Abstract
The yeast zinc cluster transcription factor Oaf1p activates transcription of target genes in response to direct binding of fatty acids in a manner analogous to the vertebrate nuclear receptor peroxisome proliferator-activated receptor α (PPARα). PPARs and other metazoan nuclear receptors productively engage several distinct LXXLL motif-containing co-activators, including p160 family members and the TRAP220/MED1 subunit of the Mediator co-activator, to promote ligand-dependent gene activation. Yeast, however, does not appear to harbor LXXLL motif co-activators, and the mechanism of fatty acid-dependent gene activation by the yeast PPARα analog Oaf1p is unknown. Here we show that the yeast Mediator subunit Gal11p/MED15 and its activator-targeted KIX domain plays a critical role in fatty acid-dependent transcriptional regulation of fatty acid β-oxidation and peroxisomal genes by Oaf1p and for the ability of yeast to utilize fatty acids as a sole carbon source. Moreover, structural studies by NMR spectroscopy reveal that the Oaf1p activation domain interacts with the Gal11p/MED15 KIX domain in a manner similar to the yeast zinc cluster family member and xenobiotic receptor Pdr1p, revealing that the Gal11p/MED15 KIX domain is a key target of several ligand-dependent transcription factors in yeast. Together with previous work showing that the Caenorhabditis elegans Gal11p/MED15 homolog MDT-15 plays a critical role in regulation of fatty acid metabolism by the nematode PPAR-like nuclear receptor NHR-49, the findings presented here provide evidence for an ancient and essential role of a Mediator co-activator subunit in regulation of fatty acid metabolism by nuclear receptor-like transcription factors in eukaryotes.
The vertebrate nuclear receptor PPARα2 activates transcription of genes involved in fatty acid β-oxidation in response to binding to certain fatty acids and their metabolites, as well as peroxisome proliferators, a class of hypolipidemic chemicals, and non-steroidal anti-inflammatory drugs (NSAIDs) (1, 2). Interestingly, the yeast zinc cluster transcription factor Oaf1p (together with its heterodimer partner Pip2p) was discovered as a fatty acid-activated transcriptional regulator of a number of peroxisomal and β-oxidation genes, many of which are homologous to PPARα-activated genes (1, 3). Importantly, a recent study demonstrated that Oaf1p could directly bind to the fatty acid oleic acid to regulate transcription of target genes; this functional behavior is thus strikingly similar to vertebrate PPARα and raised the question of whether these metazoan and fungal ligand-dependent transcription factor families are evolutionarily related (4). Additional comparisons of Oaf1p and PPARs functional characteristics, such as ligand binding specificities and transcription co-activator requirements, might shed further light on the possibility of an evolutionary relationship.
Vertebrate nuclear receptors, including PPARs, stimulate expression of target genes by recruiting different classes of transcription co-activators, including Mediator, a large co-activator complex conserved from yeast to human (5). We recently demonstrated that orthologs of the Pdr1p zinc cluster family member in Saccharomyces cerevisiae and Candida glabrata require the Mediator subunit Gal11p/MED15 for xenobiotic-dependent transactivation of multidrug resistance genes (6). This work also revealed that Gal11p/MED15 harbors an activator-targeted domain with marked structural and functional similarity to the KIX domain present in the metazoan ARC105/MED15 Mediator subunit and the CREB-binding protein/p300 acetyl-transferases (6–8). The activation domain of Pdr1p orthologs interacts with the Gal11p/MED15 KIX domain in a xenobiotic-stimulated manner and Gal11p/MED15 KIX is essential for xenobiotic-dependent activation of the multidrug resistance program in S. cerevisiae and C. glabrata. In contrast with the detailed understanding of the transactivation mechanism by Pdr1p orthologs, the molecular requirements for gene activation, including a potential role for Gal11p/MED15 and its KIX domain, by other ligand-stimulated fungal zinc cluster transcription factors, such as Oaf1p, remain unclear.
Here we show that Oaf1p binds directly to a number of fatty acids (including oleic acid, palmitic acid, myristic acid, and lauric acid), resulting in gene activation. Interestingly, Oaf1p also interacts with peroxisome proliferators/NSAIDs, albeit in a non-productive manner. These findings suggest that Oaf1p shares several functional attributes with PPARα; however, important mechanistic differences do exist. We also establish here that the Gal11p/MED15 Mediator subunit and its KIX domain are critical mediators of fatty acid- and Oaf1p-dependent transactivation and important for yeast growth on oleic acid as carbon source. Together with the finding by others that gene activation by the Caenorhabditis elegans PPARα-like nuclear receptor NHR-49 requires the Gal11p/MED15 homolog MDT-15 (9), these studies suggest that targeting of the Gal11p/MED15 KIX domain is a conserved transactivation mechanism shared by members of the fungal ligand-dependent zinc cluster transcription factor family and metazoan nuclear receptors.
EXPERIMENTAL PROCEDURES
Strains, Plasmids, and Oligonucleotides—The Escherichia coli strain DH5α was used for all the plasmid manipulations, amplifications, subcloning and isolations. The S. cerevisiae strains used in this study are listed in supplemental Table 1, and the plasmids and oligonucleotides are listed in supplemental Table 2. For the construction of pADH2-FLAG-Gal4pDBD-Oaf1pC, FLAG-Gal4pDBD was amplified by PCR from pcDNA3-HA-Gal4pDBD using the oligonucleotide pair XFG4 (5′-GCTCTAGAGACTACAAAGACGACGATGACAAGCT-ACTGTCTTC-3′), which contained FLAG sequence, and KG4 (5′-GGGGTACCCCGTAACAATGCTTTTATATC-3′). The resulting 330-bp XbaI-KpnI fragment was used to replace the 900-bp XbaI-KpnI fragment in pADH2-Oaf1p-Myc9 (10). To generate pGBKT7-Oaf1p, the 3.2-kb OAF1 open reading frame was amplified by PCR using pADH2-Oaf1p-Myc9 as template (10). The primers used were 5′-CTCGCCGTCGACTATC-TAGAGTCGAAAATAGTACGCAG-3′ and 5′-CTCG-CTCAAGCTTGCGGCCGCTTAAGCAAAGTCATTGCC-3′. The amplified fragment was digested with SalI and HindIII and ligated into appropriately cut pGBKT7. Strains overexpressing FLAG-Gal4pDBD-Oaf1pC were generated by integration of EcoRV-linearized pADH2-FLAG-Gal4pDBD-Oaf1pC into the trp1 locus of the YF120 strain. The resulting strain was obtained by selecting transformants on – Trp selective media.
