Abstract
The C termini of β-tubulin isotypes are regions of high sequence variability that bind to microtubule-associated proteins and motors and undergo various post-translational modifications such as polyglutamylation and polyglycylation. Crystallographic analyses have been unsuccessful in resolving tubulin C termini. Here, we used a stepwise approach to study the role of this region in microtubule assembly. We generated a series of truncation mutants of human βI and βIII tubulin. Transient transfection of HeLa cells with the mutants shows that mutants with deletions of up to 22 residues from βIII and 16 from βI can assemble normally. Interestingly, removal of the next residue (Ala428) results in a complete loss of microtubule formation without affecting dimer formation. C-terminal tail switching of human βI and βIII tubulin suggests that C-terminal tails are functionally equivalent. In short, residues outside of 1–429 of human β-tubulins make no contribution to microtubule assembly. Ala428, in the C-terminal sequence motif N-QQYQDA428, lies at the end of helix H12 of β-tubulin. We hypothesize that this residue is important for maintaining helix H12 structure. Deletion of Ala428 may lead to unwinding of helix H12, resulting in tubulin dimers incapable of assembly. Thr429 plays a more complex role. In the βI isotype of tubulin, Thr429 is not at all necessary for assembly; however, in the βIII isotype, its presence strongly favors assembly. This result is consistent with a likely more complex function of βIII as well as with the observation that evolutionary conservation is total for Ala428 and frequent for Thr429.
Microtubules are involved in a great variety of cellular functions. Their constituent protein tubulin is an αβ heterodimer, both α- and β-tubulin existing as multiple isotypes, encoded by different genes and differing in amino acid sequence (1). The differences among the isotypes are highly conserved in evolution. In mammals, the β isotypes are βIa, βIb, βII, βIII, βIVa, βIVb, βV, and βVI. There is evidence that the isotype differences have functional significance. For instance, the βIV isotype is found in all axonemes (2).
Structurally, both α- and β-tubulin consist of a globular region of 427 amino acids followed by a C-terminal region of 17–24 amino acids (3–5). The C-terminal region is highly negatively charged, being especially rich in glutamate residues and lacking in basic residues, and is likely to project outward from the rest of the molecule, because of its high negative charge and the electrostatic repulsion among the glutamate residues (3). The three-dimensional structure of the globular domain has been determined by electron and x-ray crystallography (4, 5). However, the C-terminal region has never been localized in the three-dimensional reconstructions except by computer modeling. The probable reasons for this are 1) that, if the C-terminal region projects out from the rest of molecule, it is likely to be very flexible with respect to the rest of the molecule and 2) the C-terminal region undergoes post-translational modification. Both of these can lead to structural heterogeneity and cause the C terminus to be invisible to crystallographic techniques.
In this work, we examine the role of the C termini of human β-tubulins to determine the minimal sequence requirement for microtubule incorporation through structure/function analyses. The human βI and βIII tubulin isotypes were utilized based on their high degree of sequence variability clustered at the C terminus (Table 1 and Fig. 1) and the fact that βI is broadly distributed among normal tissues, whereas βIII has a very narrow tissue distribution. These two isotypes share 92% sequence identity, with differences among these isotypes occurring in both the globular domain and the C-terminal region (1).
TABLE 1.
The C-terminal amino acid sequences of the human β-tubulin isotypes
| Human β-tubulin isotype | C-terminal tail sequence |
|---|---|
| βIa | QQYQDATAEEEEDFGEEAEEEA |
| βIb | QQYQDATAEEEEDFGEEAEEEA |
| βII | QQYQDATADEQGEFEEEEGEDEA |
| βIII | QQYQDATAEEEGEMYEDDEEESEAQGPK |
| βIVa | QQYQDATAEQGEFEEEAEEEVA |
| βIVb | QQYQDATAEEEGEFEEEAEEEVA |
| βV | QQYQDATANDGEEAFEDEEEEIDG |
| βVI | QQFQDAKAVLEEDEEVTEEAEMEPEDKGH |
| βVII | QQYQDATAEGEGV |
FIGURE 1.
Sequence alignment of human βIa and βIII tubulin isotypes. Human βIa and βIII tubulin isotypes were aligned with ClustalX 1.83 and processed with BioEdit. Hyphens denote identical residues between sequences.
Three attributes of potential functional significance have been assigned to the C-terminal regions of tubulin. First, the fact that it projects outward makes it likely that it can serve as a signal. For example, elegant experiments by Popodi et al. (6), working with β-tubulin isotypes from Drosophila, indicate that the C terminus is the region that determines which isotype goes into axonemal microtubules. In Tetrahymena thermophila, Duan and Gorovsky (7) demonstrated that α- and β-tubulin C-terminal tails (CTT)2 are interchangeable, and their functions are indistinguishable. In addition, a duplicated β-tubulin CTT rescued the lethal mutant lacking post-translational modification sites on β-tubulin but did not rescue the mutant lacking a 17-amino acid deletion from the β-tubulin tail (7). A significant amount of research on C-terminal tail function has utilized proteolytic digestion with a number of different endoproteinases such as subtilisin, proteinase K, and chymotrypsin among others (8–10). For example, subtilisin-digested αsβs-tubulin was found to have a higher capacity for generating microtubules than undigested (9). A single drawback to using these proteases is their site-specific nature, which limits us to distinct digestion sites in proteolysis experiments. Furthermore, the proteolyzed tail fragment could still interact with the globular body without being really separated. Thus, to elucidate the importance of amino acids flanking these digestion sites, alternative approaches must be utilized.
Second, MAPs and motor proteins such as MAP2, MAP4, tau, DMAP-85, OP18/stathmin, dynein, and kinesin have been shown to bind the C-terminal region (11–22). These proteins are known to play very important roles in cellular processes including intracellular transport and modulation of microtubule dynamics. Third, the C terminus is subject to a large number of post-translational modifications, some of which are known to have functional significance (1). These include phosphorylation (β) (23–25), poly-glutamylation (α, β) (26–30), polyglycylation (α, β) (31–34), detyrosination (α) (35–37), and deglutamylation (α) (38).
In this paper we present evidence for a fourth function for the C-terminal region, namely, that it plays a major role in controlling the conformation of the globular region of the tubulin molecule such that microtubules can form. We have found that all of the amino acid residues necessary for assembly of the βI isotype of tubulin are contained within the first 428 amino acids, ending in N-QQYQDA428; C-terminal truncations lacking Ala428 yield tubulins that are not compatible with microtubule formation. We demonstrate that the C-terminal region does not contribute to intradimer formation. Furthermore, we find that β-tubulin C-terminal tail switching does not affect incorporation and that the presence of the full chimeric tail is not necessary for functional microtubules. Finally, we have observed that residue Thr429 plays an important but not critical role in the βIII isotype becoming assembly-competent but is not at all necessary for the βI isotype to form microtubules.
EXPERIMENTAL PROCEDURES
Materials—HeLa human cervical carcinoma cells were purchased from the American Type Culture Collection (Manassas, VA). Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Dulbecco’s modified Eagle’s medium, Hanks’ balanced salt solution, trypsin-EDTA, TOPO cloning kit, pcDNA3.1/V5-His-TOPO vector, anti-V5 antibody (Mo), anti-glyceraldehyde-3-phosphate dehydrogenase (Mo), and 4′,6-diamidino-2-phenylindole were purchased from Invitrogen. Penicillin-streptomycin-fungisone antibiotics were from Gemini Bio-Products (West Sacramento, CA). Vectashield mounting medium was from Vector Laboratories (Burlingame, CA). G-418 sulfate was purchased from American Bioanalytical (Natick, MA). The Super Signal kit was from Pierce. Normal goat serum, Cy3-conjugated goat anti-mouse antibody, horse-radish peroxidase-conjugated anti-mouse antibody were purchased from Jackson Immunoresearch (West Grove, PA). FuGENE HD transfection reagent was from Roche Applied Science. Taq PCR Master Mix kit was purchased from Qiagen.
