Abstract
BACKGROUND
Respiratory failure after acute spinal cord injury (SCI) is well recognized, but data defining which patients need long-term ventilator support, and criteria for weaning and extubation are lacking. We hypothesized that many patients with SCI, even those with cervical SCI, can be successfully managed without long-term mechanical ventilation and its associated morbidity.
METHODS
Under the auspices of the Western Trauma Association Multi-Center Trials Group, a retrospective study of patients with SCI at 14 major trauma centers was conducted. Comprehensive injury, demographic, and outcome data on patients with acute SCI was compiled. The primary outcome variable was the need for mechanical ventilation at discharge. Secondary outcomes included the use of tracheostomy, and development of acute lung injury (ALI) and ventilator-associated pneumonia (VAP).
RESULTS
360 patients had SCI requiring mechanical ventilation. Sixteen patients were excluded for death within the first 2 days of hospitalization. Of the 344 patients included, 222 (64.5%) had cervical SCI. Notably, 62.6% of patients with cervical SCI were ventilator-free by discharge. 149 patients (43.3%) underwent tracheostomy and 53.7% of them were successfully weaned from the ventilator, compared to an 85.6% success rate among those with no tracheostomy (p<0.05). Patients who underwent tracheostomy had significantly higher rates of VAP (61.1% vs 20.5%, p<0.05) and ALI (12.8% vs 3.6%, p<0.05), and fewer ventilator free days (1 vs. 24 p<0.05). When controlled for injury severity, thoracic injury, and respiratory comorbidities, tracheostomy after cervical SCI was an independent predictor of ventilator dependence with an associated 14-fold higher likelihood of prolonged mechanical ventilation (OR 14.1, CI 2.78–71.67, p<0.05).
DISCUSSION
While many patients with SCI require short-term mechanical ventilation, the majority can be successfully weaned prior to discharge. In patients with SCI tracheostomy is associated with major morbidity and its use, especially among patients with cervical SCI, deserves further study.
LEVEL OF EVIDENCE
Level III, care-management/prognostic
Keywords: spinal cord injury, mechanical ventilation, tracheostomy
BACKGROUND
Traumatic spinal cord injury (SCI) represents an injury with devastating sequelae and limited treatment options, affecting 12,000 new patients every year in the United States; less than 1% of patients have complete recovery at hospital discharge(1). Given dismal prospects for complete functional recovery, the treatment of SCI is focused on preventing secondary injury and maximizing residual function. In keeping with this principle, airway and pulmonary management are of paramount importance in preventing morbidity, minimizing mortality, and promoting meaningful recovery. Multiple studies have addressed respiratory challenges related to SCI, which constitute the most common cause of morbidity and mortality (2–6) and the highest acute costs (2, 4, 7, 8) related to these injuries.
A well-established respiratory rehabilitation profession has evolved to facilitate long-term recovery from SCI. However, little data exists to guide acute ventilator management during the index hospitalization, and whether extubation of these patients can or should be attempted during initial hospitalization remains unknown. This has led to widespread variability in approach to and timing of tracheostomy placement, long-term acute care utilization, and overall outcomes in this patient demographic. Our group has previously published a single-center study reporting that 74% of patients who survive to discharge after SCI tolerated extubation and did not require tracheostomy. Specifically, over half of patients with cervical SCI were successfully extubated, leading to shorter ICU and hospital stays and a decreased incidence of ventilator-associated pneumonia (VAP) (9). These preliminary findings highlight the possibility that many patients with cervical SCI may not require long-term mechanical ventilation (MV), and may benefit from aggressive weaning and extubation. While intriguing, the small sample size and single-center nature of that study prompted skepticism from the trauma and critical-care community, given the prevalent, but not necessarily evidence-based teaching that most patients with cervical SCI should undergo tracheostomy during the index hospitalization. Inspired by these data, we hypothesized that many patients with SCI, even those with cervical SCI can be successfully managed without long-term MV and its associated morbidity. To test this, we performed a multicenter cohort study to examine the predictors of ventilator dependence at discharge in patients with acute SCI.
METHODS
The Western Trauma Association Multi-Center Trials Group performed a retrospective cohort study of patients with SCI requiring MV at 14 United States trauma centers. Institutional review board approval was obtained from the University of California San Francisco and at all contributing sites. SCI was defined as radiologically-confirmed injury to the cervical, thoracic, or lumbar spinal column, combined with clinical signs and symptoms consistent with SCI at that level. Comprehensive injury, demographic, clinical and outcome data on patients with acute SCI were compiled from medical records, ICU databases, and trauma registries. Ventilator and respiratory therapy data were collected for patients at the time of intubation, extubation (if attempted), and tracheostomy (if performed). Failed extubation was defined as unplanned re-intubation or tracheostomy at any point after initial removal of the endotracheal tube. The primary outcome variable was the need for MV at discharge. Secondary outcomes included the use of tracheostomy, acute lung injury (ALI), and VAP based on consensus definitions (10–13).
All data are presented as mean +/− standard deviation, median (inter-quartile range), or percentage. Percentages are calculated out of subgroup total (N) for each subgroup analysis. Univariate comparisons were made using Student’s t-test for normally distributed data, Wilcoxon rank-sum testing for skewed data, and Fisher’s exact test for proportions. For multiple group univariate comparisons, ANOVA was used for normally distributed and Kruskal-Wallis testing for skewed data. Multivariate comparisons were performed by logistic regression and adjusted for baseline demographics, injury severity, and respiratory comorbidities (smoking, asthma, chronic obstructive pulmonary disease, or other respiratory disease). Missing predictor data was multiply imputed using standard multivariate normal (for continuous) and logistic (for binary data) methods. For reporting purposes, missing outcome data was presumed negative to reflect the minimum possible incidence. An alpha of 0.05 was considered significant. All data was analyzed using Stata version 12 (StatCorp LP; College Station, TX).
RESULTS
360 patients with SCI requiring intubation and MV at 14 trauma centers from 2005–2009 were evaluated. 16 patients who died within the first 2 days of hospitalization from non-respiratory causes were excluded. Of the 344 patients analyzed, the majority (80.5%) were male with a mean age of 43 years (range of 18–90 years), and severely injured with a mean injury severity score (ISS) of 32 +/− 16. Blunt mechanism of injury accounted for the preponderance of SCI (85.8%). The level of SCI was primarily cervical (64.5%, 222 patients, Table 1a). Although 72 patients (20.9%) had a concomitant traumatic brain injury (TBI) diagnosis, this did not differ by level of SCI (Table 1b; p=0.17). Over half of patients had additional injuries (60.8%); this differed by level of cord injury, with the highest percentage of additional injuries occurring in patients with thoracic SCI (68.9%, p<0.05, Table 1b).
