Abstract
Problem
Vaginal epithelial cells (VEC) are the first line of defense against incoming pathogens in the female reproductive tract. Their ability to produce the anti-HIV molecules elafin and HBD2 under hormonal stimulation is unknown.
Method of study
Vaginal epithelial cells were recovered using a menstrual cup and cultured overnight prior to treatment with estradiol (E2), progesterone (P4) or a panel of selective estrogen response modulators (SERMs). Conditioned media were recovered and analyzed for protein concentration and anti-HIV activity.
Results
E2 significantly decreased the secretion of HBD2 and elafin by VEC over 48 hrs, while P4 and the SERMs (tamoxifen, PHTTP, ICI or Y134) had no effect. VEC conditioned media from E2-treated cells had no anti-HIV activity, while that from E2/P4-treated cells significantly inhibited HIV-BaL infection.
Conclusion
The menstrual cup allows for effective recovery of primary VEC. Their production of HBD2 and elafin is sensitive to E2, suggesting that innate immune protection varies in the vagina across the menstrual cycle.
Keywords: Antimicrobials, epithelial cells, estradiol, human immunodeficiency virus, hormones, innate immunity, progesterone, selective estrogen response modulators, vagina
Introduction
The female reproductive tract (FRT) is a unique mucosal environment with two essential, if sometimes contradictory, functions – host defense and reproduction. It is the primary site for the transmission of several pathogens including Neisseria gonorrhoeae, Chlamydia trachomatis, herpes simplex virus 2 (HSV-2) and human immunodeficiency virus (HIV), which severely compromise reproductive health in women.1
Protecting the mucosal surface of the lower FRT (vagina and ectocervix) are multiple layers of non-keratinized squamous epithelial cells.2 The outermost layers of the vaginal epithelium are composed of superficial cells that are very loosely bound to each other, below which are multiple layers of intermediate and basal epithelial cells. Superficial and intermediate epithelial cells of the vaginal mucosa are likely to be the first cells exposed to incoming pathogens. Therefore, the factors that govern their protective ability may be crucial to preventing the sexual transmission of HIV and other pathogens.
Overlaying the vaginal epithelium is a complex fluid composed of vaginal transudate, vaginal epithelial secretions, bacterial products, cervical mucus and secretions, and secretions from the upper FRT.3 The overall contribution of vaginal epithelial cells (VEC) to the composition of this fluid is still unclear. Within the fluid is a potent cocktail of antimicrobials including human beta-defensin 2 (HBD2), elafin and CCL20/MIP3α, which reduce HIV infection of target cells in vitro.4–6 HBD2 is expressed by immortalized VEC and upregulated in response to Toll-like receptor 2 and 4 stimulation.7 It acts upon host target cells to decrease CXCR4 expression levels and modulate cell-signaling pathways.8–10 HBD2 also exerts its effects by binding to and destabilizing the HIV viral envelope.11 Elafin, a member of the whey acidic protein family, is an endogenous protease inhibitor implicated in controlling inflammatory responses and tissue remodeling that also possesses anti-HIV activity.12,13 The production of elafin by VEC has not been studied, although it is present in secretions from the lower FRT.14 However, secretory leukocyte protease inhibitor (SLPI), another whey acidic protein with 40% homology to elafin, is secreted by immortalized VEC.15 CCL20/MIP3α is a neutrophil, lymphocyte and dendritic cell chemoattractant that inhibits HIV infection by an unknown mechanism and is expressed by the SiHa vaginal cell line and primary human VEC in extended culture.16,17 However, the contribution of VEC to the antiviral activity of lower FRT secretions is not well understood.
Hormonal balance affects antimicrobial and cytokine secretion, and potentially innate immune protection in the FRT.18 We have shown that estradiol (E2) stimulation increases the secretion of HBD2 and SLPI by polarized human UEC.19 Intriguingly, several studies have shown variations in FRT antimicrobial content associated with menstrual cycle stage, strongly suggesting an element of hormonal control. 20,21 In contrast to the upper FRT, the constitutive antimicrobial and cytokine profile of primary human VEC and the effects of hormonal stimulation are relatively unknown.
In this study, we sought to determine whether freshly isolated human VEC constitutively produce HBD2 and elafin, and whether the sex hormones, E2 and progesterone (P4) modulate their secretion. We report that HBD2 and elafin are constitutively produced by VEC. Furthermore, E2 significantly suppressed the secretion of HBD2 and elafin, while P4 had no effect on protein levels of these factors. Intriguingly, despite the decrease in HBD2 and elafin, there was no effect of E2 on the ability of VEC conditioned media to inhibit HIV infection of a reporter cell line.
Methods and materials
Study Participants
Healthy, premenopausal women (n = 7) between the ages of 35–45 were recruited at Dartmouth-Hitchcock Medical Center (DHMC), Lebanon, New Hampshire. All volunteers were free of sexually transmitted infections and were not on any form of hormonal birth control or using an intrauterine device. All investigations involving human subjects were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from the Committee for the Protection of Human Subjects (CPHS) at DHMC, and with written informed consent obtained from the volunteers.
Recovery and Isolation of Vaginal Epithelial Cells
Volunteers were provided with an Instead Softcup™ (menstrual cup) (Evofem Inc., San Diego, CA, USA) to recover VEC at three points during their menstrual cycle: days 6–8, 13–15 and 21–23 after the start of menses (day 1) corresponding respectively to the proliferative, mid-cycle and secretory stages of the menstrual cycle. The menstrual cup was inserted into the vagina as per the manufacturer’s instructions (http://www.softcup.com/video-tutorials) for 1 hr. Upon removal at DHMC, the cup was placed in a 50-mL tube and immediately brought to the laboratory where it was centrifuged (800 g; 5 min) to recover vaginal fluid and cells. The menstrual cup was then removed, washed with 5–10 mL 1× phosphate-buffered saline (PBS) to recover any remaining cells still adhered to the cup and then discarded. Vaginal fluid in the tube was measured (weight and volume), diluted in 1× PBS (4°C) at a ratio of 1 mL PBS/1 g of vaginal fluid and centrifuged (800 g; 5 min). The resulting supernatant (vaginal fluid) was stored at −80°C. The pellet, which contained a large population of VEC, was resuspended in the PBS prior to filtration through a 40-μm nylon filter (Small Parts, Miami Lakes, FL, USA). Large sheets of VEC retained on the filter were recovered with DMEM/F12 (Gibco, Grand Island, NY, USA). The resulting filtrate was then passed through a 20-μm nylon filter, which captured single or small sheets of VEC. These were also recovered with DMEM/F12 and added to the cells recovered from the 40-μm filter.