Molecular Constructs—Polymerase chain reaction was used to create chimeras and C-terminal truncations for human βI and βIII tubulin (GenBank™ accession numbers AF427491 and NM_178014, respectively) (Table 2) with the appropriate forward and reverse primers at final concentrations of 1 μm each (Table 3). Amino acid deletions made to the βIII C-terminal end were the following: Δ5, Δ10, Δ20, Δ21, Δ22, Δ23, Δ24, Δ25, and Δ30. Wild type βIII tubulin was also constructed as a positive control. For βI tubulin, C-terminal deletions resulted in Δ16 and Δ17 constructs. Taq PCR Master Mix kit and cycling parameters were performed according to Qiagen. Two rounds of PCR were required to produce βIII+CβI tubulin consisting of the first 433 amino acids from βIII tubulin and the last 11 amino acids from βI tubulin. The first round produced βIII+CβI CΔ5, which contained a five-amino acid deletion from the βI C-terminal tail, whereas the second round generated the full-length βI tail (Table 2). The reciprocal chimera βI+CβIII, containing the first 433 amino acids from βI tubulin and the last 17 amino acids from βIII tubulin, required four successive PCRs that generated βI+CβIII CΔ13, βI+CβIII CΔ9, βI+CβIII CΔ5 lacking the last 13, 9, and 5 amino acids, respectively, from the βIII tubulin tail (Table 2). The last of four rounds of PCR yielded the full-length chimera (Table 2). After each round of PCR, the product was diluted to final concentrations of 1:100 and 1:1000 dilutions prior to subsequent PCR. All of the constructs were TA cloned into pcDNA3.1/V5-His-TOPO and tagged with a C-terminal V5-His tag according to the manufacturer’s protocol (Invitrogen). Additionally, the βI+CβIII chimera was cloned into pcDNA3.1/V5-His-TOPO lacking the V5-His tag (Table 2).
TABLE 2.
Truncations and chimeras of human βI and βIII tubulin
The sequences of the desired C-terminal amino acid deletions are shown from Phe408. All of the β-tubulin constructs were cloned into the mammalian expression vector pcDNA3.1/V5-His-TOPO and tagged with a C-terminal V5-His. The assembly-critical Ala428 is shown in bold. Nine different C-terminal deletions were made, including 5, 10, 20, 21, 22, 23, 24, 25, and 30 amino acids denoted as βIII CΔ5, βIII CΔ10, βIII CΔ20, βIII CΔ21, βIII CΔ22, βIII CΔ23, βIII CΔ24, βIII CΔ25, and βIII CΔ30, from the βIII tubulin C terminus. For the βI tubulin isotype, deletions of 16 and 17 amino acids were made to the C terminus indicated as βI CΔ16 and βI CΔ17, respectively, in the table. The underlined regions represent the sequences from human βI tubulin. Five different chimeras were created from the point where variation begins in C-terminal tails at amino acid residue 434 for βI and βIII tubulin. βIII+CβI FL (full length) represents the first 433 amino acid residues from βIII tubulin with the last 11 residues from βI tubulin. FL denotes a full-length βI tubulin tail without any deletion. βI+CβIII FL (full length) represents the first 433 amino acids from βI tubulin with the last 17 amino acids from βIII tubulin generating a chimera with a full length βIII tail. βI+Cβ III CΔ5, βI+CβIII CΔ9, and βI+CβIII CΔ13 were created with the first 433 amino acids from βI tubulin with 5, 9, and 13 residues removed from the βIII C-terminal tail as indicated.
| Human β-tubulin constructs | C-terminal amino acid sequences |
|---|---|
| βIII wild type | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDDEEESEAQGPK |
| βIII CΔ5 | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDDEEESE |
| βIII CΔ10 | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDD |
| βIII CΔ20 | FTEAESNMNDLVSEYQQYQDATA |
| βIII CΔ21 | FTEAESNMNDLVSEYQQYQDAT |
| βIII CΔ22 | FTEAESNMNDLVSEYQQYQDA |
| βIII CΔ23 | FTEAESNMNDLVSEYQQYQD |
| βIII CΔ24 | FTEAESNMNDLVSEYQQYQ |
| βIII CΔ25 | FTEAESNMNDLVSEYQQY |
| βIII CΔ30 | FTEAESNMNDLVS |
| βI wild type | FTEAESNMNDLVSEYQQYQDATAEEEEDFGEEAEEEA |
| βI CΔ16 | FTEAESNMNDLVSEYQQYQDA |
| βI CΔ17 | FTEAESNMNDLVSEYQQYQD |
| βIII+CβI FL (full length) | FTEAESNMNDLVSEYQQYQDATAEEEEDFGEEAEEEA |
| βI+CβIII FL (full length) | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDDEEESEAQGPK |
| βI+CβIII CΔ5 | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDDEEESE |
| βI+CβIII CΔ9 | FTEAESNMNDLVSEYQQYQDATAEEEGEMYEDDE |
| βI+CβIII CΔ13 | FTEAESNMNDLVSEYQQYQDATAEEEGEMY |
TABLE 3.
Sequences of primer sets used to generate chimeras and deletions of βI and βIII tubulin
In this table, the human isotype is shown in the left column. The column labeled C-terminal truncation represents the number of residues truncated from the C terminus of the respective isotype (CΔ5 represents a 5-amino acid deletion from the C terminus). Forward and reverse primers are given in separate columns labeled Forward primer and Reverse primer as indicated. For βIII+CβI full length, the first round of PCR yielded a truncated tail due to a mixture of different primer lengths. A second reverse primer was constructed to complete the full-length βI tail for the βIII+CβI full length chimera.
| Human β-tubulin isotype | C-terminal truncation | Forward primer | Reverse primer |
|---|---|---|---|
| βIII | Wild type | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-CTTGGGGCCCTGGGCCTCC-3′ |
| βIII | CΔ5 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-CTCCGACTCCTCCTCGTCGTC-3′ |
| βIII | CΔ10 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GTCGTCTTCGTACATCTCGCCC-3′ |
| βIII | CΔ20 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GGCCGTGGCGTCCTGG-3′ |
| βIII | CΔ21 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-CGTGGCGTCCTGGTACTGCT-3′ |
| βIII | CΔ22 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GGCGTCCTGGTACTGCTGGTA-3′ |
| βIII | CΔ23 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GTCCTGGTACTGCTGGTACTCGG-3′ |
| βIII | CΔ24 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-CTGGTACTGCTGGTACTCGGACA-3′ |
| βIII | CΔ25 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GTACTGCTGGTACTCGGACACCAG-3′ |
| βIII | CΔ30 | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GGACACCAGGTCGTTCATGTTG-3′ |
| βI | Wild type | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-GGCCTCCTCTTCGGCCTCCTCACCGAAATCCTCCTC-3′ |
| βI | CΔ16 | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-GGCATCCTGGTACTGCTGATACTC-3′ |
| βI | CΔ17 | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-ATCCTGGTACTGCTGATACTCAGAGA-3′ |
| βIII+CβIa | Full length | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-GGCCTCCTCTTCGGCCTCCTCACCGAAATCCTCCTCTTCCTCGGCCGTGGC-3′ |
| βIII+CβIb | Full length | 5′-ATGCGGGAGATCGTGCACATC-3′ | 5′-CATCTCGCCCTCCTCTTCTGCGGTGGC-3′ |
| βI+CβIII | CΔ13 | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-GTACATCTCGCCCTCCTCTTCTGCGGTGGC-3′ |
| βI+CβIII | CΔ9 | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-CTCGTCGTCTTCGTACATCTCGCCCTCCTC-3′ |
| βI+CβIII | CΔ5 | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-CTCCGACTCCTCCTCGTCGTCTTCGTACAT-3′ |
| βI+CβIII | Full length | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-CTTGGGGCCCTGGGCCTCCGACTCCTCCTCGTC-3′ |
| βI+CβIII (no tag)c | Full length | 5′-ATGAGGGAAATCGTGCACATC-3′ | 5′-TCACTTGGGGCCCTGGGCC-3′ |
Primer set used in first round of PCR for βIII+CβI full length.
Primer set used in second round of PCR to obtain βIII+CβI full length.
βI+CβIII (no tag) is a chimera generated without the V5-His tag.
