Abstract
Background
Myxofibrosarcomas (MFS) are a historically heterogeneous group of tumors that exhibit a propensity for local recurrence. The objectives of this study were to analyze the prognostic factors and outcomes of patients with MFS treated at a single institution.
Methods
We retrospectively reviewed the records of 69 consecutive patients with pathologically confirmed MFS of the extremities or superficial trunk who underwent surgery from August 1995 to November 2010. Clinicopathologic features, treatments, and patient outcomes were reviewed.
Results
Sixty-nine patients were identified, of whom 38 were men (55 %). The median age was 62 years. Sixty-four patients (93 %) presented with primary tumors, and 5 patients (7 %) presented with locally recurrent tumors. Median tumor size was 6.0 cm, and 44 patients (64 %) had grade 3 tumors (FNCLCC [Fédération Nationale des Centres de Lutte Contre le Cancer] classification). Margins were microscopically positive in 14 patients (20 %) and negative in 55 patients (80 %), including close margins (<1 mm) in 14 patients (20 %). Fifty-three patients (77 %) received radiotherapy. At a median follow-up of 41 months, there were 11 local (16 %) and 11 distant (16 %) recurrences. The local and distant 5-year recurrence-free survival rates were 72 % and 82 %, and the 5-year overall survival was 61 %. Increased age (scaled by 0.1; hazard ratio [HR] 1.80, P = 0.002) and tumor size (HR 1.12, P = 0.004) were negatively correlated with overall survival. Positive/close (<1 mm) margin status (HR 4.34, P = 0.030) predicted worsened local recurrence-free survival.
Conclusions
MFS exhibit a propensity for local recurrence, which is predicted by resection with positive or close margins. Aggressive surgery combined with radiotherapy may contribute to more effective local control.
Myxofibrosarcoma (MFS) is a malignant mesenchymal tumor that usually arises in the extremities of elderly patients.1,2 Historically, MFS were histologically and clinically recognized as a part of the spectrum of myxoid fibroblastic sarcomas.3 However, the nomenclature for these tumors has been inconsistent.1,4,5 Furthermore, MFS were often grouped with other malignant fibrous histiocytomas of variable prognosis, thus camouflaging their innate clinical patterns of behavior.3 In 2002, this ambiguity prompted the World Health Organization (WHO) to recognize and define MFS as a discrete entity with a unique set of pathologic features.6
Given its relatively recent recognition as a distinct pathologic entity, the clinical behavior and outcomes for patients with MFS are uncertain, and there are no randomized trials to guide treatment protocols. Series of myxoid malignant fibrous histiocytomas and MFS describe these tumors as having a significant propensity for local recurrence, while exhibiting an overall better prognosis when compared to other types of sarcomas.7-9 This penchant for local recurrence is concerning, given that up to 50 % of recurrences exhibit higher-grade histology and correspondingly greater metastatic potential than the primary lesion, postulated to be related to the infiltrative growth pattern seen in MFS.8,10 Recent retrospective cohorts of MFS reported a 5-year local recurrence rate of 18–31 % and an associated overall survival (OS) of approximately 70 %.7,11,12 Tumor size at resection, tumor grade, positive surgical resection margin, percent necrosis, and mitotic rate have all been suggested as possible prognostic factors for disease-specific and metastasis-free survival, but these studies have been hampered by their small size and heterogeneous treatments.11-13
Over the past 20 years, advances in sarcoma management have encouraged the use of combined modality therapies to improve survival and promote limb-sparing surgery. However, the precise roles of pre- and postoperative chemotherapy and radiotherapy (RT) remain elusive. The goals of this study were to evaluate the clinicopathologic characteristics, treatments, and outcomes for patients with MFS and to define an optimal treatment approach.