Vaginal Epithelial Cell Culture
Freshly isolated VEC were plated at 1 × 105 cells per well in a 96-well culture plate (Fisher Scientific, Pittsburgh, PA, USA) in 0.3 mL of complete media: DMEM/F12 supplemented with 20 mM HEPES (Invitrogen, Carlsbad, CA, USA), 2 mM L-glutamine (Invitrogen), 50 mg/mL Primocin (Invivogen) and 10% charcoal–dextran-stripped heat-inactivated fetal bovine serum (FBS) (ThermoScientific, Logan, UT, USA) for 24 hrs prior to treatment.
Hormone/SERM Treatment
The sex hormones 17β-estradiol (E2) (Calbiochem, Gibbstown, NJ, USA), progesterone (P4) (Calbiochem) or the selective estrogen response modulators (SERM) tamoxifen, PHTTP, ICI and Y134 (all Calbiochem) were dissolved in 100% ethanol to an initial concentration of 1×10−3 M, of which 20 μL was subsequently evaporated to dryness in a scintillation vial and resuspended in 2 mL complete media containing charcoal–dextran-stripped FBS to a concentration of 1×10−5 M. Further dilutions were made to achieve working concentrations of hormone or SERM ranging from 1×10−7 to 1×10−9 M. As a control, 20 μL of 100% ethanol without dissolved hormone or SERM was initially evaporated to dryness and then resuspended in complete media.
Measurement of Cytokines and Antimicrobials
Cytokine and antimicrobial levels in cell culture conditioned media were measured by enzyme-linked immunosorbent assay (ELISA). Cell culture conditioned media were recovered and centrifuged (10,000 g; 5 min) to remove cellular debris. The resulting supernatant was recovered and stored at −80°C until required. The following ELISAs were performed according to the manufacturer’s instructions: CCL20 (R&D Systems, Minneapolis, MN, USA), Elafin (R&D) and HBD2 (PeproTech, Rocky Hill, NJ, USA). Protein concentration was quantified based on a standard curve after OD measurements at 450 nm on an ELISA reader (Dynex, Chantilly, VA, USA).
Measurement of Anti-HIV Activity
Two reference HIV-1 strains IIIB (X4) and BaL (R5) were kindly obtained from Dr. P. Gupta (Univ. of Pittsburgh, PA, USA). The TZM-bl indicator cell is a modified HeLa cell line that expresses high levels of the HIV receptor CD4 and co-receptors CCR5 and CXCR4. TZM-bl cells contain HIV long terminal repeat (LTR)-driven β-galactosidase and firefly luciferase reporter cassettes that are activated by HIV infection and subsequent Tat protein expression.22 For anti-HIV assays, TZM-bl cells were seeded at 2.5 × 104 cells per well in a 96-well plate and incubated at 37°C for 24 hrs. Vaginal epithelial conditioned media were diluted 1:8 in complete media and incubated with virus at a multiplicity of infection (MOI) of 0.1 or 1 for 1 hr at 37°C in a final volume of 100 μL. Following incubation, the combined virus and conditioned media (100 μL) samples were added to the TZM-bl cells for 48 hrs after which the cell culture supernatants were aspirated and the cells lysed with β-glo luciferase substrate solution (Promega, Madison, WI, USA). Controls included incubation of TZM-bl cells with virus alone, hormone alone, conditioned media alone, virus plus hormone and TZM-bl cells in complete media. Uninfected cells were used to determine background luminescence, and data were expressed in relative light units (RLU).
Cell Viability Assay
Vaginal epithelial cell viability was determined by trypan blue exclusion. Viability of TZM-bl cells was determined by the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega) as per the manufacturer’s instructions.
Statistics
ELISA data are presented as mean ± standard error. A one-way ANOVA with Tukey’s post-test or a t-test was calculated using GraphPad Prism (version 5.0, La Jolla, CA, USA) with a P value of <0.05 considered significantly different.
Results
Vaginal Epithelial Recovery
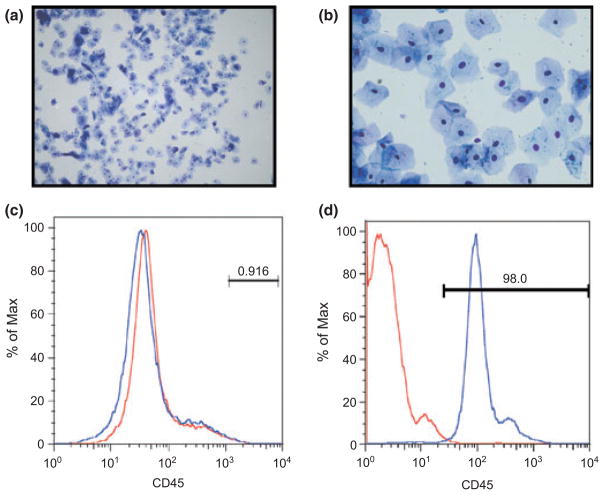
As part of our studies aimed at investigating hormonal control of innate immunity in the endometrium, we initially used the menstrual cup as an alternative to hysterectomy for recovering uterine tissues shed at menstruation as developed by Koks et al. (1997).23. Using this approach, we discovered that significant numbers of viable squamous VEC were being collected. We therefore began using the menstrual cup to recover VEC during the non-menstrual stages of the cycle. We chose three points in the cycle: proliferative (days 6–8), mid-cycle (days 13–15) and secretory (days 21–23). Day 1 was defined as the onset of menstrual bleeding. As seen in Fig. 1a,b, most of the recovered VEC were generally large (surface area), thin, polygonal in shape with high cytoplasmic/nuclear ratio characteristic of superficial VEC from the stratum corneum. However, smaller and more rounded intermediate VEC were also present in our preparations.