Yeast Media and Growth Conditions—For β-galactosidase assays, overnight cultures propagated in YPD medium (1% yeast extract, 2% bactopeptone, and 2% glucose) were diluted to an A600 of 0.3 in YP (1% yeast extract and 2% bactopeptone) supplemented with different concentrations of various fatty acids or chemicals as indicated and grown for another 16–20 h. Cultures were grown at 30 °C with vigorous aeration. For growth assay on oleic acid and YPD plates, cells cultured over-night were diluted to A600 of 0.5, and then four serial dilutions of 1/10 times were spotted on YPO (1% yeast extract, 2% bactopeptone, 0.2% oleic acid, and 0.02% Tween 80).
Ligand Binding Assay—The FLAG-tagged Oaf1p C-terminal domain (amino acids 181–1047) fused to the Gal4p DNA-binding domain (amino acids 1–94) was expressed in YF120. Yeast extract was prepared from 6 liters of culture at mid-log phase and subjected to immunoprecipitation using anti-FLAG-Sepharose beads (M2, Sigma). The beads were used for ligand binding assays as described previously (6), with anti-FLAG-Sepharose beads as control to determine nonspecific binding. Briefly, 20 μl of beads were incubated with the specified amount of 14C-labeled oleic acid (PerkinElmer Life Sciences) for 5 h at 4 °C in binding buffer (20 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.01% Nonidet P-40, 0.1 mm EDTA, 1 mm dithiothreitol, and complete protease inhibitor (Roche Applied Science)). The beads were washed four times with binding buffer followed by scintillation counting. For competition assays, 100-fold molar excess cold fatty acids or peroxisome proliferators/NSAIDs were added to the initial binding solution.
Immunopurification and Immunoblotting—Immunopurification and immunoblotting were carried out by standard procedures as described (6). The antibodies used were: anti-FLAG (Sigma), anti-hemagglutinin (HA) (Covance Research Products), and anti-Myc (antibody sc-40, Santa Cruz Biotechnology).
GST Pulldown Assays—GST fusion proteins were expressed and purified as described (6). Yeast whole cell extracts were prepared as described (6), and GST pulldowns were carried out as described (6). In vitro translated proteins (3 ml) were diluted 100-fold with the binding buffer (20 mm HEPES, pH 7.6, 140 mm NaCl, 0.1 mm EDTA, 10% glycerol, 0.05% Nonidet P-40, 1 mm dithiothreitol, 1 mm benzamidine, 0.25 mm phenylmethyl-sulfonyl fluoride, and 2 μg/ml aprotinin). A total of 1 ml of yeast whole cell extracts or diluted in vitro translated proteins was applied to 20 μl of GST fusion protein beads and incubated at 4 °C for 3 h. Beads were washed five times with 1 ml each of binding buffer containing 250 mm KCl and 0.5% Nonidet P-40. The interacting proteins were then eluted with 0.3% sarkosyl in binding buffer at 4 °C for 1 h.
NMR Experiments—15N, 15N-13C, and 15N-13C-2H (perdeu-terated) labeled samples of yeast Gal11p/MED15 KIX (amino acids 6–90) were expressed and purified as described earlier (6). Oaf1pAD-9 (LFDYDFLFG) was purchased as a synthetic peptide from the Tufts New England Medical Center peptide synthesis facility, Boston, MA. Titrations of Gal11p/MED15 KIX with the Oaf1pAD-9 were carried out in a “high phosphate buffer” (50 mm Na2HPO4, 50 mm NaH2PO4, 2 mm K2HPO4, 2.7 mm KCl, 1 mm EDTA, and 0.01% NaN3) to maintain the pH during the course of the titration. The bound conformation of Gal11p/MED15 KIX was assigned by standard triple resonance backbone experiments (HNCA and HNCACB) (11). Cross saturation transfer experiments were carried out using a perdeuterated sample of Gal11p/MED15 KIX and unlabeled Oaf1pAD-9. The methyl region of the Oaf1p peptide (i.e. 1 ppm) was excited with a WURST pulse (3 ppm of bandwidth) for the duration of 2 s followed by a standard HSQC experiment (12). The experiment was carried out in an interleaved fashion with irradiation and no irradiation in successive scans. The ratio of intensities between the saturated HSQC and the non-saturated HSQC was later analyzed. 15N-dispersed NOE spectroscopy experiments with a mixing time of 0.2 s were carried out on a perdeuterated sample of 15N-13C-enriched Gal11p/MED15 KIX and unlabeled Oaf1pAD-9 in the molar ratio 1:1 to obtain intermolecular NOEs.
Miscellaneous—The following procedures were performed according to the manufacturer’s instructions: yeast RNA preparation (Qiagen), RT-PCR analysis (Amersham Biosciences), and in vitro protein synthesis (Promega). Yeast transformation and enzyme assay for β-galactosidase activity were performed according to standard protocols.