Cell Culture and Transfection—HeLa cervical carcinoma cells were cultured at 37 °C and 5% CO2 in HeLa growth medium (Dulbecco’s modified Eagle’s medium, 10% heat-inactivated fetal bovine serum, 1% penicillin-streptomycin-fungisone antibiotics). Transfection of wild type or C-terminal truncated mutants of βI and βIII tubulin into HeLa cells were performed in six-well plates with glass coverslips containing 80–90% confluent HeLa cultures with Dulbecco’s modified Eagle’s medium and 10% heat-inactivated fetal bovine serum lacking penicillin-streptomycin-fungisone antibiotics according to the manufacturer’s protocol (Roche Applied Science) using 7 μl of FuGENE HD to 2 μg of DNA. Twenty-four hours post-transfection, the transfection medium was replaced with fresh HeLa growth medium containing 0.5 mg/ml G-418 sulfate to maintain stable, mass-transfected cultures.
Sequence Alignment—Sequence alignment was performed using ClustalX 1.83 and BioEdit software (39, 40). The human β-tubulin sequences to be aligned were βI (GenBank™ accession number NM_178014) and βIII (GenBank™ accession number AF427491) retrieved from GenBank™. In the sequence alignment, hyphens represents identical residues between sequences (Fig. 1).
Immunofluorescence Microscopy—All of the cells were grown on glass coverslips at 37 °C and 5% CO2. The cells were washed once with Hanks’ balanced salt solution, fixed for 15 min with 3.7% paraformaldehyde at room temperature, and permeabilized for 1 min with 0.5% Triton X-100 in PBS (0.15 m NaCl, 0.0027 m KCl, 0.00147 m KH2PO4, 0.01 M Na2HPO4, pH 7.2). The cells were then incubated overnight at 4 °C with the anti-V5 antibody (1:1000) diluted in PBS containing 10% normal goat serum. For HeLa cells transfected with βI+CβIII (without V5-His tag), the cells were stained with the monoclonal antibody to βIII tubulin (SDL.3D10) (1:500) diluted in PBS containing 10% normal goat serum. The cells were rinsed in PBS and labeled with Cy3-conjugated goat anti-mouse antibody (1:100) for 1 h at room temperature. The cells were then rinsed three times with PBS. For DNA detection, the cells were stained with 4′,6-diamidino-2-phenylindole (7.15 μm in PBS) for 5 min at room temperature followed by three washes with PBS. The coverslips were mounted on glass slides with Vectashield mounting medium and examined with a Olympus BX60 epifluorescence microscope coupled to a Spot camera (model 1.4.0 from Diagnostic Instruments, Inc.) using an Olympus PlanApo 60× oil immersion objective. For each image, a single cell was chosen for 2× digital expansion (120× total magnification) to view cortical microtubules. All of the images were processed using Adobe Photoshop 7.0.
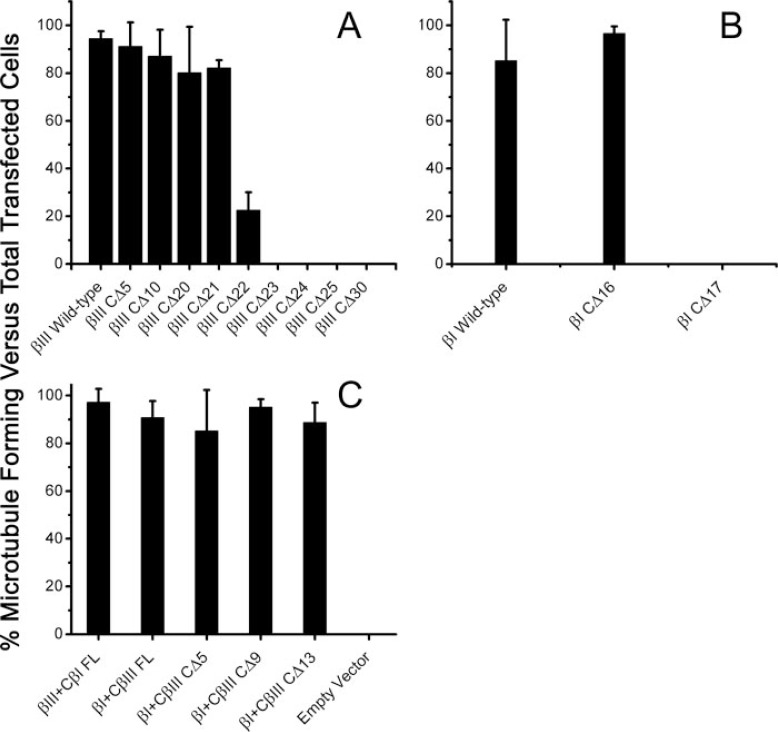
Quantitation of Microtubule-forming Cells—HeLa cells transfected with wild type, C-terminal truncated mutants, and chimeras of βI and βIII were subjected to analysis and quantitation of microtubule formation in four random fields by indirect immunofluorescence microscopy as described under “Experimental Procedures.” In each field, the transfected, microtubule-forming cells were counted and compared with the total number of transfected cells (both microtubule-forming and non-microtubule-forming cells) expressed as a percentage. For each cell line, the fields were averaged, and standard deviations are given (see Fig. 5).
FIGURE 5.
Quantitative analysis of HeLa cells transfected with βI, βIII, and chimeric β-tubulin constructs were calculated with respect to microtubule-forming versus non-microtubule-forming. Immunofluorescence of transiently transfected HeLa cells were visualized at 60× magnification and quantitated based on the ability of the ability of each transfected construct to enter microtubules. βIII tubulin truncations (A), βI tubulin truncations (B), and chimeras (C) were counted and expressed as percentages of microtubule-forming versus non-microtubule-forming cells. For each construct, four random fields were averaged with standard deviation. Notice that βIII CΔ23 and βI CΔ17 (A and B, respectively) do not form any microtubules. In addition, all chimeras, including truncated chimeras βI+CβIII CΔ5, βI+CβIII CΔ9, and βI+CβIII CΔ13, formed microtubules robustly (C). Empty vector did not form microtubules (C).
Co-immunoprecipitation of C-terminally Truncated βI and βIII Tubulin—Extracts from transfected HeLa cells were prepared with cell lysis buffer (50 mm Tris-HCl, pH 7.8, 150 mm NaCl, 1% Nonidet P-40, 1% protease inhibitor mixture, 2 mm phenylmethylsulfonyl fluoride) according to Banerjee (41). Supernatant was cleared with 200 μl of protein G-agarose beads (50% slurry) for 1 h at 4 °C. Cleared supernatant was subsequently incubated with 3 μl of anti-V5 antibody overnight at 4 °C. The immunoprecipitated complex was captured with 50 μl of protein G-agarose (50% slurry) at 4 °C for 1 h and washed twice in cell lysis buffer. The immunocomplex was resuspended in SDS-PAGE sample buffer and heated at 85 °C for 10 min. Precipitated protein was subjected to SDS-PAGE and Western analysis similar to that of Banerjee (42) utilizing anti-α-tubulin (1:5000) antibody and horseradish peroxidase-conjugated anti-mouse (1:1000) to probe the nitrocellulose membrane.
Western Blot Analysis—Cell extract preparation was done as described by Banerjee (41), followed by measurement of total protein concentration as described by Lowry et al. (43). Approximately 50 μg of total cellular protein from HeLa cells expressing βI constructs and 30 μg of total cellular protein from HeLa cells expressing βIII or chimeric β-tubulin constructs was loaded into a SDS-PAGE gel (8–16% linear gradient) and run in a manner similar to that of Banerjee (41). Immunoblotting was performed by methods described by Banerjee (42). The blots were incubated with anti-V5 antibody (1:5000). The secondary horseradish peroxidase-conjugated anti-mouse antibody (1:5000) was utilized with the Pierce Super Signal Kit to analyze expression of the transfected cell lines through chemilluminescence. The blots were reprobed with anti-glyceraldehyde-3-phosphate dehydrogenase as a loading control.