Fig. 1.
Morphology of vaginal epithelial cells (VEC). Cells were recovered from a menstrual cup after sequential filtration through a 40 and 20-μm nylon membrane. Cells were washed off the membranes, stained with Hema-3 and viewed under a light microscope at low (a) and high (b) magnification. Prior to cell culture, the freshly isolated VEC were analyzed by flow cytometry (c) for CD45 expression, an immune cell marker. As a control, freshly isolated peripheral blood mononuclear cells were also analyzed for CD45 expression (d). Figures shown are from a representative menstrual cup recovery.
To assess the purity of our VEC preparation, we analyzed CD45 expression by flow cytometry. CD45 is transmembrane tyrosine phosphatase present on all differentiated hematopoietic cells and is a commonly used marker for immune cells. We did not detect any CD45 staining in our final VEC preparations (Fig. 1c). However, to confirm the integrity of our anti-CD45 antibody, we isolated peripheral blood mononuclear cells (PBMC) and analyzed for CD45 expression. As seen in Fig. 1d, PMBC stained positive for CD45 using our antibody. On the rare occasion when immune cells were present in the final VEC preparation, it was discarded.
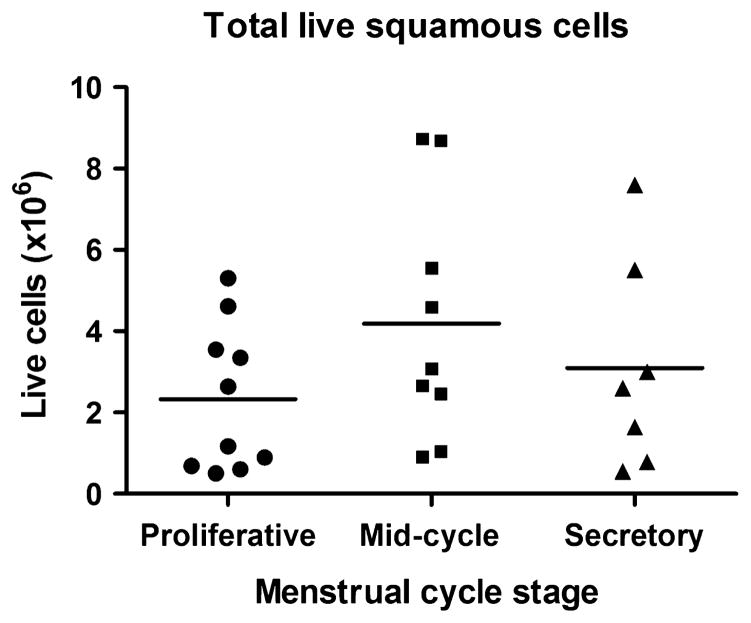
The total number and viability of purified VEC were determined by trypan blue dye exclusion. The number of live cells recovered varied between volunteers and ranged from 0.1 × 106 to 9 × 106 cells/collection (Fig. 2). Overall, there was a trend toward higher numbers of cells being recovered at mid-cycle compared to proliferative and secretory stages of the menstrual cycle. However, this difference was not significant. Approximately equal numbers of cells were recovered at the proliferative and secretory stages.
Fig. 2.
Recovery of vaginal epithelial cells. Cells were recovered from menstrual cups obtained during three stages of the menstrual cycle: proliferative (d6–8), mid-cycle (d13–15) and secretory (d21–23). Viability was determined by trypan blue exclusion. Each dot represents the number of live cells in individual menstrual cups.
Estradiol Treatment of VEC
Our past work shows that sex hormones can modulate the secretion of antimicrobials and cytokines by epithelial cells. In polarized UEC, E2 upregulates the constitutive secretion of HBD2 and elafin, but inhibits release of CCL20.19,24 As the effects of hormonal treatment on VEC secretion of antimicrobials and cytokines is relatively unknown, we recovered freshly isolated VEC, which were incubated overnight prior to treatment with E2 (5 × 10−8 M) for 48 hrs, after which cell culture conditioned media were recovered and analyzed for antimicrobial production by ELISA. We were unable to detect CCL20 under any conditions (not shown). E2 significantly inhibited the secretion of both HBD2 and elafin by VEC (Fig. 3). Recognizing that in secretory phase of the menstrual cycle both E2 and P4 are present at high concentrations, we treated VEC with P4 (1 × 10−7 M) or a combination of E2 (5 × 10−8 M) and P4 for 48 hrs. Unlike E2, P4 alone had no effect on the secretion of HBD2 or elafin. Interestingly, E2 and P4 co-treated cells had reduced secretion of HBD2 beyond that seen with E2 alone. In contrast, E2 and P4 had no effect on elafin secretion, when compared to E2 alone (Fig. 4).
Fig. 3.
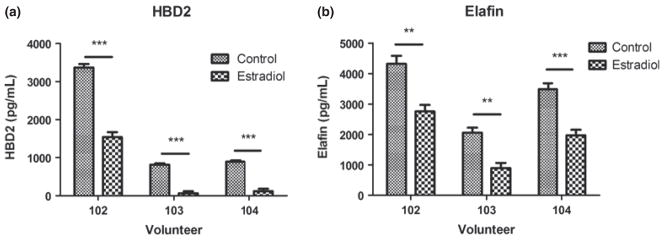
Estradiol inhibits HBD2 and elafin secretion. Freshly isolated vaginal epithelial cells from three different volunteers (102, 103 & 104) were incubated overnight in triplicate wells prior to treatment with estradiol (5×10−8 M) for 48 hrs. Conditioned media were recovered and analyzed by ELISA for the presence of HBD2 (a) and elafin (b). (n = 3). **P < 0.01, ***P < 0.001 with respect to control.
Fig. 4.