METHODS
This study was approved by the institutional review board of Partners Healthcare/Massachusetts General Hospital (MGH). Patients included in this retrospective study were treated at the MGH from January 1, 1995, through November 30, 2010, and had a histologically confirmed diagnosis of MFS or myxoid malignant fibrous histiocytoma. All pathologic specimens were initially interpreted at MGH and reviewed again in light of the current WHO diagnostic criteria before inclusion in the study cohort. Patients with primary tumors in the extremity and superficial trunk soft tissues were included. Tumors located in the head, neck, retroperitoneum, and brain were excluded. Patients who did not undergo definitive surgery at MGH, or for whom complete pathologic or follow-up data were not available, were excluded.
The electronic medical records of included patients were reviewed, and details regarding the presentation, histologic features, treatment course, and vital status were ascertained. The three-tiered FNCLCC (Fédération Nationale des Centres de Lutte Contre le Cancer) grading classification system was used, and tumors with overlapping grades (e.g., grade 1–2) were classified at the higher tier. Pathology reports of surgical specimens were evaluated in conjunction with operative reports to determine the type of resection, tumor depth, and margin status. Resections were pathologically classified as being R0 (macroscopically complete with negative microscopic margins), R1 (macroscopically complete with microscopic cells at the inked resection margin; positive margin), or R2 (macroscopically incomplete). R0 resections were further classified as negative (margin ≥1 mm) or close (margin <1 mm). Radical resection included wide surgical excision encompassing a portion of the surrounding compartmental fascia and/or excision or rearrangement of adjacent vascular or nerve structures. Wide excision included surgical excisions with intracompartmental excision of the tumor. Definitions for the extent of surgical resection, including a 1-mm threshold for negative/close margins, have previously been used in other series describing MFS.7,11
The primary outcome measures for this analysis were rates of OS, local recurrence-free survival (LRFS), and distant recurrence-free survival (DRFS). Dates of death for patients with Social Security numbers were obtained from the Social Security Death Index (SSDI). OS was calculated from the date of confirmed pathologic diagnosis to the date of documented death by SSDI. Patients who were still alive at last contact were censored for OS at this date. LRFS and DRFS were calculated from the date of confirmed pathologic diagnosis to the date of first local or distant progression or recurrence, respectively. Patients who had not experienced a local or distant recurrence as of the date of last contact (including death) were censored at this date. Overall recurrence-free survival (RFS) was calculated from the date of confirmed pathologic diagnosis to the date of first of local or distant progression or recurrence. Patients who had not experienced a local or distant recurrence as of the date of last contact (including death) were censored at this date.
Kaplan–Meier survival estimates were calculated for LRFS, DRFS, RFS, and OS. Univariate Cox proportional hazard regression modeling was used to determine clinical and histologic predictors of LRFS, DRFS, and OS. Because of the modest sample size and relatively high censoring rate, multivariate models were not constructed. All reported P-values are two-sided using a significance threshold of 0.05. Statistical analyses were performed by SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Clinicopathologic features of the 69 patients are shown in Table 1.
TABLE 1.