RESULTS
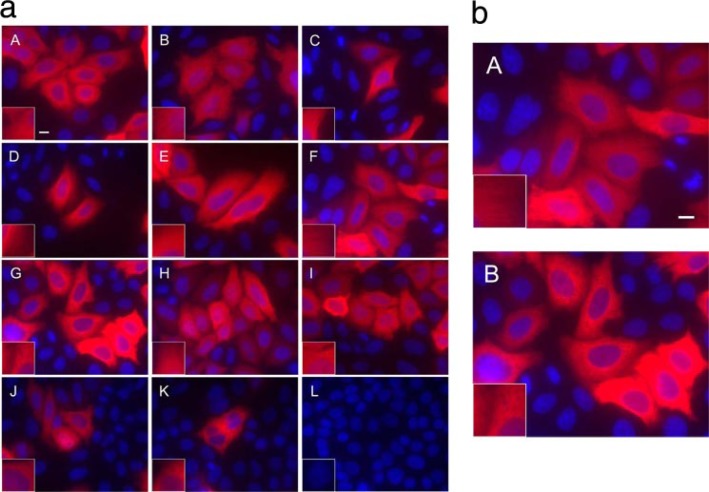
In Vivo Analysis of C-terminal Truncations of Human βI and βIII Tubulin—Deletions were made to human βI and βIII tubulin at the C-terminal end to identify the minimum sequence and number of residues required for microtubule assembly in vivo. We first addressed the question of whether it was preferable to add a reporter group to the N-terminal or the C-terminal end of βIII-tubulin. Preliminary experiments showed that constructs of wild type βIII tubulin tagged with an N-terminal green fluorescent protein or hemagglutinin were incapable of forming microtubules as visualized by immunofluorescence (data not shown). The fact that both large and small N-terminal tags (green fluorescent protein and hemagglutinin, respectively) interfered with wild type βIII incorporation is not surprising, considering the amount of conservation in this region. Across all species, there is 52 and 40% conservation in intra- and inter-dimer interface residues (44). Introducing bulky groups to this region could very well interrupt the loop T1 hydrogen-bonding interactions with the adjacent nucleotide at the interdimer interface. As a result of these experiments, we decided to add the reporter group to the C terminus of β-tubulin; constructs were created with a C-terminal V5-His tag to distinguish the transfected mutant β-tubulins from endogenous β-tubulins. Nine C-terminal truncations of varying length were made to βIII tubulin and transfected into HeLa cervical carcinoma cells along with βIII wild type as a positive control (Table 2). Of the ten, only βIII wild type, βIII CΔ5, βIII CΔ10, βIII CΔ20, βIII CΔ21, and βIII CΔ22 were incorporated into interphase microtubules as viewed by immunofluorescence (Fig. 2a). The other constructs, namely βIII CΔ23, βIII CΔ24, βIII CΔ25, and βIII CΔ30, were not found in interphase microtubules after transfection (Fig. 2a). The inflection point demonstrating forming and non-microtubule-forming constructs was surprisingly abrupt, with the βIII CΔ22 construct showing significantly less incorporation into microtubules (Fig. 2b), whereas the βIII CΔ23 (Fig. 2b) and shorter constructs showed no incorporation (Fig. 2a). Constructs of βIII that did not incorporate into microtubules appeared to form aggregates. These transfected cells lacked any form of microtubule architecture, and the truncated proteins were evenly distributed throughout the cell.
FIGURE 2.
Microtubule incorporation of βIII tubulin is abrogated with C-terminal tail truncations larger than 22-amino acids. Panel a, HeLa cells were transfected with βIII wild type (A), βIII CΔ5 (B), βIII CΔ10 (C), βIII CΔ20 (D), βIII CΔ21 (E), βIII CΔ22 (F), βIII CΔ23 (G), βIII CΔ24 (H), βIII CΔ25 (I), and βIII CΔ30 (J). The positive control was expressing V5-His tagged β-galactosidase (K), whereas the negative control was of nontransfected HeLa cells (L). Twenty-four hours post-transfection, the cells were fixed and labeled with an antibody to V5 tag followed by Cy3-conjugated goat anti-mouse and visualized at 60× magnification. Microtubules are shown in red, and nuclei are stained blue. Insets, visualization of cortical microtubules after 2× digital expansion. Panel b, inflection point of microtubule and non-microtubule-forming cells (βIII CΔ22 (A) and βIII CΔ23 (B), respectively) from transfected HeLa culture. Bar, 10 μm.
As a comparison with the βIII constructs, we also examined the βI isotype. Various constructs of βI were created and transfected into HeLa cells. Similar results were obtained as with βIII, except that it took much shorter truncations to produce similar results. However, because βIII has a longer C terminus than does than βI, this is not a surprising result. The transition between being able to form microtubules and not being able to form microtubules occurred at the corresponding point in the sequence for both βIII and βI. Clearly, all the residues required for microtubule assembly are contained within the first 428 amino acids in both βI and βIII. Interestingly, the transition between being able to incorporate into microtubules and not being able to do so was even more abrupt than was the case with βIII, with βI CΔ16 incorporating normally and βI CΔ17 not at all (Fig. 3). As was the case with βIII, the construct of βI that was unable to incorporate into microtubules appeared to form an aggregate instead (Fig. 3). Analysis through sequence alignment indicated that the first 428 amino acids of human βI and βIII tubulin, ending with the amino acid sequence N-QQYQDA428, contained the residues required for proper insertion into microtubules (Fig. 1). Digital expansion of the cortical region of microtubules clearly confirms that deletions up to βI CΔ16 and βIII CΔ22 are assembly-competent, whereas deletions beyond are not (Figs. 2 and 3).
FIGURE 3.
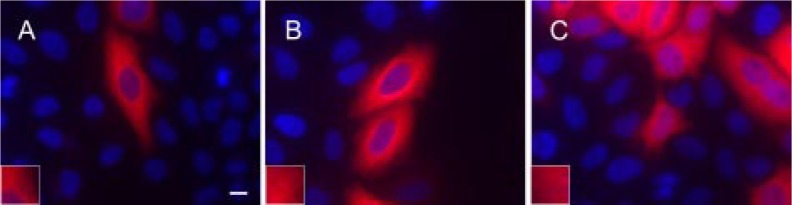
Microtubule incorporation of βI tubulin is abrogated with C-terminal tail truncations larger than 16 amino acids. Human βI wild type (A), βI CΔ16 (B), and βI CΔ17 (C) were transfected in HeLa cells. Twenty-four hours post-transfection, the cells were fixed and labeled with an antibody to V5 tag followed by Cy3-conjugated goat anti-mouse and visualized at 60× magnification. Insets, visualization of cortical microtubules after 2× digital expansion. Notice that none of the cells transfected with βI CΔ17 (C) were able to form microtubules. Bar, 10 μm.
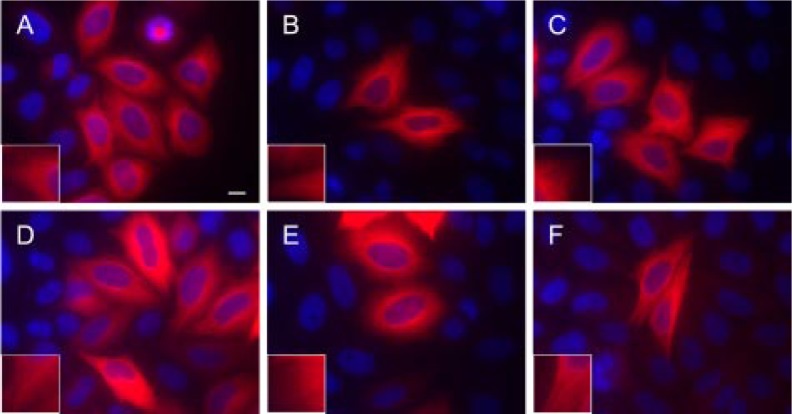
Chimeras of βI and βIII Tubulin Do Not Require the Opposing, Full-length, C-terminal Tail for Microtubule Incorporation in Vivo—The previous results indicated that residues from 429 on, in βI- or βIII-tubulin were not required for proper assembly into microtubules, but they did not address the question of whether altering the amino acid sequence of the C-terminal region could interfere with microtubule assembly. To address this point, chimeras of human βI and βIII tubulin were constructed and transfected into HeLa cells to see whether combining the region 1–433 of one isotype with the C-terminal region of another would affect incorporation into microtubules (Table 2). CTT switching resulted in functional microtubules (Fig. 4). The six variations of chimeras created were βIII+CβI FL (full length), βI+CβIII FL1, βI+CβIII CΔ5, βI+CβIII CΔ9, and βI+CβIII CΔ13 (Table 2). The first of these consists of amino acids 1–433 of βIII with the full-length C-terminal region of βI (the last 11 amino acids of βI). The second is the converse construct: 1–433 of βI with the full-length C-terminal region (last 17 amino acids) of βIII. The other constructs consist of βI with variously truncated segments of the C terminus of βIII. Our immunofluorescence results show that βIII+CβI FL and βI+CβIII FL are capable of entering microtubules (Fig. 4). Additionally, βI+CβIII CΔ5, βI+CβIII CΔ9, and βI+CβIII CΔ13, representing five-, nine-, and thirteen-amino acid deletions from the βIII C-terminal tail, showed no significant difference from the full-length chimera (βI+CβIII FL) (Fig. 4). As a control, a chimera of βI+CβIII FL was created without a V5-His tag to show that the tag does not contribute or interfere with the ability to form microtubules (Fig. 4). We conclude that CTT switching between human βI and βIII, after the first 433 amino acids, is not deleterious to microtubule formation.