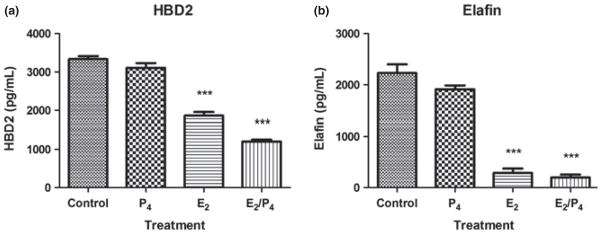
Progesterone has no effect on HBD2 and elafin secretion. Freshly isolated vaginal epithelial cells were incubated overnight in triplicate wells prior to treatment with P4 (1 × 10−7 M), E2 (5 × 10−8 M) or a combination of both for 48 hrs. Conditioned media were recovered and analyzed by ELISA for the presence of HBD2 (a) and elafin (b). Data shown are from one volunteer that is representative of two experiments from two different volunteers. ***P < 0.001 with respect to control.
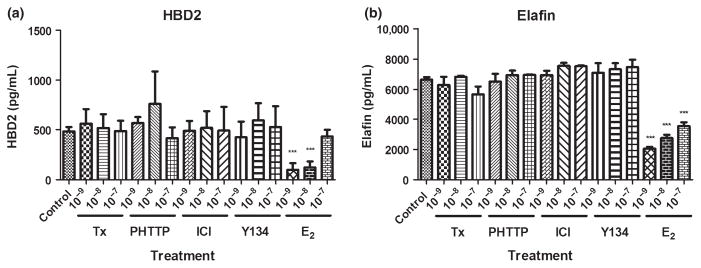
As E2 decreased HBD2 and elafin secretion by VEC, we investigated whether other modulators of estrogen receptor signaling would alter the antimicrobial production. Some of these SERMs are commonly used by women in the treatment of cancer and to alleviate the symptoms and side effects of menopause. However, their effects on innate immune protection and specifically antimicrobial secretion are unknown. We treated VEC with a panel of SERMs including tamoxifen, PHTTP, ICI and Y134 at concentrations between 1 × 10−7 and 1 × 10−9 M – levels known to have agonistic effects on antimicrobial secretions from the FRT of humans and rodents.25 As seen in Fig. 5, none of these treatments had any effect upon antimicrobial secretion by VEC over 48 hrs. In contrast, E2 maintained its inhibition of HBD2 and elafin secretion at 1 × 10−8 and 1 × 10−9 M.
Fig. 5.
Selective estrogen response modulators do not affect HBD2 and elafin secretion. Freshly isolated vaginal epithelial cells were incubated overnight in triplicate wells prior to treatment with tamoxifen (Tx), PHTTP, ICI, Y134 and estradiol (E2) (1 × 10−7–1×10−9 M) for 48 hrs. Conditioned media were recovered and analyzed by ELISA for the presence of HBD2 (a) and elafin (b). Data shown are from one volunteer that is representative of three experiments from three different volunteers. ***P < 0.001 with respect to control.
Anti-HIV Activity of VEC Secretions
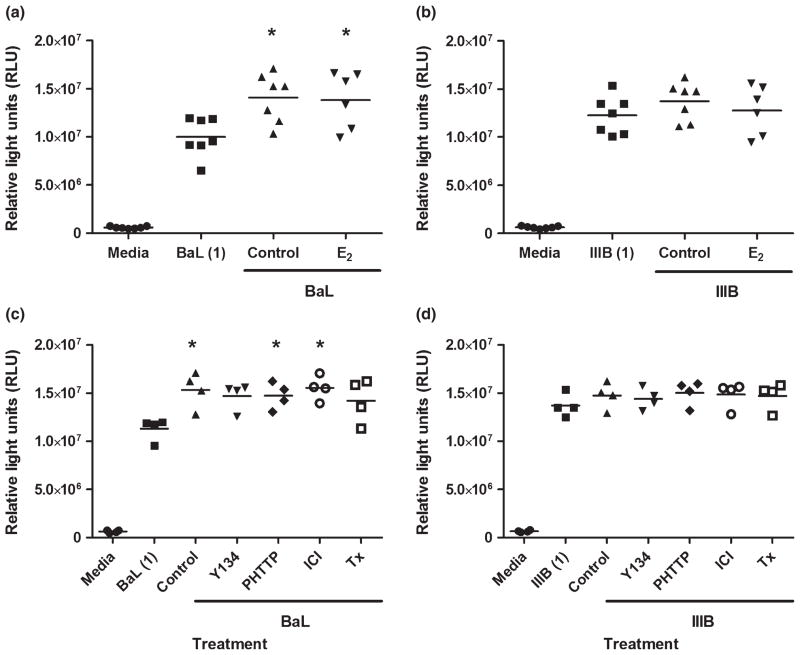
Previous studies from our laboratory have shown that UEC and ectocervical epithelial cells have limited but significant antiviral activity against BaL, an R5 laboratory-adapted strain of HIV.5 As VEC are the first cells exposed to HIV during male-to-female transmission, we determined whether VEC also displayed anti-HIV activity. We hypothesized that the decreased secretion of HBD2 and elafin by VEC in response to E2 treatment, and the absence of CCL20 from cell secretions, would lead to increased HIV infection of our TZM-bl reporter cell line in the presence of VEC conditioned media. Conditioned media were collected from VEC in culture for 48 hrs and, following a 1:8 dilution in complete media, was incubated for 1 hr with BaL or IIIB, two strains of HIV that utilize the CCR5 or CXCR4 co-receptors, respectively, prior to addition to TZM-bl cultures. As seen in Fig. 6, conditioned media from both control and E2-treated VEC had no significant inhibitory effect upon HIV infection of TZM-bl cells at either a high (MOI = 1) (Fig. 6) or low (MOI = 0.1) (not shown) concentration of virus. HIV-BaL infection of the TZM-bl cells significantly increased by 25–40% over virus alone in the presence of control and E2 conditioned media (Fig. 6a,c,e). Surprisingly, conditioned media from E2-stimulated VEC did not increase HIV infection beyond that seen in controls despite having lower levels of HBD2 and elafin (Fig. 6a,b). Conditioned media from VEC treated with SERMs generally increased HIV-BaL infection, with PHTTP and ICI reaching significance, but had no significant effect on HIV-IIIB infection of TZM-bl cells (Fig. 6c,d).