Clinicopathologic characteristics of 69 patients with myxofibrosarcoma
| Characteristic | Value |
|---|---|
| Male gender | 38 (55 %) |
| Age at diagnosis, y | |
| Mean | 62 |
| Median | 62 |
| Range | 19–94 |
| >60 y | 40 (58 %) |
| >70 y | 25 (36 %) |
| Primary vs. recurrent tumor at presentation | |
| Primary | 64 (93 %) |
| Recurrent | 5 (7 %) |
| Site | |
| Lower extremity—proximal | 28 (41 %) |
| Lower extremity—distal | 8 (12 %) |
| Upper extremity—proximal | 10 (14 %) |
| Upper extremity—distal | 6 (9 %) |
| Trunk | 12 (17 %) |
| Breast | 2 (3 %) |
| Shoulder | 2 (3 %) |
| Axilla | 1 (1 %) |
| Size (cm)a | |
| Mean | 7.4 |
| Median | 6 |
| Range | 1–27 |
| FNCLCC histologic nuclear grade | |
| 1 (low) | 9 (13 %) |
| 2 (intermediate) | 16 (23 %) |
| 3 (high) | 44 (64 %) |
| T stage | |
| 1a | 19 (28 %) |
| 1b | 7 (10 %) |
| 2a | 14(20 %) |
| 2b | 29 (42 %) |
| Surgical margin | |
| R0—negative (margin ≥1 mm) | 41 (60 %) |
| R0—close (margin < 1 mm) | 14 (20 %) |
| R1—positive | 14 (20 %) |
| Vascular invasiona | |
| No | 60 (87 %) |
| Yes | 5 (7 %) |
| Unknown | 4 (6 %) |
FNCLCC Fédération Nationale des Centres de Lutte Contre le Cancer
From surgical resection specimen
All patients underwent surgical resection. Fifty-six patients (81 %) underwent radical resection, 11 (16 %) wide excision, and 2 (3 %) amputation. Thirty-one patients (45 %) underwent complex surgical reconstruction, including a pedicled local flap with or without a split thickness skin graft in 19 patients (28 %), a free flap with microvascular anastomosis in 1 patient (1 %), a split thickness skin graft alone in 8 patients (12 %), and complex primary closure in 2 patients (3 %).
Fifty-three patients (77 %) received RT, including 11 patients with R1 resection (3 patients with grade 1 tumors and 8 patients with grade 2 or 3 tumors) and 42 patients with R0 resection. Of these 53 patients, 5 (9 %) had grade 1 tumors, 13 (25 %) had grade 2 tumors, and 35 (66 %) had grade 3 tumors. Preoperative RT was administered in 32 patients (60 %), postoperative RT was provided in 8 patients (15 %), and 13 patients (25 %) received both pre- and postoperative RT. Of these 13 patients, 2 received intraoperative electron-beam radiotherapy and 11 patients received postoperative external-beam radiotherapy boost doses from 10 to 20 Gy to the tumor bed. Examining the 46 patients who received preoperative treatment, 30 (64 %) received a total preoperative dose of 50 Gy. Eight patients (12 %) received interdigitated preoperative chemoradiotherapy with 44 Gy over 2 divided fractions. Two patients received preoperative RT doses of 20 and 22 Gy, respectively. The former patient received 20 Gy RT before definitive surgery after a previous knee arthroscopy introduced possible intra-articular contamination with tumor cells. The latter patient received 22 Gy in hospital before definitive surgery.
Of the patients who received solely postoperative RT, 7 patients received doses between 60 and 68 Gy, and 1 patient received a 14 Gy boost to the tumor bed after a R0 resection. The latter patient had a 13-cm grade 2 tumor, but because of advanced age, this patient could only tolerate a short RT course. Specifically looking at patients with grade 2 and 3 tumors who received R1 resections (8 patients), 7 patients received preoperative RT with 50 Gy, and 5 received additional postoperative RT for positive margins (range 10–20 Gy).
Receipt of radiation based on tumor grade and surgical margin status is shown in Table 2.
TABLE 2.
Number of myxofibrosarcoma patients receiving radiation (N = 53) based on margin status and grade
| Margin | Low grade (n = 9) |
Intermediate grade (n = 16) |
High grade (n = 44) |
Total |
|---|---|---|---|---|
| Negative (n = 41) |
2 | 8 | 21 | 31 |
| Close (n = 14) |
1 | 4 | 7 | 12 |
| Positive (n = 14) |
2 | 1 | 7 | 10 |
| Total | 5 | 13 | 35 | 53 |
Thirteen patients (18 %) received chemotherapy, including 11 (16 %) who underwent combined pre- and postoperative chemotherapy. Three patients (4 %) with grade 1 tumors, 4 (6 %) with grade 2 tumors, and 6 (9 %) with grade 3 tumors received chemotherapy. The most common chemotherapy regimen (7 patients) was MAID (mesna, Adriamycin, ifosfamide, dacarbazine). One patient received preoperative bevacizumab in combination with RT (50.4 Gy) on a phase II clinical trial.