FIGURE 4.
Chimeras of βI and βIII tubulin do not require the opposing, full-length, C-terminal tail for microtubule incorporation. Chimeras of human βI and βIII tubulin were constructed and transfected to show that beyond Ala428 (N-QQYQDA428), the C-terminal tails are interchangeable and do not abolish microtubule formation. Immunofluorescence of HeLa cells transfected with βIII+CβI FL (A), βI+CβIII FL (B), βI+CβIII CΔ5 (C), βI+CβIII CΔ9 (D), and βI+CβIII CΔ13 (E) indicates that all chimeric constructs enter microtubules. A chimera, βI+CβIII FL (F), was created lacking the V5 tag to show that the tag does not interfere with microtubule formation. The cells in A–E were stained with antibody to the V5 tag; the cells in F were stained with the antibody SDL.3D10 to βIII. Twenty-four hours post-transfection, the cells were fixed and labeled with an antibody to V5 tag followed by Cy3-conjugated goat anti-mouse and visualized at 60× magnification. Insets, visualization of cortical microtubules after 2× digital expansion. Microtubules are shown in red, and nuclei are shown in blue. Notice that the full-length, chimeric tail is not required for microtubule formation (C–E). Bar, 10 μm.
The βIII Tubulin Isotype Is Less Resistant to C-terminal Deletion than βI Tubulin—Quantitation of microtubule-forming cells as a percentage of the total transfected population was performed as described under “Experimental Procedures.” These data suggested that C-terminal deletions up to 21 amino acids were very well tolerated with 94.5 ± 3.1, 91.2 ± 10.1, 87.1 ± 11.0, 80.2 ± 19.2, and 82.2 ± 3.2% of transfected HeLa cells incorporating βIII wild type, CΔ5, CΔ10, CΔ15, CΔ20, and CΔ21, respectively, into microtubules (Fig. 5A). A 22-amino acid truncation of the βIII C terminus was incorporated into microtubules in only 22.6 ± 7.5% of the transfected cells (Fig. 5A). The transition from microtubule-forming to non-microtubule-forming was abrupt and absolute, occurring with the removal of the last 23 amino acids from βIII. Although the βI inflection point was the same, ending with the sequence N-QQYQDA428, quantitation between the microtubule forming (βI CΔ16) versus non-microtubule-forming (βI CΔ17) construct was even more abrupt (96.6 ± 2.9 and 0%, respectively) than was the case with βIII (Fig. 5B). This demonstrated that the extent of incorporation of different truncated forms varies greatly between βI and βIII tubulin. Specifically, the presence of Thr429 strongly promotes microtubule formation in βIII but makes absolutely no difference to βI. For both βI and βIII, deletion of the critical Ala428 completely abolished microtubules for both isotypes as expected. In addition, all of the chimeras, full-length or otherwise, assembled into microtubules as expected with the following transfects: βIII+CβI FL, βI+CβIII FL, βI+CβIII CΔ5, βI+CβIII CΔ9, and βI+CβIII CΔ13 having 97.2 ± 5.6, 90.8 ± 6.9, 85.2 ± 17.2, 95.2 ± 3.3, and 88.8 ± 8.3% of its cells making microtubules (Fig. 5C).
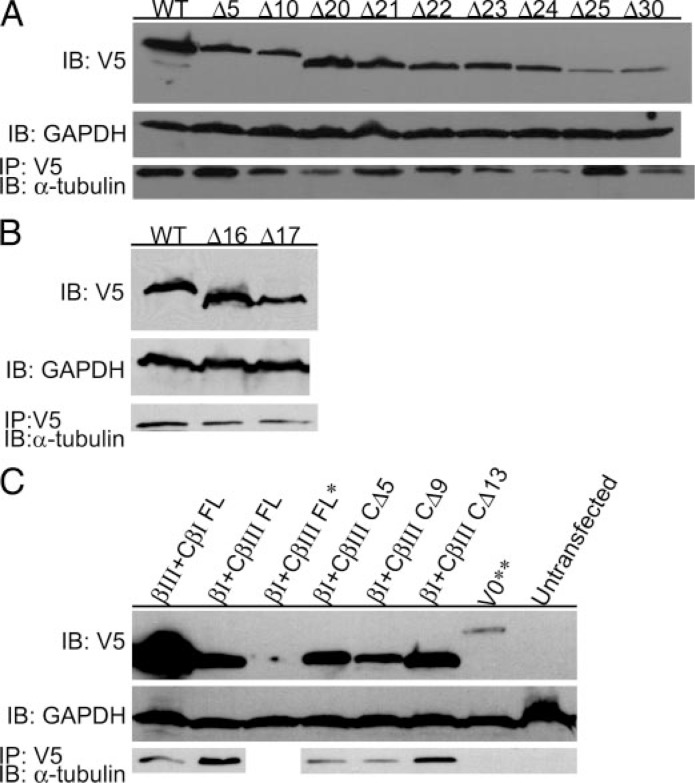
Deletion of the C-terminal Tail Does Not Affect Dimerization—The results described above indicate that incorporation into microtubules requires that the first 428 amino acids be present. Whether they are also required for incorporation into the tubulin dimer is a separate question. To address this, co-immunoprecipitation experiments were performed in which the antibody to the V5 tag was used to precipitate the β-tubulin construct to see whether α-tubulin was also precipitated. Expression analysis was performed for wild type, CTT-deleted constructs, and chimeras of βI and βIII tubulin (Fig. 6, A–C, top panels). Western analysis with anti-V5 antibody indicated that expression of the transgene was present for all transiently transfected cell lines. Glyceraldehyde-3-phosphate dehydrogenase was utilized as a loading control (Fig. 6, A–C, middle panels). The results of immunoprecipitation clearly showed that truncated tubulins lacking of up to 30 amino acids for βIII tubulin (Fig. 6A) and 17 amino acids for βI tubulin (Fig. 6B) were all able to precipitate α-tubulin. The same was true for the chimeric β-tubulins (Fig. 6C). This suggests that truncated forms of these isotypes were able to form αβ heterodimers.
FIGURE 6.
Co-immunoprecipitation and Western analysis of βI, βIII, and chimeric β-tubulin constructs. HeLa cells were transiently transfected with wild type or the indicated C-terminally truncated βIII tubulin (A), βI tubulin (B), or chimeric β-tubulin (C). Expression of βI, βIII, and chimeric β-tubulin in HeLa cells were analyzed by Western blotting with antibody to V5 (A–C, top panel) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the loading control (A–C, middle panel). The samples were immunoprecipitated and analyzed by Western blotting with antibody to β-tubulin (A–C, bottom panels). C, *, βI+CβIII FL construct that does not contain C-terminal V5-His tag. **, empty vector expressing V5-His-tagged β-galactosidase. Notice that βI CΔ17, βIII CΔ23, βIII CΔ24, βIII CΔ25, and βIII CΔ30 were all able to precipitate α-tubulin. IB, immunoblot.