Fig. 6.
Absence of anti-HIV activity in vaginal epithelial secretions. Conditioned media were recovered after 48 hrs of culture and analyzed for inhibitory activity against BaL (a, c) and IIIB (b, d), a CCR5 and CXCR4 viral strain, respectively. Conditioned media were diluted 1:8 in complete media and incubated with virus at an multiplicity of infection of 1, for 1 hr at 37°C prior to incubation with TZM-bl cells. Vaginal epithelial cells were treated for 48 hrs with 5 × 10−8 M estradiol (E2) (a, b), and a panel of selective estrogen response modulators including Y134, PHTTP, ICI and tamoxifen (Tx) at 1 × 10−7 M (c, d). Each symbol on the graph represents a single patient sample. *P < 0.05 with respect to virus alone (BaL or IIIB).
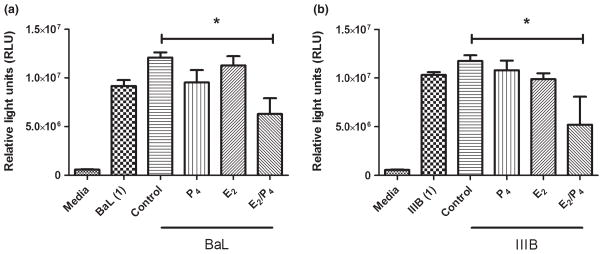
To determine whether P4 had any effect on HIV infectivity of TZM-BL cells, conditioned media from VEC incubated with P4 alone and in combination with E2 were analyzed for anti-HIV activity. There was a trend toward decreased HIV-BaL and HIV-IIIB infection by an average of 12–20% in conditioned media from VEC treated with P4 compared to control conditioned media (Fig. 7a,b). Unexpectedly, conditioned media from E2/P4 co-treated cells significantly decreased HIV-BaL infection by approximately 50% compared to control conditioned media (Fig. 7a) and significantly reduced HIV-IIIB infection by approximately 40% (Fig. 7b).
Fig. 7.
Anti-HIV activity in epithelial cells co-treated with E2/P4. vaginal epithelial cells were treated for 48 hrs with 1 × 10−7 M progesterone (P4), 5 × 10−8 M estradiol (E2) or a combination of E2 and P4. Conditioned media were recovered after 48 hrs of culture and analyzed for inhibitory activity against BaL (a) and IIIB (b). Conditioned media were diluted 1:8 in complete media and incubated with virus at an multiplicity of infection of 1, for 1 hr at 37°C prior to incubation with TZM-bl cells. Graph shown is from one volunteer that is representative of two independent experiments conducted in two different volunteers. *P < 0.05 with respect to control conditioned media.
Discussion
Vaginal epithelial cells exist in a challenging environment. They must provide a potent level of protection against incoming pathogens and modulate the growth of the vaginal microbiome, while maintaining an environment permissive for reproduction. The present study utilizes a novel technique for isolating large numbers of superficial and intermediate human VEC. These cells secrete two known antibacterial and antiviral compounds: elafin and HBD2. E2 decreases secretion of both molecules, while progesterone and a panel of SERMs (tamoxifen, PHTTP, ICI and Y134) have no effect. Despite lower HBD2 and elafin concentrations, there were no significant changes in anti-HIV activity in VEC conditioned media from E2-treated cells compared to control conditioned media. In contrast, conditioned media from VEC treated with P4 in combination with E2 inhibited HIV-BaL infection.
Our study demonstrates that the menstrual cup is suitable for collecting purified VEC at different stages of the menstrual cycle. Cell recovery is most likely due to the soft plastic rim of the cup gently shearing off the outer layers of the vaginal epithelium, which are retained in the cup until its removal from the vagina. A novel aspect of this protocol is the use of freshly isolated VEC that have not undergone any form of enzymatic digestion and are rapidly (within 24 hrs) treated after their removal from the host. This minimizes the time that cells spend in vitro and reduces the possibility of them undergoing major phenotypic changes associated with cell culture. This is not the first study to attempt to isolate fresh human VEC. Superficial VEC have been recovered by cervico-vaginal lavage (CVL) and shown to have anti-Candida albicans activity, as well as secrete MCP-1, TNFα and IL-1α into the culture media.26,27 Other studies have used a vaginal swab to brush off superficial VEC from distinct localized regions of the vagina.28 Due to the nature of the menstrual cup insertion and removal process, our protocol potentially recovers cells from throughout the vagina and not just a specific region, thus allowing us to study a broad population of VEC.
Superficial human VEC secrete the antimicrobials elafin and HBD2. Our data reflect previous immunohistochemical findings showing that both elafin and HBD2 are localized in the suprabasal layers of the human vulvo-vaginal epithelium.29,30 Furthermore, we demonstrate, for the first time, that this secretion is hormonally regulated as treatment of VEC with E2 for 48 hrs resulted in a dramatic inhibition of HBD2 and elafin secretion. This is consistent with a previous study by Han et al.31 demonstrating that E2 suppresses HBD2 mRNA expression in primary human VEC over 24 hrs in culture. However, they did not observe a concomitant decrease in protein secretion of HBD2. A potential explanation for this discrepancy between our results could lie in the duration of our E2 treatments. Whereas Han et al.31 exposed the VEC to E2 (2 × 10−9 M) for 24 hrs, we conducted our experiments over 48 hrs using a similar protocol to our studies from the upper FRT. The extra 24 hrs may have been essential for E2 to more fully exert its effects on the VEC. We have observed a similar effect in uterine stromal fibroblasts in which E2-induced upregulation of HGF secretion increases with time.32 Another explanation for the differences seen is that Han et al. used primary VEC that were obtained from enzymatically digested tissue and grown in vitro for at least 2 weeks before treatment. Previous studies have shown that the phenotype of FRT epithelial cells in culture changes with increasing passage number.33–35 In contrast, we used freshly isolated VEC that were in culture for a maximum of 72 hrs. Thus, freshly isolated VEC may substantially differ from VEC maintained in vitro over several passages. Our results complement the findings of Kumar et al.36 who demonstrated that hormonal replacement therapy suppresses expression of SLPI (like elafin, a member of the whey acidic protein family), in both the vaginal and ectocervical epithelium of post-menopausal women. E2 also inhibits other innate immune parameters in the lower FRT including the release of lactate dehydrogenase and the mRNA expression of IL-1α, IL-8, TNFα and TLR6 in the VK2 vaginal epithelial cell line.37 Thus, while our observations are consistent with prior studies demonstrating the profound regulation of multiple aspects of innate immunity in VEC by E2, we are the first to demonstrate its role in regulating antimicrobial secretion in freshly isolated human VEC.