RESULTS
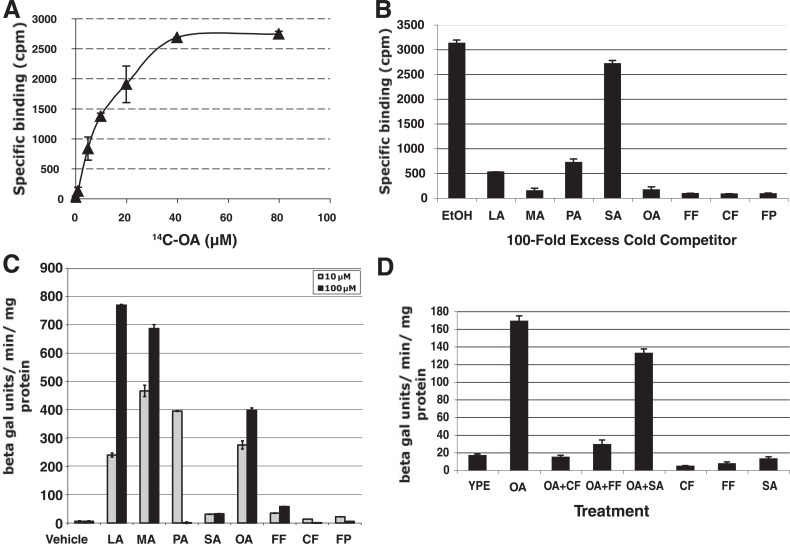
Oaf1p Binds Directly to Both Fatty Acids and Peroxisome Proliferators/NSAIDs—Oaf1p directly interacts with oleic acid (OA) to transactivate fatty acid β-oxidation and peroxisomal genes, and OA causes peroxisome proliferation in yeast in an Oaf1p-dependent manner (10, 13), suggesting intriguing functional similarities with vertebrate PPARα. We set out to determine whether peroxisome proliferators and NSAIDs might also interact with Oaf1p and affect Oaf1p transcriptional activity. We first tested whether fatty acids and peroxisome proliferators/NSAIDs interact with a C-terminal fragment of Oaf1p harboring the fatty acid-binding domain (4). FLAG-tagged Oaf1pC fused to the Gal4p DNA-binding domain expressed in yeast bound to radiolabeled OA (Fig. 1A), consistent with previous observations (4). Similar results were obtained with immunopurified, full-length, Myc-tagged Oaf1p translated in vitro, whereas several other control proteins did not significantly bind to radiolabeled OA (data not shown). Competition experiments with 100-fold excess of unlabeled fatty acids and peroxisome proliferators/NSAIDs revealed that all compounds, with the exception of stearic acid, bound strongly to immunopurified FLAG-Gal4p-Oaf1pC and full-length Myc-Oaf1p (Fig. 1B and data not shown). We next examined transactivation by Gal4p-Oaf1pC of an integrated GAL7 promoter-LacZ reporter in yeast in response to different fatty acids and peroxisome proliferators/NSAIDs. Gal4p-Oaf1pC activated the reporter strongly in response to OA, in keeping with a previous report demonstrating that the C-terminal domain of Oaf1p fused to the LexA DNA-binding domain could confer OA inducibility (Fig. 1C) (14). Similar to the broad ligand specificity of PPARα, Gal4p-Oaf1pC was also potently activated by several other fatty acids tested, including lauric acid (LA), myristic acid (MA), and palmitic acid (PA), whereas stearic acid (SA), which does not effectively bind to Oaf1p, was unable to activate Gal4p-Oaf1pC (Fig. 1C). This suggests that several fatty acids with different carbon chain lengths activate Oaf1p by direct binding to the C-terminal ligand-binding domain (Fig. 1B). Surprisingly, however, the peroxisome proliferators/NSAIDs fenofibrate (Fig. 1B, FF), clofibrate (Fig. 1B, CF), and fenoprofen (Fig. 1B, FP), which compete effectively for [14C]OA binding to Oaf1p (Fig. 1B), alone acted as very weak agonists of Gal4p-Oaf1pC and full-length Myc-Oaf1p (Fig. 1C and data not shown). Moreover, when peroxisome proliferators/NSAIDs were present in 10-fold molar excess, OA was no longer capable of stimulating transcription through Gal4p-Oaf1pC, suggesting that these drugs act as potent competitive antagonists for OA-dependent activation by Oaf1p (Fig. 1D). By contrast, SA, which does not bind to FLAG-Gal4p-Oaf1pC or Myc-Oaf1p, did not affect OA-induced Gal4p-Oaf1pC activation (Fig. 1D and data not shown). These findings demonstrate that although peroxisome proliferators/NSAIDs (which are potent agonists of PPARα) can interact directly and strongly with yeast Oaf1p, they are unable to fully activate Oaf1p, and indeed, function as antagonists of fatty acid-induced transcription.
FIGURE 1.
Oaf1p activates transcription in response to binding of fatty acids and peroxisome proliferators. A, saturation curve showing that FLAG-tagged Gal4p-Oaf1pC immunopurified from yeast bound to [14C]oleic acid with an estimated KD of ∼16 μm. B, competition of Gal4p-Oaf1pC binding to radiolabeled oleic acid with 100-fold excess of unlabeled fatty acids and peroxisome proliferators. C, several fatty acids, including LA (C12:0), MA (C14:0), PA (C16:0), and OA (C18:1n9), were able to stimulate transactivation of an integrated GAL7 promoter-LacZ reporter by the Oaf1p C terminus fused to the Gal4pDBD (Gal4p-Oaf1pC). The Gal4pDBD alone did not mediate stimulation by fatty acids (data not shown). The fatty acid SA (C18:0) and the peroxisome proliferators fenofibrate (FF) and clofibrate (CF) and the NSAID/peroxisome proliferator fenoprofen (FP) were unable to potentiate Gal4p-Oaf1pC transactivation. beta gal, β-galactosidase. D, oleic acid could not activate the GAL7 promoter-LacZ reporter in the presence of 10-fold excess of peroxisome proliferators fenofibrate, clofibrate, and fenoprofen, whereas the presence of SA did not significantly affect oleic acid-dependent gene activation. YPE represents YP medium with EtOH (vehicle).