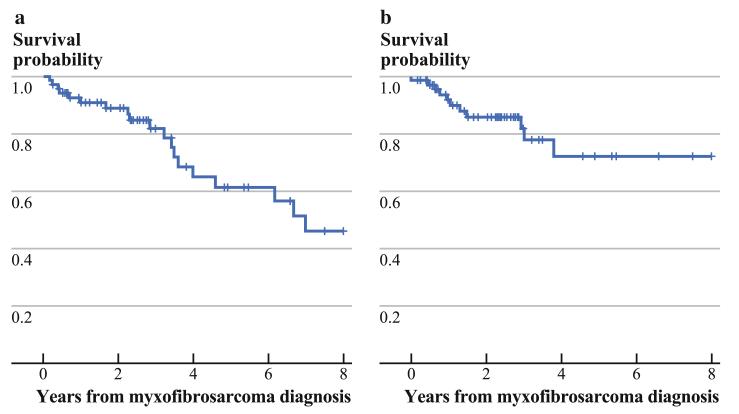
Kaplan–Meier estimates for OS and LRFS are shown in Fig. 1. At a median follow-up of 41 months (range 2–161 months), 19 patients (28 %) had died, 46 patients (67 %) were alive without evidence of local or distant recurrence, and 4 patients (6 %) were alive with locally recurrent or metastatic disease. The 5-year OS rate was 61 %, while the 5-year LRFS, DRFS, and overall RFS rates were 72 %, 82 %, and 62 %, respectively. None of the patients with grade 1 tumors experienced local or distant recurrence.
FIG. 1.
a Kaplan–Meier curve for overall survival. b Kaplan–Meier curve for local recurrence-free survival
For high-grade (grades 2 and 3) patients only, the 5-year OS was 53 %, while the LRFS, DRFS, and overall RFS rates were 66 %, 80 %, and 58 %, respectively.
Age (hazard ratio [HR] 1.80, P = 0.002), scaled by 0.1, and tumor size (HR 1.12, P = 0.004), modeled as a continuous variable, were univariately significant predictors of worsened OS. A trend was seen toward decreased OS for higher tumor grade patients (HR 2.03, P = 0.069), while a favorable trend was seen for patients receiving RT (HR 0.46, P = 0.100). Age and tumor size were found to be independently significant on multivariate analysis (both P < 0.01); however, this multivariate model was considered exploratory as a result of the modest sample size and low event rate.
For high-grade patients only, age (HR 2.64, P = 0.039), scaled by 0.1, and tumor size (HR 1.11, P = 0.010) again univariately predicted worsened OS.
Local recurrence occurred in 11 patients (16 %) at a median of 16 months (range 6–85 months) after diagnosis. Nine of these patients (82 %) experienced recurrence within 36 months of the initial diagnosis. Clinical courses of these patients are shown in Table 3. Treatment for these locally recurrent tumors included surgery alone in 4 patients (6 %), surgery and RT in 4 patients (6 %), and surgery and brachytherapy in 2 patients (3 %). One patient underwent definitive external-beam RT with complete tumor regression. Four patients (6 %) experienced multiple local recurrences.
TABLE 3.