Our results demonstrate a profound difference in hormonal regulation of HBD2 and elafin between the upper and lower FRT and are consistent with Hickey et al. (2012) who showed that E2 decreases HBD2 mRNA in murine vaginal tissue, but increases it uterine tissue. The basis for these opposite responses to E2 seen between epithelial cells from the upper and lower FRT is undetermined but several explanations can be postulated. First, the uterine columnar epithelium arises from the endoderm, while the squamous vaginal epithelium develops in the embryo from a combination of the endo-, ectoand mesoderm.38 Second, the functional characteristics of FRT epithelial cells are known to be influenced by the underlying stroma throughout development.39 It is possible that stromal–epithelial interactions within the FRT alter the phenotype of overlying VEC and UEC, thus leading to a divergence in their responses to an identical stimulus.40 For example, E2 induces P4 receptor expression in murine VEC and represses it in UEC.41 This effect of E2 in UEC is mediated via its interactions with ERα in the stromal fibroblasts and subsequent secretion of soluble paracrine factors that act on the epithelium.
Considering the large numbers of women who use SERMs to maintain their health, there is a dearth of publications regarding SERM effects on innate immune parameters in the human FRT. This is the first study to investigate the effects of SERMs on VEC-mediated immune protection in humans. The absence of a SERM effect in our system was surprising as they are known to bind and initiate signaling through ERα and/or ERβ, both of which are present in the human vaginal epithelium.42 All four SERMs used in our study decreased mouse BD2 mRNA expression in murine vaginal tissue.43 One explanation for our lack of changes may be that SERM effects are tissue specific. For example, Hickey et al.44 demonstrated that the SERMs, Y134 and ICI upregulate the expression of CCL20 in murine vaginal tissue but not in uterine tissue. In contrast, PHTTP did not have an effect at either site. Similarly, none of the SERMs altered defensin expression in the murine uterus.45 Therefore, our lack of a SERM effect on HBD2 or elafin secretion does not imply that VEC are insensitive to SERMs, but rather reflects the exquisite specificity these compounds possess when modulating gene expression in different tissue sites in both humans and rodents.
Conditioned media from VEC co-treated with E2 and P4 significantly inhibited HIV-BaL infection of TZM-bl cells but had no significant effect on HIV-IIIB. This is the first demonstration of anti-HIV activity in secretions from VEC and demonstrates that under specific hormonal conditions, VEC can potentially inhibit HIV transmission. The mechanism behind the increased antiviral activity is unclear. While E2/P4 had no effect on the secretion of HBD2 or elafin, it could conceivably have increased the production of other anti-HIV proteins, such as SLPI, and thus enhanced endogenous antiviral activity within the secretions. This finding also supports the need for future studies to investigate the effects of E2 and P4 in combination, rather than individually. This will more accurately replicate the hormonal environment in vivo where E2 and P4 are present together, especially in the secretory phase of the menstrual cycle.
Surprisingly, conditioned media from control and E2-treated cells promoted HIV-BaL infection of TZM-bl cells. This is in contrast to what we have previously observed with epithelial cells from the endometrium, endocervix and ectocervix that exhibited up to 50% inhibition against HIV-BaL (C.R. Wira, M. Ghosh and Z.S. Shen unpublished observation). 5 One explanation for the lack of anti-HIV activity in our system relates to the concentrations of CCL20, elafin and HBD2. Prior investigations have shown that HBD2 and elafin inhibit HIV infection in vitro at concentrations of 9000–20,000 ng/mL and 0.01–10 ng/mL, respectively.9,13 The maximum value we observed for HBD2 (3–4 ng/mL) was substantially lower than the minimum inhibitory dose. In contrast, the maximal value for elafin was between 4 and 5 ng/mL. However, active elafin is generated by the enzymatic cleavage of trappin-2. Our ELISA does not differentiate between the two forms. Recognizing that CCL20 was absent, HBD2 very low and levels of active elafin unknown in our conditioned media, the lack of anti-HIV activity is not surprising.
Recognizing that BaL is an R5-tropic strain of HIV, which is the predominant sexually transmitted form of the virus, our results show that antiviral activity against BaL varies with hormonal treatment. P4, either alone or in combination with E2, tended to reduce HIV-BaL infection of TZM-bl cells, while E2 had no effect. This demonstrates a fundamental difference in the effect of either hormone upon secreted anti-HIV activity in VEC secretions and raises the possibility that VEC-mediated innate immune protection against R5 viruses may be enhanced when P4 levels are elevated in the secretory phase of the menstrual cycle.
It would be premature to conclude that 48-hr constitutive and E2-treated VEC secretions have no effect on HIV transmission in the vagina. While our data suggest that under these conditions, the protein components within VEC secretions have no direct inhibitory effect on HIV, it is possible that they could modulate the phenotype of local target cells in vivo and alter their susceptibility to HIV infection. Previous studies by our group have shown that secretions from UEC can alter the phenotype of dendritic cells (DC).46,47 For example, DC cultured in the presence of UEC basolateral secretions reduce their expression of DC-SIGN, a potential receptor for HIV, leading to decreased DC-mediated HIV trans-infection of TZM-bl cells.47 It is likely that VEC secretions also alter the phenotype of immune cells in the vagina and thus their potential susceptibility to HIV infection. As these are the first immune cells exposed to HIV, it will be important for future studies to determine whether and how this phenotypic change occurs.