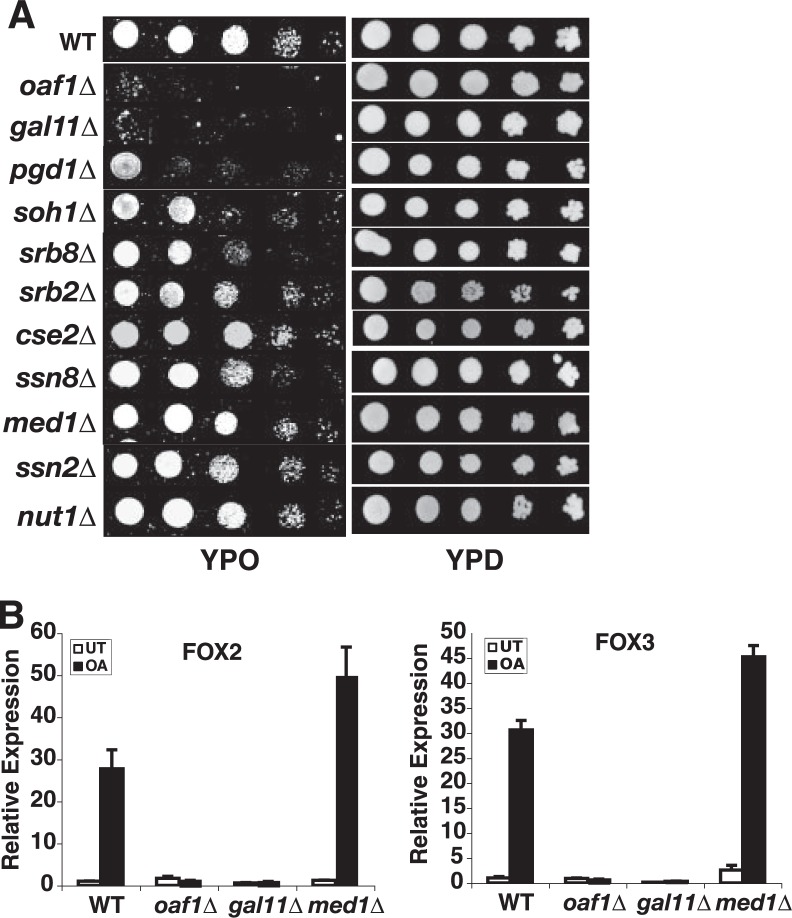
Gal11p/MED15 Is Required for Oaf1p-dependent Transactivation and Yeast Growth on Oleic Acid—The Mediator co-activator complex is conserved from yeast to human and plays a critical role in ligand-dependent gene activation both by members of the fungal zinc cluster transcription factor family and by members of the metazoan nuclear receptor family (6, 15, 16). We have examined here the functional requirement for Mediator subunits in fatty acid-regulated and Oaf1p-dependent activation of peroxisomal and fatty acid β-oxidation genes and in the ability of yeast to utilize fatty acids as the sole carbon source. Although deletion of most Mediator subunits caused little or modest effects on yeast growth on OA plates, a strain deleted for Gal11p/MED15 was deficient for growth on OA as carbon source, similar to the strain harboring a deletion of the gene encoding Oaf1p (Fig. 2A) (10, 13). The PGD1/MED3 deletion strain also exhibited a somewhat reduced capacity to grow on OA (Fig. 2A), consistent with the notion that the Pgd1p/MED3 subunit (also known as Hrs1p) is a part of the Gal11p/MED15 tail module of Mediator (17, 18). None of the deletions affected growth on rich medium with glucose as carbon source (YPD) (Fig. 2A). Our results thus suggest that the activator-targeted Gal11p/MED15 Mediator subunit is required for the ability of yeast to utilize fatty acids as carbon source and that Gal11p/MED15 could play an important role in Oaf1p- and fatty acid-dependent activation of genes in the fatty acid β-oxidation pathway. We therefore examined the requirement for Gal11p/MED15 in fatty acid-dependent transactivation of FOX2 and FOX3 (3), two well characterized Oaf1p target genes encoding peroxisomal enzymes required for fatty acid β-oxidation. Indeed, deletion of the gene encoding Gal11p/MED15 resulted in complete loss of oleic acid-stimulated transcription of both FOX2 and FOX3, similar to results with the OAF1 deletion strain, whereas deletion of MED1 and several other Mediator subunits had no significant effect on the expression of Oaf1p target genes in response to oleic acid (Fig. 2B and data not shown). These results indicate that the Gal11p/MED15 Mediator subunit is specifically and critically involved in Oaf1p- and oleic acid-dependent transactivation of fatty acid β-oxidation enzymes.
FIGURE 2.
The Gal11p/MED15 subunit of the Mediator co-activator is required for Oaf1p-dependent transactivation of peroxisomal genes and growth on oleic acid. A, Gal11p/MED15 is specifically required for growth on oleic acid as a sole carbon source, whereas other Mediator subunits tested were not essential. An OAF1 deletion strain was used as a positive control. Serially diluted cultures were applied to the plates. WT, wild type. B, quantitative real-time RT-PCR analysis reveals a specific requirement for Gal11p/MED15 for oleic acid-dependent activation of the FOX2 and FOX3 genes. By contrast, deletion of the Med1p Mediator subunit had no effect on oleic acid-induced transcription. The OAF1 deletion strain was used as positive control strain. The mean value of three independent experiments is shown, and the error bars represent standard deviation. UT represents vehicle (EtOH)-treated.