DISCUSSION
The C-terminal region of tubulin, here defined as the region downstream of the empirically determined Helix H12, is an intriguing and not well understood region of the tubulin molecule. The structure of the C-terminal region has not been determined by any kind of crystallographic analysis, although its secondary structure has been modeled (45). This region is known to be a binding site for microtubule-associated proteins and motors (11–22) and to be the site of a variety of important post-translational modifications including phosphorylation, polyglycylation, polyglutamylation, tyrosination/detyrosination, and deglutamylation (23–29, 32, 33, 35–38, 41). Our results raise the possibility that part of the C-terminal region in β-tubulin may have yet another function, namely to create or maintain a conformation in the rest of the tubulin molecule that permits microtubule assembly. It is clear from our results that Ala428 is absolutely required for microtubule formation for both βI and βIII. When Ala428 is removed, no microtubules form. The overall structure of the rest of the tubulin is likely to be perturbed as shown by the formation of aggregates that accompanies a lack of microtubule formation. The nature of the C terminus beyond residue 430 appears to have little effect, as indicated by the fact that the chimeric tubulins form microtubules normally.
It is interesting that βI and βIII differ in the roles of the residues beyond Ala428. The presence or absence of Thr429 makes no difference at all to assembly of βI. However, the absence of Thr429 strongly inhibits formation of microtubules from βIII. Even removal of Ala430 appears to slightly inhibit microtubule formation in βIII (Fig. 5A).
One could argue that the V5 reporter group, which consists of 45 residues, could create artifactual results in that its bulk could either provide inertial force or steric hindrance that could alter the natural properties of the tubulin molecule. In the former case one could imagine that the bulky V5 group might cause some partial unfolding of the globular domain, just enough to destabilize its conformation and prevent the tubulin molecule from incorporating into the microtubule. In the latter case, one could imagine that, as the V5 group might physically block the tubulin-tubulin interactions that are required for microtubule assembly and that, hence, the tubulin molecule involved would not be able to incorporate into the microtubule. One could further imagine that in a series of truncated mutants, either of these effects would occur at a specific chain length.
There are several arguments that militate against either of these hypotheses and indicate that the results reported here are not likely to be artifacts because of the presence of the V5 group. First, the inertial force hypothesis is weakened by the fact that the projecting C-terminal domain is really quite long, beginning at residue 427 (shown in Fig. 7). It is difficult, although not impossible, to imagine that the putative inertial force exerted by the V5 domain would be such that at a residue exactly 23 or 17, for βIII or βI, respectively, positions into the C-terminal domain, but presumably well removed from the globular domain, the inertial force would suddenly make a difference and cause partial unfolding of the globular domain. Second, it is interesting that the transition from allowing microtubule assembly to preventing it occurs at the precise same residue in βI as in βIII. The inertial force hypothesis presupposes that the globular domain exerts some force on the C-terminal domain that is overcome when the V5 group approaches just close enough to the globular domain. It is unlikely that the globular domains of βI and βIII would exert the exact same force on the C-terminal domain such that removal of the exact same residue on βI and βIII would cause destabilization. Although the stability of βI has never been measured directly, the stabilities of βII, βIII, and βIV have been measured. βIII is far more stable than βII, and βII is much more stable than βIV (45, 46). βI is very similar in sequence to βIV (Table 1), in both its globular domain and C-terminal domain and is therefore likely to be much less stable than βIII. In short, if the inertial force hypothesis were to be correct, the transition in βI from permitting to preventing microtubule formation would probably not occur at the same residue as it does in βIII.
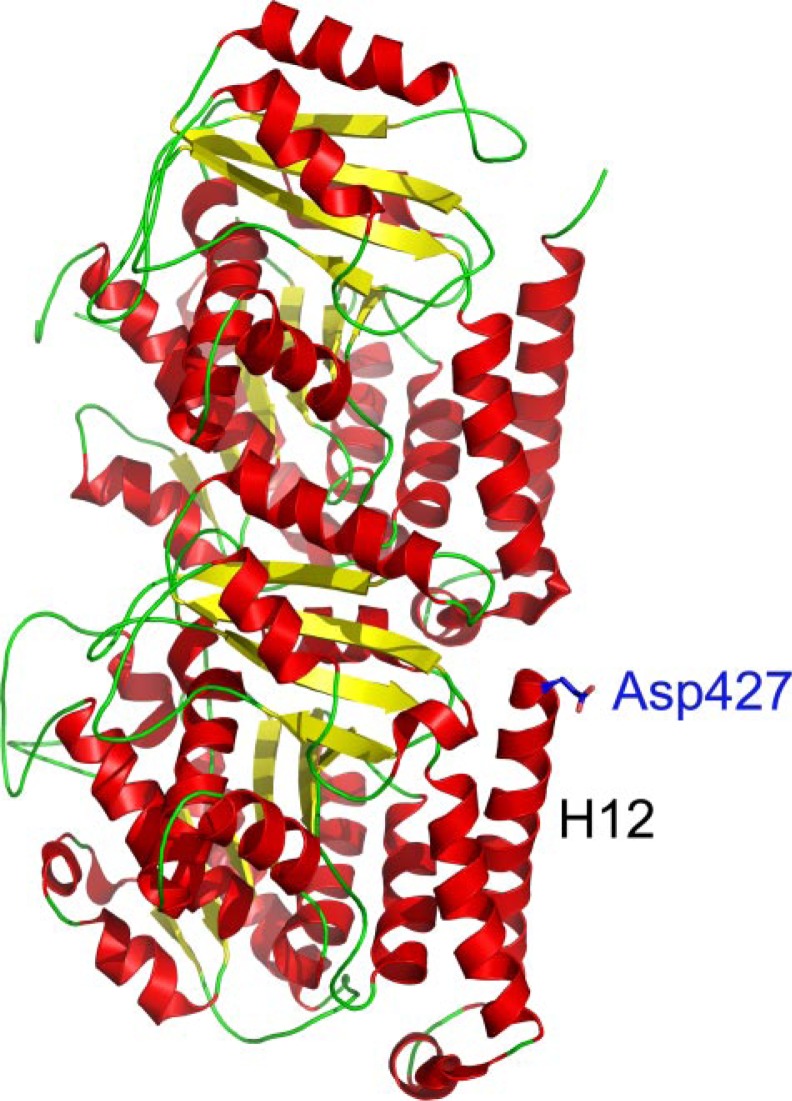
FIGURE 7.
Location of Asp427 at the C terminus of β-tubulin. This representation of the αβ-tubulin dimer, taken from the structure by Lowe et al. (5) (Protein Data Bank code 1JFF), indicates the location of Asp427 near the end of helix H12. The Asp427 side chain of β-tubulin is shown in blue. This structure was generated using PyMOL (72).
Although this argument may rule out the inertial hypothesis as a source of artifact, they do not rule out the steric hindrance hypothesis, namely that V5 could be blocking assembly once the distance between the V5 domain and the globular domain of tubulin becomes sufficiently short and that the critical truncation is the one that removes Ala428. If that were the case, then there is no reason to expect βI and βIII to give different effects. The effect should simply be a matter of length. However, our finding that Thr429 strongly promotes the ability of βIII to form microtubules but makes no difference for βI argues against the steric hindrance hypothesis. By either argument, the fact that the βI chimeric mutant containing the βIII C terminus (βI+CβIII FL) but lacking the V5 tag, assembles perfectly well into microtubules (Fig. 4F) suggests that the 45-residue V5 tag is not influencing the results and corroborates our finding that the nature of the C-terminal region beyond Thr429 has little or no effect on the likelihood of microtubule incorporation.