TABLE 1a.
Patient Demographics/Outcomes
| N=344 | |
|---|---|
| Age | 43 (18–90) |
| Male | 277 (80.5%) |
| Blunt mechanism | 295 (85.8%) |
| Mean injury severity score | 32 +/− 16 |
| Mean AIS-head | 3 +/− 2 |
| Mean AIS-chest | 2 +/− 2 |
| Median arrival Glasgow coma scale | 14 (8–15) |
| Cervical injury | 222 (64.5%) |
| Thoracic injury | 90 (26.2%) |
| Lumbar injury | 32 (9.3%) |
| Complete injury | 172 (20.0%) |
| Traumatic brain injury | 72 (20.9%) |
| Other injury | 209 (60.8%) |
| Operative stabilization | 229 (66.6%) |
| Ventilator associated pneumonia | 131 (38.1%) |
| Acute lung injury | 26 (7.6%) |
| Acute respiratory distress syndrome | 30 (8.7%) |
| ICU days | 12 (6–22) |
| Hospital days | 20 (11–32) |
| Ventilator-free days (to 28 days) | 15 (0–25) |
| Extubation attempted | 187 (54.4%) |
| Successfully extubated, never received tracheostomy | 168 (48.8%) |
| Tracheostomy | 149 (43.3%) |
| Not mechnically ventilated at discharge | 247 (71.8%) |
| Cause of death secondary to respiratory failure | 6 (1.7%) |
| Expired | 32 (9.3%) |
Patient demographics for the 344 mechanically ventilated, spinal cord injured patients. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days.
TABLE 1b.
Demographics by injury group: cervical, thoracic, lumbar
| Cervical (N=222) | Thoracic (N=90 ) | Lumbar (N=32) | p | |
|---|---|---|---|---|
| Age | 47 (18–90) | 38 (19–83) | 37 (18–83) | 0.0001 |
| Male | 175 (78.8%) | 76 (84.4%) | 26 (81.3%) | 0.5460 |
| Blunt mechanism | 204 (91.9%) | 64 (71.1%) | 27 (84.4%) | 0.0000 |
| Mean injury severity score | 31 +/− 17 | 36 +/− 13.0 | 36 +/− 16.0 | 0.0160 |
| Mean AIS-head | 4 +/− 2 | 2 +/− 2 | 1 +/− 2 | 0.0000 |
| Mean AIS-chest | 1 +/− 2 | 4 +/− 1 | 2 +/− 2 | 0.0000 |
| Median arrival Glasgow coma scale | 14 (6–15) | 14 (9–15) | 15 (14–15) | 0.0311 |
| Complete injury | 111 (50.0%) | 53 (58.9%) | 8 (25.0%) | 0.0050 |
| Traumatic brain injury | 47 (21.2%) | 20 (22.2%) | 5 (15.6%) | 0.1720 |
| Other injury | 137 (61.7%) | 62 (68.9%) | 10 (31.3%) | 0.0000 |
| Operative stabilization | 161 (72.5%) | 47 (52.2%) | 21 (65.6%) | 0.0070 |
| Ventilator associated pneumonia | 100 (45.1%) | 29 (32.2%) | 2 (6.3%) | 0.0000 |
| Acute lung injury | 17 (7.7%) | 7 (7.8%) | 2 (6.3%) | 0.1140 |
| Acute respiratory distress syndrome | 22 (9.9%) | 7 (7.8%) | 1 (3.1%) | 0.4990 |
| ICU days | 14 (7–24) | 9 (5–21) | 6 (3–8) | 0.0001 |
| Hospital days | 20 (12–32) | 18 (11–33) | 16 (9–27) | 0.3557 |
| Ventilator-free days (to 28 days) | 10 (0–23) | 21 (6–26) | 26 (19–26) | 0.0001 |
| Extubation attempted | 95 (42.8%) | 65 (72.2%) | 27 (84.4%) | 0.0000 |
| Successfully extubated | 80 (36.0%) | 60 (66.7%) | 28 (87.5%) | 0.0000 |
| Tracheostomy | 122 (55%) | 24 (26.7%) | 3 (9.4%) | 0.0000 |
| Not mechnically ventilated at discharge | 139 (62.6%) | 78 (86.7%) | 30 (93.8%) | 0.0000 |
| Cause of death secondary to respiratory failure | 5 (2.3%) | 0 (0.0%) | 1 (3.1%) | 0.4410 |
| Expired | 22 (9.9%) | 8 (8.9%) | 2 (6.3%) | 0.9180 |
Patient demographics, injury characteristics, ventilatory status at discharge or transfer, and outcomes for patients by level of spinal cord injury. Significance assessed by Kruskal-Wallis for continuous and Fisher’s exact test for dichotomous outcomes among groups. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days.
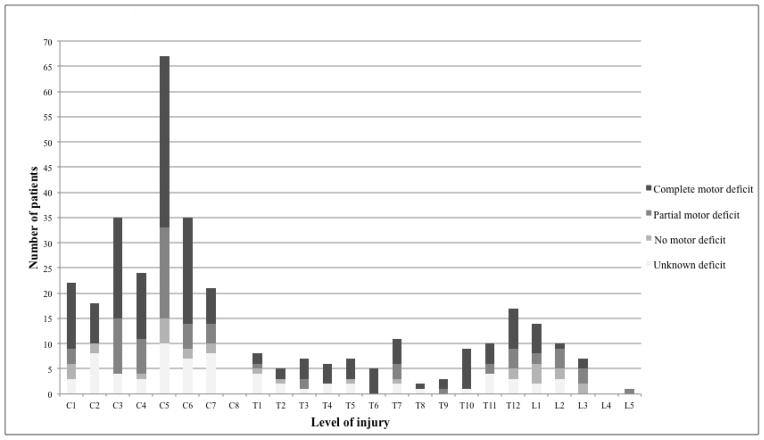
Of the 344 patients included, the majority (71.8%) did not require MV at the time of discharge. Although extubation was only attempted in half of patients overall, it was successful in the majority of attempts (89.8%, Table 1a). This was consistent even in the cervical SCI subpopulation, in which only 42.8% of patients underwent attempted extubation with 84.2% ultimately successful (Table 1b). In addition, extubation occurred early: the median time to extubation for cervical SCI patients was 2 days. The overall cohort had a high rate of VAP (38.1%), and patients with cervical SCI had significantly higher rates of VAP than those with thoracic or lumbar injuries (cervical 45.1%, thoracic 32.2%, lumbar 6.3%, p<0.05, Table 1b). The rates of ALI and ARDS did not differ significantly by injury level (Table 1b). Distribution of injuries by anatomical level and extent of motor deficit is shown in Figure 1.
