Abstract
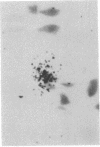
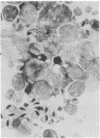
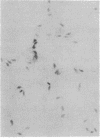
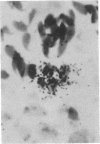
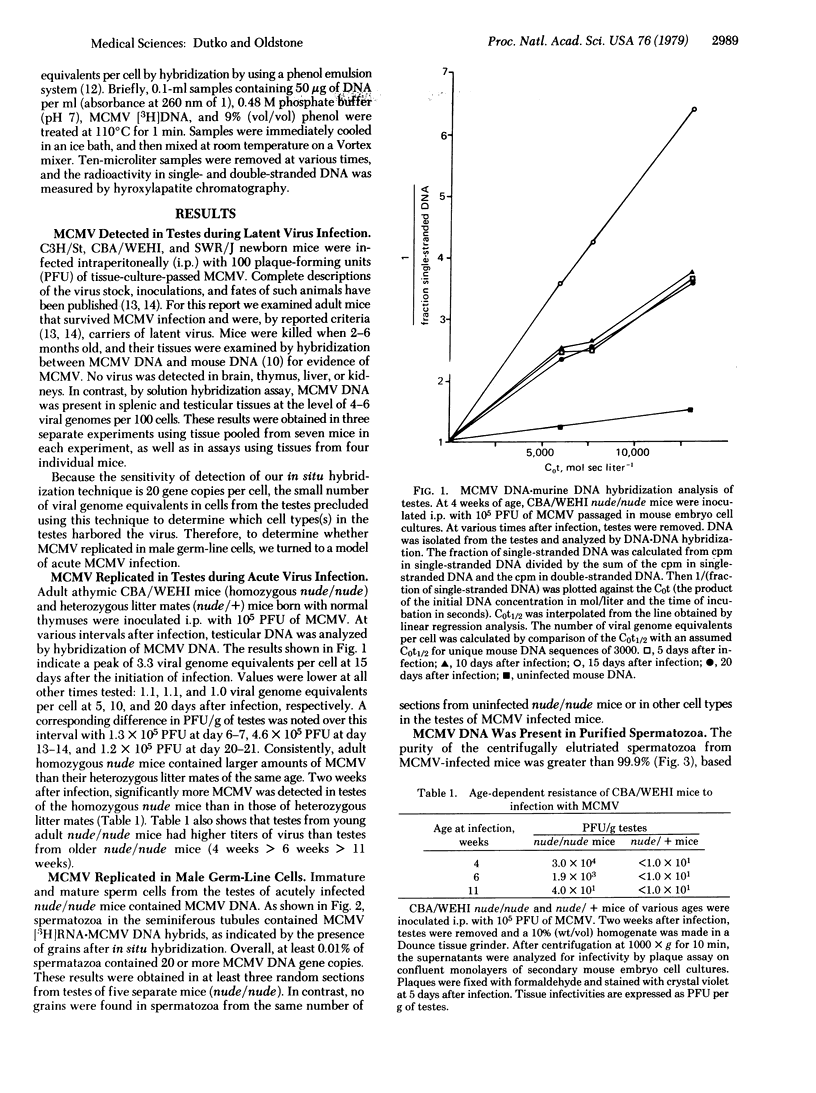
Murine cytomegalovirus replicated in reproductive tissue of male mice infected with the virus. We examined three strains of mice latently infected by injection at birth with 100 plaque-forming units of the virus. As adults, these mice contained within their testes 4--6 viral genomic equivalents per 100 cells, as tested by hybridization between mouse DNA and cytomegalovirus DNA. Acutely infected male adult CBA mice homozygous for the nude gene (athymic: nude/nude) produced infectious virus in their testes, the amounts of which varied according to the animal's age at the time of infection. Heterozygous (nude/+) litter mates contained significantly less virus than nude/nude mice. At the peak of virus replication hybridization between virus DNA and mouse DNA indicated the presence of 3.3 viral genome equivalents per testicular cell. Both in situ hybridization studies and phenol emulsion reassociation of virus DNA to DNA from purified spermatozoa localized this viral DNA to immature and mature sperm cells. Hence, murine cytomegalovirus can be harbored in testes during both acute and latent infections and can replicate in male germ-line cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chantler J. K., Misra V., Hudson J. B. Vertical transmission of murine cytomegalovirus. J Gen Virol. 1979 Mar;42(3):621–625. doi: 10.1099/0022-1317-42-3-621. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Lang D. J. Detection of latent cytomegalovirus in murine salivary and prostate explant cultures and cells. Infect Immun. 1977 Feb;15(2):568–575. doi: 10.1128/iai.15.2.568-574.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E., Hanshaw J. B., Carpenter C. B. Cytomegalovirus infection after renal allotransplantation. JAMA. 1967 Sep 4;201(10):725–728. [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Somatic cell hybrids between mouse peritoneal macrophages and SV40-transformed human cells. I. Positive control of the transformed phenotype by the human chromosome 7 carrying the SV40 genome. J Exp Med. 1974 Nov 1;140(5):1221–1229. doi: 10.1084/jem.140.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. N., Saslow A. R., Armstrong J. A., Ho M. The relationship of immunosuppression to cytomegalovirus infection. Yale J Biol Med. 1976 Mar;49(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Duvall C. P., Casazza A. R., Grimley P. M., Carbone P. P., Rowe W. P. Recovery of cytomegalovirus from adults with neoplastic disease. Ann Intern Med. 1966 Mar;64(3):531–541. doi: 10.7326/0003-4819-64-3-531. [DOI] [PubMed] [Google Scholar]