There are only two mechanisms by which the C-terminal domain can regulate the ability of tubulin to form microtubules. Either the C-terminal domain spends at least a fraction of the time lying on the surface of the globular domain and maintaining its conformation so that it can enter the microtubule or the C-terminal domain somehow reaches over to another tubulin molecule, bonding with it so as to stabilize the microtubule. If the latter mechanism is correct, then the isotypic nature of the C-terminal domain should make no difference, because it is known that different isotypes of tubulin can co-polymerize (47). Our data indicate that there is no specific relationship between the isotypic nature of the C terminus and that of the globular domain, because βIII with the βI C terminus and βI with the βIII C terminus polymerizes into the microtubule. Hence, our data support the second mechanism.
Although the larger C-terminal truncations beyond Ala428 did not assemble into microtubules, our data suggest that the C-terminal domain and even part of Helix H12 play no part in maintaining αβ dimer formation. This is not surprising, because the crystal structure (5) indicates that the dimer-forming region is not near the CTT. The C-terminal domain of tubulin has already been postulated to serve as a signal, as a site for binding of MAPs and motors, and as a site for post-translational modifications, all of these with great functional significance. Eliminating this region is likely to cause severe alterations in dynamic behavior. Because immunoprecipitation suggests that α- and β-tubulin are associated, the fluorescence data from microtubule inhibiting truncations are likely a display of the free dimer pool. Perhaps these deletions, lacking the C terminus, have caused a shift to a higher critical concentration for microtubule assembly.
The differences between βI and βIII are highly conserved in evolution, implying some degree of functional significance to those differences (1). It is striking that βIII lacks Cys239 and contains Cys124. Cys239 is present in all of the vertebrate β isotypes (except for βV and βVI). It is also present in all other animal β isotypes and in most plant and protist (but not fungal) β isotypes. A few plants and protists have Cys238 instead of Cys239, some have both, but, outside of vertebrate βIII, βV, and βVI and fungal β-tubulins, there is always a cysteine in that area (1). Cys124 is even more striking. It is present in βIII, βV, and βVI, but not in any other β-tubulin (except for octopus (48)). It is likely that βIII may have a unique function that involves these particular residues. It is known that βIII has a conformation different from that of either βII or βIV, the latter, as already argued, being very likely to have a conformation very similar to that of βI (46, 49). It is likely, therefore, that the conformations of the globular domains of the intact βI and βIII molecules are different and that, in the case, of βIII, this may permit βIII to carry out its function. It should be noted that the presence of αβIII dimer in the outer mitochondrial membranes of certain tumor cells raises the possibility that βIII may perform functions when it is not part of the microtubule (50). One could even speculate that a potentially different conformation of βIII may require slightly more of the C-terminal region (e.g. Thr429) to, in a sense, “corral” the conformation of βIII into one consistent with microtubule assembly.
A survey of β-tubulin sequences indicates that Ala428 is universally conserved. However, the same is not true of Thr429. Thr429 occurs in all of the human β-tubulin isotypes except for βVI. It also occurs in a variety of protists (e.g. Moneuplotes (51) and Paramecium (52)), fungi (e.g. Trichoderma (53)), plants (e.g. lupine (54), Eleusine (55), carrot (56), and tobacco (57)), and animals (e.g. the nematode Cooperia (58), the sea urchin Strongylocentrotus (59), the squid Loligo (60), the Antarctic fish Notothenia (61), Xenopus (62), the chicken (63), and the mouse (64)). However, Thr429 is replaced by valine in cotton (65), by serine in the moss Physcomitrella (66), and by glycine in the platyhelminth Echinococcus (67), the parabasalid Trichonympha (68), and Giardia (69). Interestingly, in the human βVI isotype, Thr429 is replaced by a lysine (National Center for Biotechnology accession number CAC09371). The other replacements of Thr429 involve small amino acids. Lysine is considerably larger. Conceivably, Lys429 may play a role in the formation of the unusual microtubules of blood platelets, which have a high propensity to form curved structures that constitute the marginal bands of these cells (70, 71). In short, our results are consistent with the phylogenetic distributions of Ala428 and Thr429; Ala428 is absolute required for microtubule assembly because it is absolutely conserved in evolution, and Thr429 strongly promotes microtubule assembly in at least one but not all types of tubulin because it is strongly but not absolutely conserved in evolution.
Acknowledgments
We are grateful to John A. Garza for helpful discussions and Veena Prasad for skilled technical assistance.
Footnotes
The abbreviations used are: CTT, C-terminal tail; MAP, microtubule-associated protein; FL, full length; PBS, phosphate-buffered saline.
This work was supported by United States Army Breast Cancer Research Program Grant W81XWH-05-1-0238, San Antonio Cancer Institute Grant P30 CA54174, the State of Texas Higher Coordinating Board, and Welch Foundation Grant AQ-0726 (to R. F. L.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Luduena RF. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 2.Jensen-Smith HC, Luduena RF, Hallworth R. Cell Motil. Cytoskeleton. 2003;55:213–220. doi: 10.1002/cm.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sackett DL, Bhattacharyya B, Wolff J. J Biol Chem. 1985;260:43–45. [PubMed] [Google Scholar]
- 4.Nogales E, Wolf SG, Downing KH. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 5.Lowe J, Li H, Downing KH, Nogales E. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 6.Popodi EM, Hoyle HD, Turner FR, Raff EC. Cell Motil. Cytoskeleton. 2005;62:48–64. doi: 10.1002/cm.20085. [DOI] [PubMed] [Google Scholar]
- 7.Duan J, Gorovsky MA. Curr Biol. 2002;12:313–316. doi: 10.1016/s0960-9822(02)00651-6. [DOI] [PubMed] [Google Scholar]
- 8.Serrano L, Wandosell F, de la Torre J, Avila J. Biochem J. 1988;252:683–691. doi: 10.1042/bj2520683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano L, de la Torre J, Maccioni RB, Avila J. Proc Natl Acad Sci U S A. 1984;81:5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jimenez MA, Evangelio JA, Aranda C, Lopez-Brauet A, Andreu D, Rico M, Lagos R, Andreu JM, Monasterio O. Protein Sci. 1999;8:788–799. doi: 10.1110/ps.8.4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Sheetz MP. Biophys J. 2000;78:1955–1964. doi: 10.1016/S0006-3495(00)76743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. J Biol Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- 13.Henriquez JP, Cambiazo V, Maccioni RB. Mol Cell Biochem. 1996;158:149–159. doi: 10.1007/BF00225841. [DOI] [PubMed] [Google Scholar]
- 14.Knipling L, Wolff J. Biochem Biophys Res Commun. 2006;341:433–439. doi: 10.1016/j.bbrc.2005.12.201. [DOI] [PubMed] [Google Scholar]
- 15.Moreno FJ, Bagnat M, Lim F, Avila J. Eur J Biochem. 1999;262:557–562. doi: 10.1046/j.1432-1327.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- 16.Priel A, Tuszynski JA, Woolf NJ. Eur Biophys J. 2005;35:40–52. doi: 10.1007/s00249-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 17.Serrano L, Avila J, Maccioni RB. Biochemistry. 1984;23:4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- 18.Serrano L, Montejo de Garcini E, Hernandez MA, Avila J. Eur J Biochem. 1985;153:595–600. doi: 10.1111/j.1432-1033.1985.tb09342.x. [DOI] [PubMed] [Google Scholar]