Figure 1. Injuries by anatomical and functional level.
Number of patients with each level of spinal cord injury. Stacked bars represent extent of motor loss.
In addition, analysis of patients with cervical SCI grouped by high (C1–3) versus low (C4–7) level of injury demonstrated that the 75 patients (33.8%) with high cervical SCI were older, more severely injured, had a higher percentage of blunt injuries, and had lower GCS scores. However, they had a lower rate of VAP compared to those with low cervical SCI (41.3% vs. 46.9%, p<0.05). The patients with high cervical SCI underwent tracheostomy 57.3% of the time, compared to 53.7% of the time in low cervical SCI patients (p<0.05), a statistically but not clinically significant difference. Impressively, over half of the patients with high cervical SCI were off the ventilator at discharge (53.3%), and of the 34.7% that had extubation attempted, 88.4% were successfully extubated (Online supplement 1).
We next examined patients who underwent tracheostomy versus those who did not. Median time to tracheostomy was 7.5 days (IQR 5–11 days, Table 2a). Patients who underwent tracheostomy had less severe thoracic injury (mean AIS-chest score 2 vs. 3, p<0.05), but a higher incidence of TBI (23.5% vs. 19%, p<0.05; mean AIS-head score 4 vs. 3, p<0.05; median GCS 13 vs. 15, p<0.05; Table 2a). Patients who underwent tracheostomy had higher rates of VAP (61.1% vs. 20.5%, p<0.05) and ALI (12.8% vs. 3.6%, p<0.05, Table 2a), as well as significantly longer median ICU stays (22 vs. 6 days, p<0.05), hospital stay (30 vs. 13 days, p<0.05), and fewer ventilator-free days (1 vs. 24, p<0.05; Table 2a). Only 15.4% of tracheostomy patients had an extubation attempt, versus 84.1% of those patients who never underwent a tracheostomy (Table 2a, p<0.05). Tracheostomy was not associated with discontinuation of MV: a higher percentage of patients were on MV at discharge in the tracheostomy group compared to those who never underwent a tracheostomy (85.6% vs. 53.7%, p<0.05). No statistical difference in death due to respiratory complications (p=0.191) or overall mortality (p=0.091, Table 2a) was found. Analysis of patients who underwent early tracheostomy (<7days, 48%) versus late (>7days, 52%) found no major clinical differences in demographics. Those who underwent late tracheostomy had higher rates of VAP (70.5% vs. 59.7%, p<0.05), ALI (18.0% vs. 8.8%, p<0.05), and ARDS (16.4% vs. 10.5%, p<0.05). However, they had no difference in ventilator-free days. Only 10.5% of patients who underwent early tracheostomy ever had an extubation attempt. Early tracheostomy was not associated with freedom from MV at discharge, as only 52.6% of the patients who underwent early tracheostomy were off the ventilator by discharge compared to 45.9% in the late tracheostomy group, a statistically significant but clinically insignificant difference (Online supplement 2).
TABLE 2a.
Demographics/Outcomes for tracheostomy versus no tracheostomy
| Tracheostomy (N=149) | No Tracheostomy (N=195) | p | |
|---|---|---|---|
| Age | 43 (18–82) | 44 (18–90) | 0.9355 |
| Male | 122 (81.9%) | 155 (79.5%) | 0.6800 |
| Blunt mechanism | 129 (86.6%) | 166 (85.1%) | 0.7570 |
| Mean injury severity score | 34 +/− 17 | 31 +/− 15 | 0.0553 |
| Mean AIS-head | 4 +/− 2 | 3 +/− 2 | 0.0006 |
| Mean AIS-chest | 2 +/− 2 | 3 +/− 2 | 0.0297 |
| Median arrival Glasgow coma scale | 13 (3–15) | 15 (10–15) | 0.0029 |
| Cervical injury | 122 (81.9%) | 100 (51.3%) | |
| Thoracic injury | 24 (16.1%) | 66 (33.9%) | 0.0000 |
| Lumbar injury | 3 (2.0%) | 29 (14.9%) | |
| Complete injury | 93 (62.4%) | 79 (40.5%) | 0.0000 |
| Traumatic brain injury | 35 (23.5%) | 37 (19.0%) | 0.0220 |
| Other injury | 96 (64.4%) | 113 (58.0%) | 0.0150 |
| Operative stabilization | 101 (67.8%) | 128 (65.6%) | 0.2490 |
| Ventilator associated pneumonia | 91 (61.1%) | 40 (20.5%) | 0.0000 |
| Acute lung injury | 19 (12.8%) | 7 (3.6%) | 0.0020 |
| Acute respiratory distress syndrome | 18 (12.1%) | 12 (6.2%) | 0.0650 |
| ICU days | 22 (16–33) | 6 (4–11) | 0.0001 |
| Hospital days | 30 (20–41) | 13 (8–23) | 0.0001 |
| Ventilator-free days (to 28 days) | 1 (0–11) | 24 (15–26) | 0.0001 |
| Extubation attempted (prior to tracheostomy) | 23 (15.4%) | 164 (84.1%) | 0.0000 |
| Not mechanically ventilated at discharge | 80 (53.7%) | 167 (85.6%) | 0.0000 |
| Cause of death secondary to respiratory failure | 2 (1.3%) | 4 (2.1%) | 0.1910 |
| Expired | 9 (6.0%) | 23 (11.8%) | 0.0910 |
Patient demographics, injury characteristics, ventilatory status at discharge or transfer, and outcomes for patients by tracheostomy vs. no tracheostomy. Significance assessed by Kruskal-Wallis for continuous and Fisher’s exact test for dichotomous outcomes among groups. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days. For patients who received tracheostomy, median time to tracheostomy was 7.5 days (IQR 5–11 days).
Next, in the subset of patients who were free from MV at discharge, the role of tracheostomy was investigated. There were no demographic differences between those with and without tracheostomy (Table 2b); however, tracheostomy was associated with higher rates of VAP (48.8% vs. 19.2%, p<0.05), ALI (10% vs. 2.4%, p<0.05), and ARDS (12.5% vs. 3.6%, p<0.05), as well as longer median ICU stay (21 vs. 6 days, p<0.05), hospital stay (30 vs. 14 days, p<0.05), and fewer ventilator-free days (10 vs. 25 days, p<0.05; Table 2b).
TABLE 2b.
