, Koji T. Identification of estrogen receptor beta-positive intraepithelial lymphocytes and their possible roles in normal and tubal pregnancy oviducts. Hum Reprod. 2006;21:2281–2289. doi: 10.1093/humrep/del176. [DOI] [PubMed] [Google Scholar]
- 68.Cretoiu SM, Cretoiu D, Suciu L, Popescu LM. Interstitial Cajal-like cells of human Fallopian tube express estrogen and progesterone receptors. J Mol Hist. 2009;40:387–394. doi: 10.1007/s10735-009-9252-z. [DOI] [PubMed] [Google Scholar]
- 69.Jones RK, Bulmer JN, Searle RF. Immunohistochemical characterization of proliferation, oestrogen receptor and progesterone receptor expression in endometriosis: comparison of eutopic and ectopic endometrium with normal cycling endometrium. Hum Reprod. 1995;10:3272–3279. doi: 10.1093/oxfordjournals.humrep.a135901. [DOI] [PubMed] [Google Scholar]
- 70.Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. Presence of estrogen receptor beta in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab. 2001;86:1379–1386. doi: 10.1210/jcem.86.3.7322. [DOI] [PubMed] [Google Scholar]
- 71.Mylonas I, Jeschke U, Shabani N, Kuhn C, Balle A, Kriegel S, Kupka MS, Friese K. Immunohistochemical analysis of estrogen receptor alpha, estrogen receptor beta and progesterone receptor in normal human endometrium. Acta Histochem. 2004;106:245–252. doi: 10.1016/j.acthis.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Remoue F, Jacobs N, Miot V, Boniver J, Delvenne P. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol. 2003;189:1660–1665. doi: 10.1016/s0002-9378(03)00852-4. [DOI] [PubMed] [Google Scholar]
- 73.Fu X, Blaydes BS, Weis CC, Latendresse JR, Muskhelishvili L, Sutter TR, Delclos KB. Effects of dietary soy and estrous cycle on adrenal cytochrome p450 1B1 expression and DMBA metabolism in adrenal glands and livers in female Sprague–Dawley rats. Chem Biol Interact. 2003;146:273–284. doi: 10.1016/j.cbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Hodgins MB, Spike RC, Mackie RM, MacLean AB. An immunohistochemical study of androgen, oestrogen and progesterone receptors in the vulva and vagina. Br J Obstet Gynaecol. 1998;105:216–222. doi: 10.1111/j.1471-0528.1998.tb10056.x. [DOI] [PubMed] [Google Scholar]
- 75.Murphy AJ, Guyre PM, Wira CR, Pioli PA. Estradiol regulates expression of estrogen receptor ERalpha46 in human macrophages. PLoS ONE. 2009;4:e5539. doi: 10.1371/journal.pone.0005539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Schust DJ, Anderson DJ, Hill JA. Progesterone-induced immunosuppression is not mediated through the progesterone receptor. Hum Reprod. 1996;11:980–985. doi: 10.1093/oxfordjournals.humrep.a019335. [DOI] [PubMed] [Google Scholar]
- 78.Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Matsuyama T, Ishimaru T. Estrogen and progesterone receptor expression in macrophages and regulation of hepatocyte growth factor by ovarian steroids in women with endometriosis. Hum Reprod. 2005;20:2004–2013. doi: 10.1093/humrep/deh897. [DOI] [PubMed] [Google Scholar]
- 79.Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–2882. [PubMed] [Google Scholar]
- 80.Sapino A, Cassoni P, Ferrero E, Bongiovanni M, Righi L, Fortunati N, Crafa P, Chiarle R, Bussolati G. Estrogen receptor alpha is a novel marker expressed by follicular dendritic cells in lymph nodes and tumor-associated lymphoid infiltrates. Am J Pathol. 2003;163:1313–1320. doi: 10.1016/s0002-9440(10)63490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aerts J, Wetzels Y, Cohen N, Aerssens J. Data mining of public SNP databases for the selection of intragenic SNPs. Hum Mutat. 2002;20:162–173. doi: 10.1002/humu.10107. [DOI] [PubMed] [Google Scholar]
- 82.Molero L, García-Durán M, Diaz-Recasens J, Rico L, Casado S, López-Farré A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: regulation by estrogen. Cardiovasc Res. 2002;56:43–51. doi: 10.1016/s0008-6363(02)00505-9. [DOI] [PubMed] [Google Scholar]
- 83.Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, Fainboim L. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol. 2008;180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- 84.Henderson TA, Saunders PTK, Moffett-King A, Groome NP, Critchley HOD. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88:440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 85.Szekeres-Bartho J, Szekeres G, Debre P, Autran B, Chaouat G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol. 1990;125:273–283. doi: 10.1016/0008-8749(90)90083-4. [DOI] [PubMed] [Google Scholar]