Our results indicate that superficial VEC do not secrete CCL20 under any of our treatment conditions. This is consistent with Hickey et al.44 who demonstrated that murine VEC do not secrete detectable levels of CCL20 during in vitro culture, in contrast to murine UEC that secrete significant quantities of CCL20. However, our findings of no CCL20 production differ from previously published data reporting that human VEC constitutively produce CCL20.16,48 This may be due to, as discussed earlier, the use of different experimental conditions between our studies. Alternatively, the lack of measureable CCL20 in our culture system may indicate that superficial VEC do not contribute to the pool of CCL20 present in CVL.4 Our studies suggest that CCL20 in cervico-vaginal secretions are derived from a combination of upper (uterine and cervical) FRT secretions, possible basal VEC production, as well as from resident immune cells in the lower FRT. This hypothesis is supported by data from murine studies that demonstrate constitutive CXCL1 production in vitro by UEC but not VEC.44 However, similar to CCL20 in human CVL, CXCL1 is still present in vaginal lavage taken from these mice thus demonstrating the contribution of uterine secretions to the pool of CXCL1 in the murine vagina.43 Given that antimicrobial production is probably increasing in the upper FRT and decreasing in the lower FRT in response to rising E2 levels,49 it is likely that CCL20 and other antimicrobials enter the lower FRT and partially compensate for lowered VEC production. Further studies are needed to determine the relative contributions of the upper FRT to lower FRT immune protection.
In conclusion, we report a novel approach for isolating human VEC that is non-invasive and allows for multiple recoveries from the same individual and at specific stages of the menstrual cycle. We were able to generate purified cultures of epithelial cells that were free of immune cell contamination. VEC were responsive to E2 stimulation in that hormone treatments at concentrations comparable to that seen in the human FRT during the menstrual cycle inhibit the secretion of HBD2 and elafin – both implicated in inhibition of HIV infection. Overall, these findings suggest a novel approach for analyzing in vitro the effects of hormones on VEC function as they relate to our understanding of their role in the prevention of sexually transmitted infections including HIV, the maintenance of the FRT microbiome and the ways in which the lower FRT contributes to reproductive function in the upper FRT.
Acknowledgments
This study was supported by NIH grants AI013541, AI102838 and AI071761 (CRW) as well as partial stipend support from the Department of Microbiology and Immunology (MVP), Geisel School of Medicine at Dartmouth. We would like to thank Linda Hallock for recruiting the volunteers and organizing menstrual cup collections. We also thank Matthew Deberge for assistance with the microscopy. Finally, we extend our profound gratitude to the volunteers for expending their time and energy to donate samples for this study.
References
- 1.World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. 2011. [Google Scholar]
- 2.Patton DL, Thwin SS, Meier A, Hooton TM, Stapleton AE, Eschenbach DA. Epithelial cell layer thickness and immune cell populations in the normal human vagina at different stages of the menstrual cycle. Am J Obstet Gynecol. 2000;183:967–973. doi: 10.1067/mob.2000.108857. [DOI] [PubMed] [Google Scholar]
- 3.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, Mayer K, Wright PF, Kappes JC, Ochsenbauer C, Wira CR. Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS ONE. 2010;5:e11366. doi: 10.1371/journal.pone.0011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wira CR, Ghosh M, Smith JM, Shen L, Connor RI, Sundstrom P, Frechette GM, Hill ET, Fahey JV. Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol. 2011;4:335–342. doi: 10.1038/mi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.John M, Keller MJ, Fam EH, Cheshenko N, Hogarty K, Kasowitz A, Wallenstein S, Carlucci MJ, Tuyama AC, Lu W, Klotman ME, Lehrer RI, Herold BC. Cervicovaginal secretions contribute to innate resistance to herpes simplex virus infection. J Infect Dis. 2005;192:1731–1740. doi: 10.1086/497168. [DOI] [PubMed] [Google Scholar]
- 7.Pivarcsi A, Nagy I, Koreck A, Kis K, Kenderessy-Szabo A, Szell M, Dobozy A, Kemeny L. Microbial compounds induce the expression of pro-inflammatory cytokines, chemokines and human β-defensin-2 in vaginal epithelial cells. Microbes Infect. 2005;7:1117–1127. doi: 10.1016/j.micinf.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 9.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, Mirza M, Jiang B, Kiser P, Medvik K, Sieg SF, Weinberg A. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Finnegan CM, Kish-Catalone T, Blumenthal R, Garzino-Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino-Demo A. Human β-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–14329. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinberg A, Quinones-Mateu ME, Lederman MM. Role of human beta-defensins in HIV infection. Adv Dent Res. 2006;19:42–48. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- 12.Reading JL, Meyers AF, Vyakarnam A. Whey acidic proteins (WAPs): novel modulators of innate immunity to HIV infection. Curr Opin HIV AIDS. 2012;7:172–179. doi: 10.1097/COH.0b013e32835005d9. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-human immunodeficiency virus-1 molecule of the human female reproductive tract. Immunology. 2010;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal SM, Ball TB, Levinson P, Maranan L, Jaoko W, Wachihi C, Pak BJ, Podust VN, Broliden K, Hirbod T, Kaul R, Plummer FA. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS. 2009;23:1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 15.Fichorova RN, Anderson DJ. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod. 1999;60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 16.Cremel M, Berlier W, Hamzeh H, Cognasse F, Lawrence P, Genin C, Bernengo J-C, Lambert C, Dieu-Nosjean M-C, Delézay O. Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction. J Leukoc Biol. 2005;78:158–166. doi: 10.1189/jlb.0305147. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3a is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickey DK, Patel MV, Fahey JV, Wira CR. Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol. 2011;88:185–194. doi: 10.1016/j.jri.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, Wira CR. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunol. 2008;1:317–325. doi: 10.1038/mi.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135:739–749. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 21.Moriyama A, Shimoya K, Ogata I, Kimura T, Nakamura T, Wada H, Ohashi K, Azuma C, Saji F, Murata Y. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Hum Reprod. 1999;5:656–661. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- 22.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koks CAM, Dunselman GAJ, de Goeij AFPM, Arends JW, Evers JLH. Evaluation of a menstrual cup to collect shed endometrium for in vitro studies. Fertil Steril. 1997;68:560–564. doi: 10.1016/s0015-0282(97)00250-1. [DOI] [PubMed] [Google Scholar]