Demographics/Outcomes for patients with tracheostomy and off MV at discharge vs. no tracheostomy and off MV at discharge
| Tracheostomy & off MV at D/C (N=80) | No tracheostomy & off MV at D/C (N=167) | p | |
|---|---|---|---|
| Age | 40 (18–82) | 41 (18–83) | 0.7793 |
| Male | 66 (82.5%) | 130 (77.8%) | 0.5110 |
| Blunt mechanism | 67 (83.8%) | 140 (83.8%) | 0.2490 |
| Mean injury severity score | 32 +/− 16 | 30 +/− 13 | 0.1769 |
| Mean AIS-head | 3 +/− 2.0 | 2 +/− 2 | 0.0197 |
| Mean AIS-chest | 2 +/− 2 | 3 +/− 2 | 0.2652 |
| Median arrival Glasgow coma scale | 14 (3–15) | 15 (11–15) | 0.0069 |
| Cervical injury | 60 (75%) | 79 (47.3%) | |
| Thoracic injury | 18 (22.5%) | 60 (35.9%) | 0.0000 |
| Lumbar injury | 2 (2.5%) | 28 (16.8%) | |
| Complete spinal cord injury | 45 (56.3%) | 64 (38.3%) | 0.0000 |
| Traumatic brain injury | 15 (18.8%) | 29 (17.4%) | 0.0690 |
| Other injury | 51 (63.8%) | 93 (55.7%) | 0.0080 |
| Operative stabilization | 50 (62.5%) | 115 (68.9%) | 0.4920 |
| Ventilator Associated Pneumonia | 39 (48.8%) | 32 (19.2%) | 0.0000 |
| Acute lung Injury | 8 (10.0%) | 4 (2.4%) | 0.0000 |
| Acute respiratory distress syndrome | 10 (12.5%) | 6 (3.6%) | 0.0060 |
| ICU days | 21 (14–28) | 6 (4–11) | 0.0001 |
| Hospital days | 30 (22–41) | 14 (9–25) | 0.0001 |
| Ventilator-free days (to 28 days) | 10 (1–16) | 25 (21–26) | 0.0001 |
Significance assessed by Kruskal-Wallis for continuous and Fisher’s exact test for dichotomous outcomes among groups. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days.
As tracheostomy was not associated with improved respiratory outcomes, we then examined potential predictors of the need for MV at discharge. The 63 patients requiring ventilation at discharge had higher ISS (36 vs. 31, p<0.05) compared to the 247 ventilator-free patients; higher mean AIS head scores (4.0 vs. 3, p<0.05), but lower mean AIS chest scores (2 vs. 3, p<0.05, Table 3a). As expected, patients requiring MV at discharge had significantly higher rates of VAP (77.8% vs. 28.7%, p<0.05) and ALI (17.5% vs. 4.9%, p<0.05), and longer ICU (25 vs. 10 days, p<0.05) and hospital stays (28 vs. 19 days, p<0.05, Table 3a). Tracheostomy was performed in 93.7% of the patients still requiring ventilation on discharge, with initial extubation attempted in only 11.1% (Table 3a). No major differences in respiratory parameters at the time of intubation were identified for patients on and off MV at discharge (Online supplement 3). More importantly, we found no statistically significant differences in ventilatory parameters or arterial blood gas values at the time of extubation for patients that tolerated extubation versus those who failed (Table 3b). The vast majority of patients (93.7%) requiring MV at discharge had cervical SCI (Table 3a); yet, over half (62.6%) of the 222 patients who presented with cervical SCI were off MV at discharge (Table 1b and Figure 2).
TABLE 3a.
Demographics/Outcomes for patients off MV at discharge vs. on MV at discharge
| Off ventilation at D/C (N=247) | On ventilation at D/C (N=63) | p | |
|---|---|---|---|
| Age | 41 (18–83) | 45 (18–80) | 0.0744 |
| Male | 196 (79.4%) | 50 (79.4%) | 0.2630 |
| Blunt mechanism | 207 (83.8%) | 56 (88.9%) | 0.2080 |
| Mean injury severity score | 31 +/− 14 | 36 +/− 17 | 0.0129 |
| Mean AIS-head | 3 +/− 2 | 4 +/− 2 | 0.0000 |
| Mean AIS-chest | 3 +/− 2 | 2 +/− 2 | 0.0109 |
| Median arrival Glasgow coma scale | 15 (10–15) | 13 (8–15) | 0.1009 |
| Cervical injury | 139 (56.3%) | 59 (93.7%) | |
| Thoracic injury | 78 (31.6%) | 4 (6.4%) | 0.0000 |
| Lumbar injury | 30 (12.2%) | 0 (0.0%) | |
| Complete injury | 109 (44.1%) | 47 (74.6%) | 0.0000 |
| Traumatic brain injury | 44 (17.8%) | 19 (30.2%) | 0.0570 |
| Other injury | 144 (58.3%) | 39 (61.9%) | 0.1030 |
| Operative stabilization | 165 (66.8%) | 49 (77.8%) | 0.0040 |
| Ventilator associated pneumonia | 71 (28.7%) | 49 (77.8%) | 0.0000 |
| Acute lung injury | 12 (4.9%) | 11 (17.5%) | 0.0010 |
| Acute respiratory distress syndrome | 16 (6.5%) | 8 (12.7%) | 0.1300 |
| ICU days | 10 (5–18) | 25 (17–36) | 0.0001 |
| Hospital days | 19 (11–31) | 28 (18–40) | 0.0002 |
| Ventilator-free days (to 28 days) | 22 (11–26) | 0 (0-0) | 0.0001 |
| Extubation attempted | 177 (71.7%) | 7 (11.1%) | 0.0000 |
| Successfully extubated (never received tracheostomy) | 167 (67.6%) | 0 (0.0%) | 0.0000 |
| Tracheostomy | 80 (32.4%) | 59 (93.7%) | 0.0000 |
Patient demographics, injury characteristics, ventilatory status at discharge or transfer, and outcomes for patients on and off MV at discharge. Significance assessed by Kruskal-Wallis for continuous and Fisher’s exact test for dichotomous outcomes among groups. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days.
TABLE 3b.