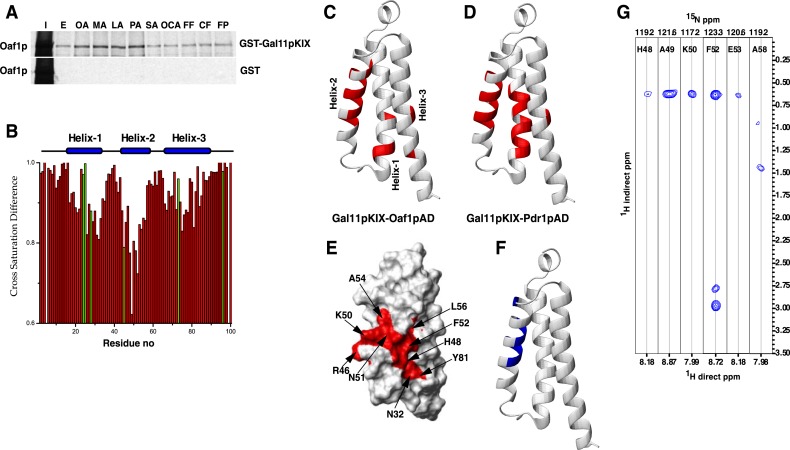
The Oaf1p Activation Domain Interacts with the KIX Domain of Gal11p/MED15—We have recently shown that the N terminus of yeast Gal11p/MED15 harbors a structurally conserved KIX domain that interacts with Pdr1p orthologs in fungi and is required for xenobiotic-dependent gene activation by Pdr1p orthologs (6–8). Based on the functional requirement for Gal11p/MED15 in Oaf1p gene activation, we examined whether the Gal11p/MED15 KIX domain could serve as a target of Oaf1p. Indeed, both full-length Oaf1p and Gal4p-Oaf1pC bound to the Gal11p/MED15 KIX domain fused to GST in an OA-stimulated manner but not to GST alone (Fig. 3A and data not shown). Fatty acids that activate Oaf1p (OA, MA, LA, and PA) all enhance the interaction between the Gal11p/MED15 KIX domain and 35S-labeled, in vitro synthesized, full-length Oaf1p, whereas SA, which does not bind to nor activate Oaf1p, did not stimulate Oaf1p interaction with the Gal11p/MED15 KIX domain (Fig. 3A). Importantly, although they are capable of binding to Oaf1p, peroxisome proliferators/NSAIDs were also unable to enhance the interaction between the Gal11p/MED15 KIX domain and Oaf1p (Fig. 3A), suggesting that their association with Oaf1p does not result in a proper conformational change allowing interaction between Oaf1p and its cognate co-activator. Oaf1p contains a short activation domain in its distal C terminus (14, 19), and we have investigated whether a fragment of Oaf1p containing the activation domain could bind to the Gal11p/MED15 KIX domain. Indeed, we find that the Oaf1p activation domain fragment alone bound effectively to GST-Gal11p/MED15 KIX but not to GST alone (data not shown).
FIGURE 3.
The Gal11p/MED15 KIX domain interacts with Oaf1p in a fatty acid-stimulated manner. A, activating fatty acids (OA, MA, LA, and PA) enhance interaction between Oaf1p and the Gal11p/MED15 KIX domain, whereas non-activating fatty acids SA and oleoyl-CoA (OCA), as well as peroxisome proliferators/NSAIDs, do not significantly affect this interaction. The 35S-labeled in vitro synthesized Oaf1p was subjected to GST pulldown experiments using glutathione-Sepharose beads bound to GST or GST-Gal11p/MED15 KIX proteins in the presence of fatty acids or peroxisome proliferators/NSAIDs as mentioned above. I represents 10% input, and E (for EtOH) represents vehicle control. B, cross saturation transfer difference (ratio of saturated to non-saturated) of a complex of Gal11p/MED15 KIX with Oaf1pAD-9 in the molar ratio 1:1. The resonances of the residues represented in green exhibit significant overlap. Residue no, residue number. C, ribbon representation of the Gal11p/MED15 KIX domain, with the residues that are significantly affected (>15% reduction in intensity) in cross saturation transfer experiments with Oaf1pAD-9 peptide highlighted in red. D, ribbon representation of Gal11p/MED15 KIX highlighting the residues affected in cross saturation transfer experiments with Pdr1pAD-12. E, surface representation of Gal11p/MED15 KIX highlighting some of the residues significantly affected by cross saturation transfer with Oaf1pAD-9. F, ribbon representation of Gal11p/MED15 KIX with residues that have intermolecular NOE cross peaks with Oaf1pAD-9 highlighted in blue. G, strips from the 15N-dispersed NOESY spectrum showing intermolecular to the amide protons in helix 2 of Gal11p/MED15 KIX.
We have recently used NMR spectroscopy to determine the solution structure of the Gal11p/MED15 KIX domain and performed detailed characterization of the Pdr1p-binding site on the KIX domain (6). To further investigate the mechanistic similarities and differences of Oaf1p and Pdr1p, we have initially employed NMR to study the interaction of a 9-amino acid Oaf1p activation domain peptide (Oaf1pAD-9) with the Gal11p/MED15 KIX domain (19). Gal11p/MED15 KIX forms a tight three-helix bundle packing around an extensively hydrophobic core (6). Cross saturation experiments (12), where the resonances of the peptide are selectively saturated and amide protons of the KIX domain are monitored for saturation transfer, show that Oaf1pAD-9 binding occurs at the interface between the three helices with strong interactions with helix 2 (Fig. 3B and supplemental Fig. S1). Direct NOE contacts are observed to the amide protons of residues 48–50, 52, 53, and 58, all of which are located in helix 2 (Fig. 3, F and G and supplemental Fig. S1). The binding mode of Oaf1pAD-9, including extensive contacts with the residues that together constitute the hydrophobic core of Gal11p/MED15 KIX, parallels that of Pdr1p (Fig. 3, C and D, and supplemental Fig. S1) (6). Both activators harbor hydrophobic residues in their C-terminal tails, which yield favorable van der Waals interactions with the Gal11p/MED15 KIX domain.