- 24.Crane-Godreau MA, Wira CR. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infect Immun. 2005;73:4231–4237. doi: 10.1128/IAI.73.7.4231-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fahey JV, Bodwell JE, Hickey DK, Ghosh M, Muia MN, Wira CR. New approaches to making the microenvironment of the female reproductive tract hostile to HIV. Am J Reprod Immunol. 2011;65:334–343. doi: 10.1111/j.1600-0897.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barousse MM, Steele C, Dunlap K, Espinosa T, Boikov D, Sobel JD, Fidel PL. Growth inhibition of Candida albicans by human vaginal epithelial cells. J Infect Dis. 2001;184:1489–1493. doi: 10.1086/324532. [DOI] [PubMed] [Google Scholar]
- 27.Steele C, Fidel PL. Cytokine and chemokine production by human oral and vaginal epithelial cells in response to Candida albicans. Infect Immun. 2002;70:577–583. doi: 10.1128/IAI.70.2.577-583.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderete JF, Demeś P, Gombosova A, Valent M, Fabusová M, Jánoska A, Stefanovic J, Arroyo R. Specific parasitism of purified vaginal epithelial cells by Trichomonas vaginalis. Infect Immun. 1988;56:2558–2562. doi: 10.1128/iai.56.10.2558-2562.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfundt R, van Ruissen F, van Vlijmen-Willems IM, Alkemade HA, Zeeuwen PL, Jap PH, Dijkman H, Fransen J, Croes H, van Erp PE, Schalkwijk J. Constitutive and inducible expression of SKALP/elafin provides anti-elastase defense in human epithelia. J Clin Invest. 1996;98:1389–1399. doi: 10.1172/JCI118926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erhart W, Alkasi Ö, Brunke G, Wegener F, Maass N, Arnold N, Arlt A, Meinhold-Heerlein I. Induction of human β-defensins and psoriasin in vulvovaginal human papillomavirus–associated lesions. J Infect Dis. 2011;204:391–399. doi: 10.1093/infdis/jir079. [DOI] [PubMed] [Google Scholar]
- 31.Han JH, Kim MS, Lee MY, Kim TH, Lee M-K, Kim HR, Myung SC. Modulation of human β-defensin-2 expression by 17β-estradiol and progesterone in vaginal epithelial cells. Cytokine. 2010;49:209–214. doi: 10.1016/j.cyto.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Coleman KD, Ghosh M, Crist SG, Wright JA, Rossoll RM, Wira CR, Fahey JV. Modulation of hepatocyte growth factor secretion in human female reproductive tract stromal fibroblasts by poly (I:C) and estradiol. Am J Reprod Immunol. 2012;67:44–53. doi: 10.1111/j.1600-0897.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benali R, Dupuit F, Chevillard M, Jacquot J, Haye B, Puchelle E. Modulations of the epithelial phenotype and functional activity of cultured bovine tracheal gland cells: dependence on the culture medium and passage number. Biol Cell. 1991;73:49–56. doi: 10.1016/0248-4900(91)90008-b. [DOI] [PubMed] [Google Scholar]
- 34.Miessen K, Einspanier R, Schoen J. Establishment and characterization of a differentiated epithelial cell culture model derived from the porcine cervix uteri. BMC Vet Res. 2012;8:31. doi: 10.1186/1746-6148-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato S, Espinoza N, Lange S, Villalon M, Cuello M, Owen GI. Characterization and phenotypic variation with passage number of cultured human endometrial adenocarcinoma cells. Tissue Cell. 2008;40:95–102. doi: 10.1016/j.tice.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Kumar R, Vicari M, Gori I, Achtari C, Fiche M, Surbeck I, Damnon F, Canny GO. Compartmentalized secretory leukocyte protease inhibitor expression and hormone responses along the reproductive tract of postmenopausal women. J Reprod Immunol. 2011;92:88–96. doi: 10.1016/j.jri.2011.06.103. [DOI] [PubMed] [Google Scholar]
- 37.Wagner RD, Johnson SJ. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. J Biomed Sci. 2012;19:58. doi: 10.1186/1423-0127-19-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farage MA, Maibach HI. Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol. 2011;40:9–19. doi: 10.1159/000321042. [DOI] [PubMed] [Google Scholar]
- 39.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 40.Grant-Tschudy KS, Wira CR. Hepatocyte growth factor regulation of uterine epithelial cell transepithelial resistance and tumor necrosis factor alpha release in culture. Biol Reprod. 2005;72:814–821. doi: 10.1095/biolreprod.104.035618. [DOI] [PubMed] [Google Scholar]
- 41.Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 42.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 43.Hickey DK, Fahey JV, Wira CR. Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, α-/β-defensins and TLRs. Innate Immun. 2012 doi: 10.1177/1753425912454026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickey DK, Fahey JV, Wira CR. Estrogen receptor α antagonists mediate changes in CCL20 and CXCL1 secretions in the murine female reproductive tract. Am J Reprod Immunol. 2012;69:159–167. doi: 10.1111/aji.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickey DK, Wira CR. Beta-defensin 1–4 mRNA expression in the murine female reproductive tract is regulated by estrogen receptor ligand interactions. 2012. [Google Scholar]
- 46.Ochiel DO, Ghosh M, Fahey JV, Guyre PM, Wira CR. Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands. J Leukoc Biol. 2010;88:435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PLoS ONE. 2010;5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berlier W, Cremel M, Hamzeh H, Levy R, Lucht F, Bourlet T, Pozzetto B, Delezay O. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod. 2006;21:1135–1142. doi: 10.1093/humrep/dei496. [DOI] [PubMed] [Google Scholar]
- 49.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]