Ventilatory parameters/arterial blood gas values at time of extubation for patients who failed extubation vs. tolerated extubation
| Failed Extubation (N=38) | Successful Extubation (N=142) | p | |
|---|---|---|---|
| PH at extubation | 7.41 +/− 0.05 | 7.42 +/− 0.04 | 0.0921 |
| Paco2 at extubation (mmHg) | 41 +/− 6 | 39 +/− 6 | 0.1485 |
| Pao2 at extubation (mmHg) | 114 +/− 53 | 130 +/− 65 | 0.1786 |
| Hco3 at extubation (mmol/L) | 25 +/− 4 | 25 +/− 4 | 0.9744 |
| Base deficit at extubation | 0.4 +/− 3.4 | 1.1 +/− 3.8 | 0.4557 |
| Glasgow coma scale at extubation | 10 (9–12) | 10 (10–11) | 0.4830 |
| Fio2 at extubation | 0.39 +/− 0.08 | 0.41 +/− 0.13 | 0.2984 |
| Respiratory rate at extubation (bpm) | 19 +/− 7 | 18 +/− 6 | 0.3547 |
| Ventilator rate at extubation (bpm) | 12 +/− 7 | 10 +/− 5 | 0.2734 |
| Tidal volume at extubation (mL) | 570 +/− 185 | 568 +/− 177 | 0.9580 |
| Minute volume at extubation (L/min) | 8.6 +/− 1.6 | 8.0 +/− 2.8 | 0.2306 |
| Peak pressure at extubation (cmH2O) | 22 +/− 16 | 19 +/− 8 | 0.4095 |
| Plateau pressure at extubation (cmH2O) | 16 +/− 7 | 17 +/−7 | 0.7241 |
| PEEP at extubation (cmH2O) | 5 +/− 1 | 5 +/− 1 | 0.2872 |
| Spo2 at extubation (%) | 99 (97–100) | 99 (98–100) | 0.0565 |
| Mean airway pressure at extubation (L/cmH2O) | 9 +/− 3 | 9 +/− 3 | 0.8398 |
| Compliance at extubation (L/cmH2O) | 0.055 +/− 0.012 | 0.049 +/− 0.018 | 0.2927 |
| P/F ratio at extubation | 338 +/− 224 | 314 +/− 116 | 0.6045 |
Ventilator parameters and arterial blood gas values at time of extubation for patients who tolerated vs. failed extubation. Significance assessed by Kruskal-Wallis for continuous outcomes among groups. Data are mean +/− SD for normally distributed data or median (inter-quartile range) for not-normally distributed data. Parameters at time of initial extubation attempt. 19 patients additionally had a 2nd extubation attempt (of which 4 failed and 15 were successfuly extubated)
Figure 2. Freedom from mechanical ventilation at hospital discharge by injury level.
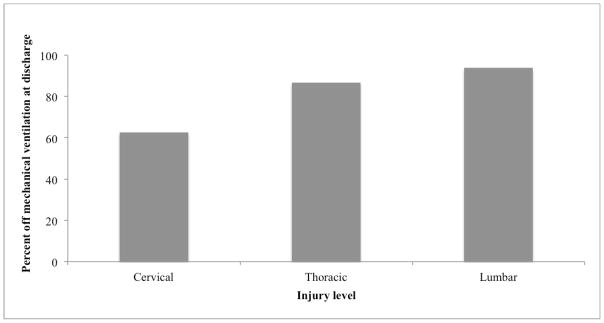
Percentage of patients who did not require mechanical ventilation on hospital discharge classified by level of spinal cord injury. *p < 0.05 by two-sided Fisher’s exact testing comparing the rate of ventilator independence on discharge between cervical vs. thoracic vs. lumbar injury.
Given the surprising incidence of freedom from MV in patients with cervical SCI, we then specifically analyzed this subgroup. Although similar in age, gender, and mechanism of injury, patients with cervical SCI who required MV at discharge had higher mean ISS (36 vs. 27, p<0.05) and higher rates of TBI (30.5% vs. 17.3%, p<0.05, Table 4a); no significant differences in severity of thoracic injury existed (Table 4a). Notably, only 11.9% of cervical SCI patients who still required MV at discharge ever had extubation attempted, compared to 61.2% of ventilator-free patients (p<0.05, Table 4a). Not surprisingly, ventilator-free cervical SCI patients had lower rates of VAP (31.0% vs. 81.4%, p<0.05) and ALI (5.0% vs. 15.3%, p<0.05), as well as shorter median ICU (12 vs. 27 days, p<0.05) and hospital stays (19 vs. 29 days, p<0.05) and more ventilator-free days (20 vs. 0 days, p<0.05; Table 4a).
TABLE 4a.
Demographics/Outcomes for patients with cervical SCI, off MV at discharge vs. on MV at discharge
| Off ventilation at D/C (N=138) | On ventilation at D/C (N=59) | p | |
|---|---|---|---|
| Age | 44 (18–82) | 44 (18–80) | 0.9584 |
| Male | 107 (77.0%) | 46 (78.0%) | 0.2820 |
| Blunt mechanism | 127 (91.4%) | 53 (89.8%) | 0.3430 |
| Mean injury severity score | 27 +/− 14 | 36 +/−17 | 0.0002 |
| Mean AIS-head | 4 +/− 2 | 4 +/− 2 | 0.0356 |
| Mean AIS-chest | 1 +/− 2 | 2 +/− 2 | 0.5217 |
| Median arrival Glasgow coma scale | 14 (6–15) | 13 (8–15) | 0.9085 |
| Complete injury | 55 (39.6%) | 44 (74.6%) | 0.0000 |
| Traumatic brain injury | 24 (17.3%) | 18 (30.5%) | 0.0410 |
| Other injury | 86 (61.9%) | 35 (59.3%) | 0.9730 |
| Operative stabilization | 103 (74.1%) | 46 (78.0%) | 0.0220 |
| Ventilator associated pneumonia | 43 (31.0%) | 48 (81.4%) | 0.0000 |
| Acute lung injury | 7 (5.0%) | 9 (15.3%) | 0.0060 |
| Acute respiratory distress syndrome | 12 (8.6%) | 7 (11.9%) | 0.7770 |
| ICU days | 12 (6–19) | 27 (17–37) | 0.0001 |
| Hospital days | 19 (11–30) | 29 (18–41) | 0.0004 |
| Ventilator-free days (to 28 days) | 20 (11–25) | 0 (0-0) | 0.0001 |
| Extubation attempted | 85 (61.2%) | 7 (11.9%) | 0.0000 |
| Tracheostomy | 60 (43.2%) | 55 (93.2%) | 0.0000 |
Patient demographics, injury characteristics, ventilatory status at discharge or transfer, and outcomes for cervical SCI patients on and off MV at discharge. Significance assessed by Kruskal-Wallis for continuous and Fisher’s exact test for dichotomous outcomes among groups. Data are mean +/− SD, median (inter-quartile range), or n (%) as indicated. For not-normally distributed variables reported as median with inter-quartile ranges. Ventilator-free days are counted to 28 days.
TABLE 4b.