- 19.Skiniotis G, Cochran JC, Muller J, Mandelkow E, Gilbert SP, Hoenger A. EMBO J. 2004;23:989–999. doi: 10.1038/sj.emboj.7600118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker C, Goldstein LS. J Biol Chem. 1997;272:9481–9488. doi: 10.1074/jbc.272.14.9481. [DOI] [PubMed] [Google Scholar]
- 21.Aizawa H, Emori Y, Mori A, Murofushi H, Sakai H, Suzuki K. J Biol Chem. 1991;266:9841–9846. [PubMed] [Google Scholar]
- 22.Chapin SJ, Bulinski JC. J Cell Sci. 1991;98:27–36. doi: 10.1242/jcs.98.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JE, Hunt DF, Lee MK, Shabanowitz J, Michel H, Berlin SC, MacDonald TL, Sundberg RJ, Rebhun LI, Frankfurter A. Proc Natl Acad Sci U S A. 1991;88:4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luduena RF, Zimmermann HP, Little M. FEBS Lett. 1988;230:142–146. doi: 10.1016/0014-5793(88)80658-6. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Nido J, Serrano L, Lopez-Otin C, Vandekerckhove J, Avila J. J Biol Chem. 1990;265:13949–13954. [PubMed] [Google Scholar]
- 26.Ikegami K, Mukai M, Tsuchida J, Heier RL, Macgregor GR, Setou M. J Biol Chem. 2006;281:30707–30716. doi: 10.1074/jbc.M603984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gagnon C, White D, Cosson J, Huitorel P, Edde B, Desbruyeres E, Paturle-Lafanechere L, Multigner L, Job D, Cibert C. J Cell Sci. 1996;109:1545–1553. doi: 10.1242/jcs.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 28.Rudiger A, Rudiger M, Weber K, Schomburg D. Anal Biochem. 1995;224:532–537. doi: 10.1006/abio.1995.1083. [DOI] [PubMed] [Google Scholar]
- 29.Rudiger M, Plessman U, Kloppel KD, Wehland J, Weber K. FEBS Lett. 1992;308:101–105. doi: 10.1016/0014-5793(92)81061-p. [DOI] [PubMed] [Google Scholar]
- 30.Redeker V, Melki R, Prome D, Le Caer JP, Rossier J. FEBS Lett. 1992;313:185–192. doi: 10.1016/0014-5793(92)81441-n. [DOI] [PubMed] [Google Scholar]
- 31.Dossou SJ, Bre MH, Hallworth R. Cell Motil. Cytoskeleton. 2007;64:847–855. doi: 10.1002/cm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bre MH. Science. 1994;266:1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- 33.Bre MH, Redeker V, Vinh J, Rossier J, Levilliers N. Mol. Biol. Cell. 1998;9:2655–2665. doi: 10.1091/mbc.9.9.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Banerjee A. J Biol Chem. 2002;277:46140–46144. doi: 10.1074/jbc.M208065200. [DOI] [PubMed] [Google Scholar]
- 35.Deanin GG, Preston SF, Hanson RK, Gordon MW. Eur J Biochem. 1980;109:207–216. doi: 10.1111/j.1432-1033.1980.tb04786.x. [DOI] [PubMed] [Google Scholar]
- 36.Gundersen GG, Bulinski JC. J Cell Biol. 1986;102:1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gundersen GG, Kalnoski MH, Bulinski JC. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 38.Audebert S, Desbruyeres E, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Edde B. Mol. Biol. Cell. 1993;4:615–626. doi: 10.1091/mbc.4.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall TA. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- 41.Banerjee A. Biochem Biophys Res Commun. 2002;293:598–601. doi: 10.1016/S0006-291X(02)00269-3. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee A. Biochemistry. 1999;38:5438–5446. doi: 10.1021/bi981572n. [DOI] [PubMed] [Google Scholar]
- 43.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 44.Nogales E. Cell Mol Life Sci. 1999;56:133–142. doi: 10.1007/s000180050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luchko T, Huzil JT, Stepanova M, Tuszynski J. Biophys J. 2008;94:1971–1982. doi: 10.1529/biophysj.107.115113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz PM, Liggins JR, Luduena RF. Biochemistry. 1998;37:4687–4692. doi: 10.1021/bi972763d. [DOI] [PubMed] [Google Scholar]
- 47.Lewis SA, Gu W, Cowan NJ. Cell. 1987;49:539–548. doi: 10.1016/0092-8674(87)90456-9. [DOI] [PubMed] [Google Scholar]
- 48.Tomarev SI, Zinovieva RD, Piatigorsky J. Biochim. Biophys. Acta. 1993;1216:245–254. doi: 10.1016/0167-4781(93)90151-3. [DOI] [PubMed] [Google Scholar]
- 49.Sharma J, Luduena RF. J Protein Chem. 1994;13:165–176. doi: 10.1007/BF01891975. [DOI] [PubMed] [Google Scholar]
- 50.Carre M, Andre N, Carles G, Borghi H, Brichese L, Briand C, Braguer D. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- 51.Harper DS, Jahn CL. Proc Natl Acad Sci U S A. 1989;86:3252–3256. doi: 10.1073/pnas.86.9.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupuis P. EMBO J. 1992;11:3713–3719. doi: 10.1002/j.1460-2075.1992.tb05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee M, Hadar R, Mukherjee PK, Horwitz BA. J Appl Microbiol. 2003;95:861–867. doi: 10.1046/j.1365-2672.2003.02061.x. [DOI] [PubMed] [Google Scholar]
- 54.Vassilevskaia TD, Ricardo CP, Rodrigues-Pousada C. Plant Mol Biol. 1993;22:715–718. doi: 10.1007/BF00047413. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto E, Baird WV. Plant Mol Biol. 1999;39:45–61. doi: 10.1023/a:1006108412801. [DOI] [PubMed] [Google Scholar]
- 56.Okamura S, Naito K, Sonehara S, Ohkawa H, Kuramori S, Tatsuta M, Minamizono M, Kataoka T. Cell Struct Funct. 1997;22:291–298. doi: 10.1247/csf.22.291. [DOI] [PubMed] [Google Scholar]
- 57.Okamura S, Okahara K, Iida T, Ozaki M, Asano S, Morita M, Imanaka T. Cell Struct Funct. 1999;24:117–122. doi: 10.1247/csf.24.117. [DOI] [PubMed] [Google Scholar]
- 58.Njue AI, Prichard RK. Parasitology. 2003;127:579–588. doi: 10.1017/s0031182003004086. [DOI] [PubMed] [Google Scholar]
- 59.Harlow P, Litwin S, Nemer M. J Mol Evol. 1988;27:56–64. doi: 10.1007/BF02099730. [DOI] [PubMed] [Google Scholar]
- 60.Gioio AE, Lavina ZS, Jurkovicova D, Zhang H, Eyman M, Giuditta A, Kaplan BB. Eur J Neurosci. 2004;20:865–872. doi: 10.1111/j.1460-9568.2004.03538.x. [DOI] [PubMed] [Google Scholar]
- 61.Detrich HW, 3rd, Parker SK. Cell Motil. Cytoskeleton. 1993;24:156–166. doi: 10.1002/cm.970240303. [DOI] [PubMed] [Google Scholar]
- 62.Good PJ, Richter K, Dawid IB. Nucleic Acids Res. 1989;17:8000. doi: 10.1093/nar/17.19.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan KF, Lau JT, Cleveland DW. Mol Cell Biol. 1985;5:2454–2465. doi: 10.1128/mcb.5.9.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis SA, Lee MG, Cowan NJ. J Cell Biol. 1985;101:852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji S, Lu Y, Li J, Wei G, Liang X, Zhu Y. Biochem Biophys Res Commun. 2002;296:1245–1250. doi: 10.1016/s0006-291x(02)02069-7. [DOI] [PubMed] [Google Scholar]
- 66.Jost W, Baur A, Nick P, Reski R, Gorr G. Gene (Amst) 2004;340:151–160. doi: 10.1016/j.gene.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Brehm K, Kronthaler K, Jura H, Frosch M. Mol. Biochem. Parasitol. 2000;107:297–302. doi: 10.1016/s0166-6851(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 68.Gerbod D, Sanders E, Moriya S, Noel C, Takasu H, Fast NM, Delgado-Viscogliosi P, Ohkuma M, Kudo T, Capron M, Palmer JD, Keeling PJ, Viscogliosi E. Mol Phylogenet Evol. 2004;31:572–580. doi: 10.1016/j.ympev.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Kirk-Mason KE, Turner MJ, Chakraborty PR. Nucleic Acids Res. 1988;16:2733. doi: 10.1093/nar/16.6.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartwig J, Italiano J., Jr J. Thromb. Haemostasis. 2003;1:1580–1586. doi: 10.1046/j.1538-7836.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 71.Schwer HD, Lecine P, Tiwari S, Italiano JE, Jr, Hartwig JH, Shivdasani RA. Curr Biol. 2001;11:579–586. doi: 10.1016/s0960-9822(01)00153-1. [DOI] [PubMed] [Google Scholar]
- 72.DeLano WL. PyMOL. DeLano Scientific; San Carlos, CA: 2002. [Google Scholar]