Together, these findings indicate that the Gal11p/MED15 Mediator subunit is an essential target and transducer of fatty acid-regulated and Oaf1p-dependent transactivation of peroxisomal target genes. Moreover, the KIX domain in Gal11p/MED15 appears to be sufficient for binding to Oaf1p and its activation domain. Furthermore, our findings suggest that binding of peroxisome proliferators cannot relieve intramolecular inhibitory contacts that prevent the Oaf1p activation domain from interacting with the Gal11p/MED15 KIX domain, explaining why these drugs are incapable of effectively inducing transcription of Oaf1p target genes.
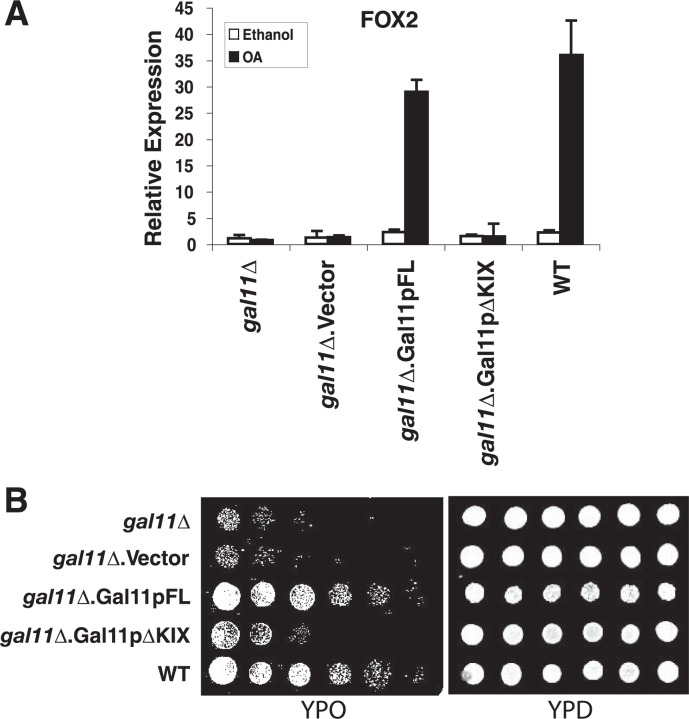
The Gal11p/MED15 KIX Domain Is Required for Oleic Acid-dependent Transactivation by Oaf1p—Following up the observation that the Gal11p/MED15 KIX domain interacts with the Oaf1p activation domain in vitro, we investigated the functional role of Gal11p/MED15 KIX in oleic acid-induced transcription of Oaf1p target genes in vivo. A gal11/med15Δstrain was transformed with plasmids harboring either the full-length wild-type GAL11/MED15 cDNA (Gal11pFL) or a variant of GAL11/MED15 lacking the sequences coding for the KIX domain (Gal11pΔKIX) (6). In yeast cells lacking endogenous Gal11p/MED15, ectopic expression of Gal11p/MED15 deleted for the KIX domain could not rescue oleic acid-induced expression of the Oaf1p target genes FOX2 and FOX3, whereas expression of full-length Gal11p/MED15 complemented Gal11p/MED15 loss, as expected (Fig. 4A and data not shown). To further determine the functional significance of the Gal11p/MED15 KIX domain in fatty acid metabolism in yeast, we cultured the Gal11pFL and Gal11pΔKIX strains on oleic acid as the sole carbon source. Yeast cells expressing KIX-deleted Gal11p/MED15 grew poorly on oleic acid, similar to the gal11/med15Δ parental strain, whereas full-length Gal11p/MED15 was able to fully rescue the gal11/med15Δ growth phenotype on oleic acid (Fig. 4B). These results together indicate that the Oaf1p-binding KIX domain of Gal11p/MED15 is essential for Oaf1p- and oleic acid-dependent transactivation of peroxisomal genes coding for fatty acid β-oxidation enzymes, as well as Oaf1p-dependent growth of yeast cells with oleic acid as a sole carbon source.
FIGURE 4.
The Gal11p/MED15 KIX domain is required for Oaf1p- and ligand-dependent transactivation of peroxisomal genes and growth on oleic acid. A, the KIX domain of Gal11p/MED15 is necessary for the growth of yeast on the medium with oleic acid as a sole carbon source. The gal11/med15-deleted yeast strain was transformed with plasmids harboring either full-length Gal11p/MED15 or Gal11p/MED15 lacking the KIX domain (amino acids 1–100) or vector alone. Wild-type (WT) yeast was used as a positive control. The right panel shows yeast cells grown on normal YPD medium. B, the Gal11p/MED15 KIX domain is required for OA-induced transcription of the Oaf1p target genes FOX2 and FOX3. The yeast strains mentioned in A were used for this experiment. Quantitative real-time RT-PCR was performed in triplicates, and the mean values are shown. Error bars represent standard deviation.