Predictors of ventilator dependence for patients with cervical spinal cord injuries
| OR | CI | p | OR adjust | CI | p | |
|---|---|---|---|---|---|---|
| Age | 1.00 | 0.99–1.01 | 0.938 | 1.00 | 0.99–1.01 | 0.935 |
| Gender | 1.05 | 0.44–2.57 | 0.901 | 0.95 | 0.49–1.86 | 0.885 |
| Blunt mechanism | 1.19 | 0.38–3.74 | 0.756 | 0.55 | 0.09–3.34 | 0.516 |
| Body mass index | 1.00 | 0.96–1.05 | 0.883 | 1.01 | 0.97–1.05 | 0.725 |
| Injury severity score | 1.04 | 1.02–1.05 | 0.000 | 1.05 | 1.03–1.08 | <0.001 |
| AIS head | 1.29 | 0.65–2.52 | 0.465 | 1.10 | 0.62–1.93 | 0.746 |
| AIS chest | 1.06 | 0.86–1.31 | 0.568 | 0.85 | 0.67–1.06 | 0.154 |
| Glasgow coma scale on arrival | 0.99 | 0.91–1.07 | 0.756 | 0.99 | 0.92–1.05 | 0.686 |
| TBI | 1.96 | 0.83–4.60 | 0.124 | 1.57 | 0.64–3.82 | 0.323 |
| Other injury | 0.98 | 0.42–2.28 | 0.957 | 0.66 | 0.15–2.83 | 0.578 |
| Operative stabilization | 1.34 | 0.67–2.69 | 0.412 | 1.60 | 0.78–3.28 | 0.196 |
| Respiratory comorbidities | 1.14 | 0.56–2.33 | 0.714 | 1.10 | 0.29–4.19 | 0.886 |
| Tracheostomy | 18.10 | 5.53–59.22 | 0.000 | 14.11 | 2.78–71.67 | 0.001 |
| PH at intubation | 0.44 | 0.01–15.10 | 0.651 | 0.92 | 0.01–67.72 | 0.970 |
| Paco2 at intubation | 0.99 | 0.96–1.02 | 0.495 | 0.99 | 0.94–1.04 | 0.715 |
| Pao2 at intubation | 1.00 | 1.00-1.00 | 0.917 | 1.00 | 1.00-1.00 | 0.633 |
| Hco3 at intubation | 0.95 | 0.85–1.07 | 0.414 | 0.99 | 0.85–1.15 | 0.892 |
| Base deficit at intubation | 0.97 | 0.89–1.05 | 0.458 | 0.99 | 0.89–1.11 | 0.911 |
| Glasgow coma scale at intubation | 1.03 | 0.96–1.10 | 0.477 | 1.07 | 0.98–1.15 | 0.113 |
| Fio2 at intubation | 2.64 | 0.93–7.5 | 0.068 | 1.15 | 0.23–5.78 | 0.867 |
| Respiratory rate at intubation | 1.04 | 0.98–1.11 | 0.186 | 1.05 | 0.96–1.15 | 0.322 |
| Ventilator rate at intubation | 1.02 | 0.91–1.14 | 0.706 | 1.03 | 0.84–1.25 | 0.809 |
| Tidal volume at intubation | 1.00 | 1.00-1.00 | 0.082 | 1.00 | 1.00-1.00 | 0.440 |
| Minute volume at intubation | 0.99 | 0.88–1.12 | 0.921 | 0.82 | 0.69–0.97 | 0.022 |
| Peak pressure at intubation | 1.02 | 0.95–1.10 | 0.514 | 1.03 | 0.97–1.09 | 0.291 |
| Plateau pressure at intubation | 1.07 | 0.95–1.20 | 0.298 | 1.09 | 0.96–1.24 | 0.188 |
| PEEP at intubation | 0.87 | 0.70–1.07 | 0.188 | 0.83 | 0.57–1.21 | 0.336 |
| Spo2 at intubation | 1.06 | 0.96–1.18 | 0.225 | 1.06 | 0.97–1.16 | 0.198 |
| Mean airway pressure at intubation | 0.99 | 0.95–1.03 | 0.535 | 0.89 | 0.69–1.14 | 0.366 |
| Compliance at intubation | 1.34 | 0.64–2.82 | 0.436 | 1.58 | 1.12–2.46 | 0.045 |
| P/F ratio at intubation | 1.00 | 1.00-1.00 | 0.462 | 1.00 | 1.00-1.00 | 0.966 |
OR adjust= odds ratio adjusted for age, arrival GCS, injury severity score, AIS-chest score, respiratory comorbidities
OR and OR adjust for compiance for a change of 0.1Liter/cmH20
The cervical SCI cohort was then analyzed for independent predictors of ventilator dependence using logistic regression analysis. In both unadjusted and adjusted analysis, the most notable predictor of ventilator dependence was the presence of tracheostomy. When controlled for injury severity, thoracic injury, and respiratory comorbidities (smoking, asthma, chronic obstruction pulmonary disease, or any other respiratory disease), we found tracheostomy to be associated with a 14.1-fold higher odds of prolonged MV (OR 14.1, CI 2.78–71.67, p<0.05, Table 4b).
DISCUSSION
Here we show that a high number of patients with cervical SCI and respiratory failure can successfully be weaned and extubated without empiric tracheostomy, and describe the association of tracheostomy with increased respiratory morbidity. These findings have important implications for improving the outcome of cervical SCI patients. In 2012, up to 370,000 people in the United States were estimated to be living with SCI, and the lifetime costs of cervical tetraplegia are estimated at nearly $2.5 million (1). SCI carries significant morbidity, mortality, and hospital costs associated with short and long-term ventilator dependence (2, 4, 8, 14); as such, careful airway management and avoidance of pulmonary complications are central focuses of appropriate management (2, 3).
Although chronic respiratory care, ventilator management, and respiratory rehabilitation for patients with SCI have been broadly explored in the literature, successful acute weaning and extubation strategies have not been elucidated (2–6, 8, 14). Two small studies have examined weaning and extubation attempts in this setting. Chiodo et al. demonstrated in a small series of 26 tetraplegic patients that negative inspiration force diaphragm needle electromyography was the best predictor of the ability of patients with SCI to wean from the ventilator; however this small study did not examine standard clinical predictors (15). Claxton et al. demonstrated in another small series of 72 patients that copious sputum production and pneumonia were independent predictors of the need for MV after SCI, but they did not examine predictors of ventilator weaning or extubation (7). Combined with the pervasive clinical teaching that cervical SCI will always result in ventilatory paralysis, many trauma centers do not consider weaning or extubation prior to tracheostomy in patients with cervical SCI. Contrary to this belief, we previously demonstrated in a small single-center study that the majority of patients with SCI who survived to discharge tolerated extubation, and never required tracheostomy (9). We therefore hypothesized that many patients with cervical SCI can be successfully managed without long-term MV and its associated morbidity, and sought to identify predictors of ventilator dependence in this population.