- Grabske R. J., Lake S., Gledhill B. L., Meistrich M. L. Centrifugal elutriation: separation of spermatogenic cells on the basis of sedimentation velocity. J Cell Physiol. 1975 Aug;86(1):177–189. doi: 10.1002/jcp.1040860119. [DOI] [PubMed] [Google Scholar]
- Ho M., Dowling J. N., Armstrong J. A., Suwansirikul S., Wu B., Youngblood L. A., Saslow A. Factors contributing to the risk of cytomegalovirus infection in patients receiving renal transplants. Yale J Biol Med. 1976 Mar;49(1):17–26. [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohne D. E., Levison S. A., Byers M. J. Room temperature method for increasing the rate of DNA reassociation by many thousandfold: the phenol emulsion reassociation technique. Biochemistry. 1977 Nov 29;16(24):5329–5341. doi: 10.1021/bi00643a026. [DOI] [PubMed] [Google Scholar]
- Lang D. J., Kummer J. F. Demonstration of cytomegalovirus in semen. N Engl J Med. 1972 Oct 12;287(15):756–758. doi: 10.1056/NEJM197210122871508. [DOI] [PubMed] [Google Scholar]
- Lang D. J., Kummer J. F., Hartley D. P. Cytomegalovirus in semen. Persistence and demonstration in extracellular fluids. N Engl J Med. 1974 Jul 18;291(3):121–123. doi: 10.1056/NEJM197407182910303. [DOI] [PubMed] [Google Scholar]
- Neighbour P. A., Fraser L. R. Murine cytomegalovirus and fertility: potential sexual transmission and the effect of this virus on fertilization in vitro. Fertil Steril. 1978 Aug;30(2):216–222. doi: 10.1016/s0015-0282(16)43463-1. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Jensen F. C., Oldstone M. B. Pathogenesis of of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J Exp Med. 1975 Mar 1;141(3):561–572. doi: 10.1084/jem.141.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olding L. B., Kingsbury D. T., Oldstone M. B. Pathogenesis of cytomegalovirus infection. Distribution of viral products, immune complexes and autoimmunity during latent murine infection. J Gen Virol. 1976 Nov;33(2):267–280. doi: 10.1099/0022-1317-33-2-267. [DOI] [PubMed] [Google Scholar]
- Pagano J. S. Diseases and mechanisms of persistent DNA virus infection: latency and cellular transformation. J Infect Dis. 1975 Aug;132(2):209–223. doi: 10.1093/infdis/132.2.209. [DOI] [PubMed] [Google Scholar]
- Reynolds D. W., Stagno S., Stubbs K. G., Dahle A. J., Livingston M. M., Saxon S. S., Alford C. A. Inapparent congenital cytomegalovirus infection with elevated cord IgM levels. Casual relation with auditory and mental deficiency. N Engl J Med. 1974 Feb 7;290(6):291–296. doi: 10.1056/NEJM197402072900601. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962 Jun 14;266:1233–1244. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]