DISCUSSION
Metazoan nuclear receptors represent a large family of ligand-regulated transcription factors that respond to diverse lipophilic ligands, including steroid hormones and dietary derivatives such as fatty acids, vitamin D3, retinoids, as well as xenobiotics (20–22). The evolutionary origin of metazoan nuclear receptors has been obscure, although bioinformatics studies have suggested that the ancestor emerged in an urmetazoan subsequent to the split of animals from fungi and plants (23–25). Recent findings by others and us have suggested that members of the fungal zinc cluster transcription factor family behave in a manner functionally analogous to metazoan nuclear receptors, raising the question of whether these small molecule-regulated transcription factor families are evolutionarily related (4, 6). Indeed, Oaf1p, a zinc cluster family member first discovered as a transcription regulator of peroxisomal and fatty acid β-oxidation genes in response to fatty acids, was shown to directly bind to the fatty acid oleic acid (4, 10, 13, 26); these functional characteristics are remarkably similar to the vertebrate nuclear receptor PPARα, which also regulates β-oxidation genes in response to binding to fatty acids (1). PPARα is additionally activated by synthetic chemicals referred to as peroxisome proliferators, including fibrates, as well as NSAIDs, such as fenoprofen and indomethacin, and several studies have shown that PPARα binds directly to these compounds (1, 2, 22, 27–30). Extending the report by Phelps et al. (4) where they showed direct binding of oleic acid to Oaf1p, we have here demonstrated that Oaf1p also binds directly to several other fatty acids (such as myristic acid, palmitic acid, and lauric acid), as well as peroxisome proliferators and NSAIDs such as clofibrate, fenofibrate, and fenoprofen. These new findings suggest further mechanistic similarities of yeast Oaf1p and vertebrate PPARα. Interestingly, although peroxisome proliferators/NSAIDs can bind to Oaf1p, they are by contrast with fatty acids unable to promote effective Oaf1p-dependent gene activation. Instead, these compounds compete with fatty acids for the same ligand-binding site in Oaf1p and act as potent antagonists of fatty acid-dependent gene activation. Thus, although Oaf1p and PPARα exhibit extensive functional similarities, there are apparent differences in their responses to specific ligands. Ligand binding to nuclear receptors results in conformational alterations that liberate the C-terminal activation domain from intramolecular inhibitory contacts, allowing binding of the activation domain to co-activators (5). We thus speculate that binding of fatty acids, but not peroxisome proliferators/NSAIDs, to Oaf1p may also cause a functionally productive conformational change in Oaf1p. It will be interesting to investigate the resultant structural differences in Oaf1p after binding to different ligands such as fatty acids and peroxisome proliferators/NSAIDs.
Together with the striking functional similarities discussed above and the fact that Oaf1p and PPARα regulate many homologous genes, our findings support the hypothesis that these families of ligand-regulated transactivators may share a common ancestor. Recently, we demonstrated that Pdr1p, another zinc cluster transcription factor, regulates expression of genes constituting the multidrug resistance network in S. cerevisiae and C. glabrata in response to direct binding of diverse xenobiotics, and thus, is functionally analogous to the vertebrate xenobiotic nuclear receptor pregnane X receptor (PXR) (6, 31). These results provide further support for the notion that these fungal and metazoan ligand-regulated transcription factor families might be evolutionarily related. However, despite the compelling structural and functional similarities between Oaf1p and PPARα, we cannot exclude the possibility of convergent evolution. Additional studies of possible functional analogy of other nuclear receptors and yeast zinc cluster family members should further clarify the issue of evolutionary divergence versus convergence of these families of eukaryotic ligand-responsive transcription factors.
Mammalian nuclear receptors, including PPARs, have been found to interact with co-activators containing the LXXLL motif, such as the p160 family and the TRAP220/MED1 Mediator subunit (5, 16, 32). The yeast MED1 subunit, however, does not contain an LXXLL motif and is not required for fatty acid-activated transcription by the yeast PPARα analog Oaf1p or for growth on oleic acid as a sole carbon source. We find that Oaf1p instead functionally requires a distinct Mediator subunit, Gal11p/MED15, and interacts with its conserved KIX domain. Moreover, activating fatty acids such as OA, MA, PA, and LA enhance the interaction between Oaf1p and the Gal11p/MED15 KIX domain, whereas poorly binding fatty acids (e.g. SA) do not. Although peroxisome proliferators and NSAIDs do bind strongly to Oaf1p, they do not stimulate interaction between the Oaf1p activation domain and the Gal11p/MED15 KIX domain, explaining the inability of these compounds to induce transcription of Oaf1p target genes.
The reliance of Oaf1p on the Gal11p/MED15 Mediator subunit for transactivation would seem to indicate that the gene activation mechanism of Oaf1p is dissimilar to that of nuclear receptors. Interestingly, however, a recent study showed that the Gal11p/MED15 homolog in C. elegans (MDT-15) can serve as a target of NHR-49, a C. elegans orphan nuclear receptor that regulates genes involved in fatty acid and glucose homeostasis and that has been suggested to be functionally similar to vertebrate PPARα (supplemental Fig. S2) (9). Moreover, sequences containing the predicted KIX domain in MDT-15 were sufficient for interaction with NHR-49 (9). Hence, the mechanistic similarity of C. elegans NHR-49 and yeast Oaf1p lend further credence to the notion that at least a subset of metazoan nuclear receptors that are functionally related to PPARα may indeed share a common evolutionary origin with Oaf1p (supplemental Fig. S2). Fungal Pdr1p orthologs, which are functionally analogous to metazoan PXR, also require Gal11p/MED15 Mediator subunits and their KIX domains for expression of multidrug resistance genes (6). Moreover, a recent study indicated that MDT-15 might also play a role in detoxification/multidrug resistance in C. elegans (33). These findings together suggest that Gal11p/MED15 orthologs may represent primordial targets and conserved mediators of nuclear receptor-like transcription factors in eukaryotes.
Footnotes
Supplemental material: http://www.jbc.org/content/suppl/2008/12/16/M808263200.DC1.html
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains two supplemental figures and two supplemental tables.
The abbreviations used are: PPAR, peroxisome proliferator-activated receptor; NSAID, non-steroidal anti-inflammatory drug; GST, glutathione S-transferase; CREB, cAMP-response element-binding protein; OA, oleic acid; LA, lauric acid; MA, myristic acid; SA, stearic acid; PA, palmitic acid; NOE, nuclear Overhauser effect; HSQC, heteronuclear single quantum correlation; RT-PCR, reverse transcription-PCR.