Our data here confirm the previous result that many patients with SCI and respiratory failure can successfully extubated during their acute hospitalization (9). Despite the prevalence of cervical SCI in this cohort, the majority of patients were free from MV at discharge. Specifically, extubation attempts were successful 84.2% of the time in cervical SCI patients. Despite this impressive successful extubation rate, there remains a pervasive clinical bias toward avoiding attempts at extubation and proceeding directly to tracheostomy. Clinical intuition would seem to dictate that not all SCI patients are appropriate for attempts at extubation. However, our study did not demonstrate statistical differences in ventilator settings or arterial blood gas values at the time of extubation in those who were successfully extubated versus those who were not, highlighting the paucity of objective data available to support this clinical decision.
An area of respiratory management in SCI patients that has been well-studied is the use of tracheostomy. Several studies have examined tracheostomy in SCI with regards to potential benefits, timing, and resource utilization, and sought to identify predictors of the need for tracheostomy (16–21). Despite identifying that early tracheostomy led to shorter duration of MV and ICU stay, Romero et al. were unable to demonstrate that tracheostomy protected against VAP or decreased mortality rates (16). In contrast, we demonstrate here that tracheostomy was a significant independent predictor of ventilator dependence at discharge. Not surprisingly, over half of cervical SCI patients in this cohort underwent tracheostomy, remained ventilated at discharge, and had concerning rates of VAP. Of those patients, few had any documented weaning or extubation attempts prior to tracheostomy. In fact, 90% of patients who underwent an early tracheostomy never had an extubation attempt prior, and over half of them remained MV on discharge. Specifically in patients with cervical SCI, tracheostomy was the only notable predictor of ventilator dependence (OR 14.1, CI 2.78–71.67, p-value<0.05), while no demographics, ventilator parameters, or arterial blood gas measurements at the time of extubation were predictive of ventilator dependence. We interpret this result as an injunction to rethink the clinical paradigm of empiric tracheostomy after cervical SCI. We believe there are patients with SCI that do require tracheostomy, but that further study may identify evidence-based criteria for patient selection allowing a reduction in empiric tracheostomy and an associated decrease in morbidity and costs associated with SCI.
This study shares the limitations inherent to all retrospective and multi-center studies. We did not identify differences between successfully extubated SCI patients and those who were not, despite the fact that bedside gestalt often clearly identifies patients that are poor candidates for weaning; in a retrospective cohort, these unmeasured confounders seen by the experienced clinical eye cannot be accounted for. In fact, the clearly poor candidate for trial extubation is not who we are interested in; our overall aim is to identify patients whose clinical appearance and objective data are indeterminate, and elucidate clinical parameters to provide decision support for assessment in determining appropriateness of trial extubation versus empiric tracheostomy. The critical lack of weaning data from individual sites could be due to a clinical bias toward not performing any weaning attempts in patients with SCI prior to tracheostomy, or due to missing data. In addition, the timing of development of VAP in relation to tracheostomy and the clinician reason for tracheostomy are unknown. Although our data suggest that patients did not undergo tracheostomy based off of objective differences, the answer to this is unknown. These data are crucial to conclusively answering our objective and the serial standardized data collection of a prospective study would elucidate these.
In conclusion, while many patients with SCI require short-term MV, we demonstrate that the majority can be successfully weaned from ventilation prior to discharge without the use of empiric tracheostomy. This is of paramount importance, as we also identified that tracheostomy is associated with major morbidity in this population, especially among patients with cervical SCI. Tracheostomy and its use in cervical SCI patients deserves further study to identify the subset best served by this procedure. This data does not suggest that empiric tracheostomy should be abandoned in all patients with cervical SCI. We believe there are cervical SCI patients that do require early tracheostomy, but that there is need for a prospective trial to identify optimal clinical guidelines for weaning and extubation attempts in patients with SCI. Clinical equipoise is supported for such a trial by the high rates of successful extubation, the lack of clear differences between patients who succeed and fail, and the morbidity associated with tracheostomy shown here.
Supplementary Material
Acknowledgments
Funding: Supported by NIH GM-085689 (M.J.C), NIH T32 GM-08258-20 (M.E.K), and NIH T32 GM-008258-25 (L.Z.K).
Footnotes
AUTHOR CONTRIBUTIONS:
Literature search: LZK, MEK, MJC
Study design: LZK, MEK, MJC, BJR
Data collection: LZK, MEK, RAC, BJR, CKH, THC, CCB, MLS, CCB, KLK, MAD, JMH, CHK, SJZ, SDG, DVS, DBP, MJC
Data analysis: LZK, MEK, RAC, BJR, MJC
Data interpretation: LZK, MEK, RAC, MJC
Manuscript writing: LZK, MEK, RAC, BJR, MJC
Critical revision: LZK, MEK, RAC, BJR, CKH, THC, CCB, MLS, CCB, KLK, MAD, JMH, CHK, SJZ, SDG, DVS, DBP, MJC
Conflicts of interest: None
Meetings: Podium presentation: Western Trauma Association Meeting March 2013
Members of the Western Trauma Association Study Group
Data Coordinating Center, University of California, San Francisco: Mitchell J. Cohen, MD; Lucy Z. Kornblith, MD; Matthew E. Kutcher, MD; Rachael A. Callcut, MD: Britt J. Redick, BA; Mary Nelson, RN, MPA; M. Margaret Knudson, MD; Ryan C. McCreery, BS; Molly D. Greenberg, BS; Leslie M. Cachola, BA; Kimen S. Balhotra, BA; Amanda S. Conroy, BA; Jeremy M. Crane, BS; Melissa R. Forde, BS. Scottsdale Healthcare Osborn Medical Center: Charles K. Hu, MD; Veronica Back, MSN. Gundersen Lutheran Medical Foundation: Thomas H. Cogbill, MD; Christina M. Reichgelt, BA. Virginia Tech Carilion School of Medicine: Christopher C. Baker, MD; Amanda H. VanLaeken, MS. Duke University Medical Center: Mark L. Shapiro, MD; Carlos A. Bagley, MD. Denver Health Medical Center: Clay C. Burlew, MD; Ernest E. Moore, MD. Community Regional Medical Center/University of California San Francisco, Fresno: Krista L. Kaups, MD; Christopher R. Smith, MD. Massachusetts General Hospital and Harvard Medical School: Marc A. deMoya, MD; Gwendolyn M. van der Wilden, MSc. Via Christi Regional Medical Center: James M. Haan, MD; Jeanette Ward, BA. New York University Langone Medical Center: Christopher H. Koontz, MD; Samual R. Todd, MD. University of Pittsburgh Medical Center: Samuel J. Zolin, BS; Jason L. Sperry, MD. Oregon Health Sciences University: Stephanie D. Gordy, MD; Loic J. Fabricant, MD. University of California, Davis Medical Center: David V. Shatz, MD.
Grant Medical Center: Doug B. Paul, DO; Forrest O. Moore, MD.
Contributor Information
LZ Kornblith, Email: lucy.kornblith@ucsfmedctr.org.
ME Kutcher, Email: matthew.kutcher@ucsfmedctr.org.
RA Callcut, Email: callcutr@sfghsurg.ucsf.edu.
BJ Redick, Email: redickb@sfghsurg.ucsf.edu.
CK Hu, Email: chu@shc.org.
TH Cogbill, Email: thcogbil@gundluth.org.
CC Baker, Email: ccbaker@carilionclinic.org.
ML Shapiro, Email: ml.shapiro@duke.edu.
CC Burlew, Email: clay.cothren@dhha.org.
KL Kaups, Email: kkaupsmd@communitymedical.org.
MA DeMoya, Email: mdemoya@partners.org.
JM Haan, Email: james_haan@via-christi.org.
CH Koontz, Email: christopher.koontz@nyumc.org.
SJ Zolin, Email: zolins@upmc.edu.
SD Gordy, Email: gordys@ohsu.edu.
DV Shatz, Email: dvshatz@ucdavis.edu.
DB Paul, Email: dpaul2@ohiohealth.com.
MJ Cohen, Email: mcohen@sfghsurg.ucsf.edu.
References
- 1.Spinal cord injury facts and figures at a glance. The journal of spinal cord medicine. 2012;35(4):197–8. doi: 10.1179/1079026812Z.00000000063. Epub 2012/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. The journal of spinal cord medicine. 2007;30(4):319–30. doi: 10.1080/10790268.2007.11753947. Epub 2007/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidl RO, Wolf D, Nusser-Muller-Busch R, Niedeggen A. Airway management in acute tetraplegics: a retrospective study. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2010;19(7):1073–8. doi: 10.1007/s00586-010-1328-7. Epub 2010/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Archives of physical medicine and rehabilitation. 1994;75(3):270–5. doi: 10.1016/0003-9993(94)90027-2. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 5.Hassid VJ, Schinco MA, Tepas JJ, Griffen MM, Murphy TL, Frykberg ER, Kerwin AJ. Definitive establishment of airway control is critical for optimal outcome in lower cervical spinal cord injury. The Journal of trauma. 2008;65(6):1328–32. doi: 10.1097/TA.0b013e31818d07e4. Epub 2008/12/17. [DOI] [PubMed] [Google Scholar]
- 6.Berney S, Bragge P, Granger C, Opdam H, Denehy L. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: a systematic review. Spinal cord. 2011;49(1):17–29. doi: 10.1038/sc.2010.39. Epub 2010/04/21. [DOI] [PubMed] [Google Scholar]
- 7.Claxton AR, Wong DT, Chung F, Fehlings MG. Predictors of hospital mortality and MV in patients with cervical spinal cord injury. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 1998;45(2):144–9. doi: 10.1007/BF03013253. Epub 1998/03/26. [DOI] [PubMed] [Google Scholar]
- 8.Winslow C, Bode RK, Felton D, Chen D, Meyer PR., Jr Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest. 2002;121(5):1548–54. doi: 10.1378/chest.121.5.1548. Epub 2002/05/15. [DOI] [PubMed] [Google Scholar]
- 9.Call MS, Kutcher ME, Izenberg RA, Singh T, Cohen MJ. Spinal cord injury: outcomes of ventilatory weaning and extubation. The Journal of trauma. 2011;71(6):1673–9. doi: 10.1097/TA.0b013e31821e87c2. Epub 2011/07/20. [DOI] [PubMed] [Google Scholar]
- 10.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA: the journal of the American Medical Association. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. Epub 2012/07/17. [DOI] [PubMed] [Google Scholar]
- 11.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical-care medicine. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 12.Guideline for prevention of nosocomial pneumonia. Centers for Disease Control and Prevention. Respiratory care. 1994;39(12):1191–236. Epub 1994/12/01. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Guidelines for prevention of nosocomial pneumonia. MMWR Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 1997;46(RR-1):1–79. Epub 1997/01/03. [PubMed] [Google Scholar]
- 14.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respiratory care. 2006;51(8):853–68. discussion 69–70. Epub 2006/07/27. [PMC free article] [PubMed] [Google Scholar]
- 15.Chiodo AE, Scelza W, Forchheimer M. Predictors of ventilator weaning in individuals with high cervical spinal cord injury. The journal of spinal cord medicine. 2008;31(1):72–7. doi: 10.1080/10790268.2008.11753984. Epub 2008/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero J, Vari A, Gambarrutta C, Oliviero A. Tracheostomy timing in traumatic spinal cord injury. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2009;18(10):1452–7. doi: 10.1007/s00586-009-1097-3. Epub 2009/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanna B, Ayman HA, Soni A. Early tracheostomy in intensive care trauma patient improves resource utilization: a cohort study and literature review. Crit Care. 2005;9(4):414–6. doi: 10.1186/cc3043. author reply -6; discussion -6. Epub 2005/09/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrop JS, Sharan AD, Scheid EH, Jr, Vaccaro AR, Przybylski GJ. Tracheostomy placement in patients with complete cervical spinal cord injuries: American Spinal Injury Association Grade A. Journal of neurosurgery. 2004;100(1 Suppl Spine):20–3. doi: 10.3171/spi.2004.100.1.0020. Epub 2004/01/30. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243. doi: 10.1136/bmj.38467.485671.E0. Epub 2005/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Como JJ, Sutton ER, McCunn M, Dutton RP, Johnson SB, Aarabi B, Scalea TM. Characterizing the need for MV following cervical spinal cord injury with neurologic deficit. The Journal of trauma. 2005;59(4):912–6. doi: 10.1097/01.ta.0000187660.03742.a6. discussion 6. Epub 2005/12/24. [DOI] [PubMed] [Google Scholar]
- 21.Branco BC, Plurad D, Green DJ, Inaba K, Lam L, Cestero R, Bukur M, Demetriades D. Incidence and clinical predictors for tracheostomy after cervical spinal cord injury: a National Trauma Databank review. The Journal of trauma. 2011;70(1):111–5. doi: 10.1097/TA.0b013e3181d9a559. Epub 2010/06/08. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.