REFERENCES
- 1.Desvergne B, Wahli W. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 2.Issemann I, Green S. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 3.Hiltunen JK, Mursula AM, Rottensteiner H, Wierenga RK, Kastaniotis AJ, Gurvitz A. FEMS Microbiol Rev. 2003;27:35–64. doi: 10.1016/S0168-6445(03)00017-2. [DOI] [PubMed] [Google Scholar]
- 4.Phelps C, Gburcik V, Suslova E, Dudek P, Forafonov F, Bot N, MacLean M, Fagan RJ, Picard D. Proc Natl Acad Sci U S A. 2006;103:7077–7081. doi: 10.1073/pnas.0510080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass CK, Rosenfeld MG. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 6.Thakur JK, Arthanari H, Yang F, Pan SJ, Fan X, Breger J, Frueh DP, Gulshan K, Li DK, Mylonakis E, Struhl K, Moye-Rowley WS, Cormack BP, Wagner G, Näär AM. Nature. 2008;452:604–609. doi: 10.1038/nature06836. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan I, Perez-Alvarado GC, Parker D, Dyson HJ, Montminy MR, Wright PE. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Vought BW, Satterlee JS, Walker AK, Jim Sun ZY, Watts JL, DeBeaumont R, Saito RM, Hyberts SG, Yang S, Macol C, Iyer L, Tjian R, van den Heuvel S, Hart AC, Wagner G, Näär AM. Nature. 2006;442:700–704. doi: 10.1038/nature04942. [DOI] [PubMed] [Google Scholar]
- 9.Taubert S, Van Gilst MR, Hansen M, Yamamoto KR. Genes Dev. 2006;20:1137–1149. doi: 10.1101/gad.1395406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rottensteiner H, Kal AJ, Hamilton B, Ruis H, Tabak HF. Eur J Biochem. 1997;247:776–783. doi: 10.1111/j.1432-1033.1997.00776.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferentz AE, Wagner G. Q Rev Biophys. 2000;33:29–65. doi: 10.1017/s0033583500003589. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Nakanishi T, Kami K, Arata Y, Shimada I. Nat Struct Mol Biol. 2000;7:220–223. doi: 10.1038/73331. [DOI] [PubMed] [Google Scholar]
- 13.Karpichev IV, Luo Y, Marians RC, Small GM. Mol Cell Biol. 1997;17:69–80. doi: 10.1128/mcb.17.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumgartner U, Hamilton B, Piskacek M, Ruis H, Rottensteiner H. J Biol Chem. 1999;274:22208–22216. doi: 10.1074/jbc.274.32.22208. [DOI] [PubMed] [Google Scholar]
- 15.Kornberg RD. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Malik S, Roeder RG. Trends Biochem Sci. 2005;30:256–263. doi: 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Björklund S, Gustafsson CM. Trends Biochem Sci. 2005;30:240–244. doi: 10.1016/j.tibs.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Lee YC, Park JM, Min S, Han SJ, Kim YJ. Mol Cell Biol. 1999;19:2967–2976. doi: 10.1128/mcb.19.4.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piskacek S, Gregor M, Nemethova M, Grabner M, Kovarik P, Piskacek M. Genomics. 2007;89:756–768. doi: 10.1016/j.ygeno.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Handschin C, Meyer UA. Arch Biochem Biophys. 2005;433:387–396. doi: 10.1016/j.abb.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kliewer SA, Xu HE, Lambert MH, Willson TM. Recent Prog Horm Res. 2001;56:239–263. doi: 10.1210/rp.56.1.239. [DOI] [PubMed] [Google Scholar]
- 23.Bertrand S, Brunet FG, Escriva H, Parmentier G, Laudet V, Robinson-Rechavi M. Mol Biol Evol. 2004;21:1923–1937. doi: 10.1093/molbev/msh200. [DOI] [PubMed] [Google Scholar]
- 24.Escriva H, Bertrand S, Laudet V. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- 25.Escriva H, Delaunay F, Laudet V. BioEssays. 2000;22:717–727. doi: 10.1002/1521-1878(200008)22:8<717::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Karpichev IV, Small GM. Mol Cell Biol. 1998;18:6560–6570. doi: 10.1128/mcb.18.11.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottlicher M, Widmark E, Li Q, Gustafsson JA. Proc Natl Acad Sci U S A. 1992;89:4653–4657. doi: 10.1073/pnas.89.10.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issemann I, Prince RA, Tugwood JD, Green S. Mol Endocrinol. 1993;11:37–47. doi: 10.1677/jme.0.0110037. [DOI] [PubMed] [Google Scholar]
- 29.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 31.Kliewer SA, Goodwin B, Willson TM. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 32.Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Björklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, Conaway JW, Conaway RC, Emmons SW, Fondell JD, Freedman LP, Fukasawa T, Gustafsson CM, Han M, He X, Herman PK, Hinnebusch AG, Holmberg S, Holstege FC, Jaehning JA, Kim YJ, Kuras L, Leutz A, Lis JT, Meisterernest M, Näär AM, Nasmyth K, Parvin JD, Ptashne M, Reinberg D, Ronne H, Sadowski I, Sakurai H, Sipiczki M, Sternberg PW, Stillman DJ, Strich R, Struhl K, Svejstrup JQ, Tuck S, Winston F, Roeder RG, Kornberg RD. Mol. Cell. 2004;14:553–557. doi: 10.1016/j.molcel.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Taubert S, Hansen M, Van Gilst MR, Cooper SB, Yamamoto KR. PLoS Genet. 2008;4:e1000021. doi: 10.1371/journal.pgen.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]