Tumor characteristics and treatment courses for 11 patients with local recurrence
| Site | Max size (cm) |
Margin status |
Grade | RT | CT | Time to first local recurrence (mo) |
No. of local recurrences |
Distant metastatic disease |
Treatment of local recurrence | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| UE-P | 10 | <1 mm | 3 | Pre | – | 13 | 3 | – | (1) EBRT/resection/brachytherapy, (2) resection, (3) amputation |
DOD |
| LE-P | 15.5 | >1 mm | 3 | Pre | Pre/post | 16 | 1 | Lung | Resection | DOD |
| LE-P | 2.2 | >1 mm | 3 | Pre/post | Post | 45 | 1 | Lung, brain | Resection | DOD |
| LE-P | 10.5 | Positive | 3 | Pre/post | – | 36 | 2 | Lung | (1) Resection + IORT, (2) amputation | DOD |
| Trunk | 10 | <1 mm | 2 | Pre/IORT | – | 10 | 1 | – | Open biopsy only | DOD |
| Breast | 7 | >1 mm | 3 | – | – | 17 | 1 | – | Resection/EBRT | NED |
| UE-D | 2.5 | Positive | 3 | – | – | 6 | 1 | – | Resection/brachytherapy | NED |
| UE-D (R) | 5.5 | Positive | 2 | – | – | 12 | 2 | – | (1) Resection, (2) amputation | DOD |
| LE-P (R) | 2 | Positive | 3 | – | – | 85 | 3 | – | (1) EBRT/resection/boost, (2) resection/ brachytherapy, (3) resection |
NED |
| LE-D | 1.5 | <1 mm | 2 | – | – | 8 | 1 | – | EBRT/resection | NED |
| Trunk | 6 | Positive | 3 | Pre/post | – | 35 | 1 | – | EBRT only | NED |
RT radiotherapy, CT chemotherapy, Pre/Post sequencing with surgery (before or after), UE-P upper extremity—proximal, UE-D upper extremity—distal, LE-P lower extremity—proximal, LE-D lower extremity—distal, (R) recurrent tumor, IORT intraoperative radiotherapy, EBRT external-beam radiotherapy, DOD dead of disease, NED no evidence of disease
Looking specifically at patients with high-grade tumors, local recurrence occurred in 8 patients (12 %) with grade 3 tumors and 3 patients (4 %) with grade 2 tumors, including 1 patient whose tumor grade increased from 2 to 3 upon recurrence. Of the 8 patients with grade 3 tumors, 3 had negative surgical margins, 1 had a close margin, and 4 had positive margins. Of the 3 patients with grade 2 tumors, 2 had had close margins and 1 had a positive margin.
Close/positive margin status (HR 4.34, P = 0.030) was the sole significant predictor of local recurrence on univariate analysis, while R status (HR 3.08, P = 0.064) showed a trend toward significance. There were no univariately predictive variables of local recurrence when considering high-grade patients alone.
Distant metastatic disease occurred in 11 patients (16 %) at a median of 10 months (range 3–64 months) after diagnosis, including 3 patients (4 %) who also developed local recurrences. Distant recurrence occurred in 8 patients (12 %) with grade 3 tumors and 3 patients (4 %) with grade 2 tumors. Only one patient who had a documented grade of the metastatic site had an increase in the grade of his disease from 2 to 3. Of those who developed distant metastases, 4 patients (6 %) developed metastases at multiple sites. Ten patients (15 %) developed lung metastases, and 4 underwent surgical metastasectomy. Four patients (6 %) developed brain metastases, 2 of whom underwent surgery with whole-brain RT and 2 of whom received chemotherapy alone. Other sites of metastases included small bowel, pelvis, retroperitoneum, stomach, liver, and adrenal gland. Chemotherapeutic regimens provided after the development of metastases varied widely and included gemcitabine/docetaxel, vinorelbine, liposomal doxorubicin, ifosfamide/etoposide, trabectedin, MAID, gemcitabine/vinorelbine, and Adriamycin.
Tumor size (HR 1.16, P = 0.004) and receipt of chemotherapy (HR 3.50, P = 0.048) were the only univariately significant predictors of distant metastatic disease, while tumor location (lower extremity vs. remaining sites, HR 4.43, P = 0.057) and tumor grade (HR 2.92, P = 0.106) trended toward significance (Table 4). An exploratory multivariate analysis considering all four of these variables, and stepwise selection showed that only tumor size maintained significance in the multivariate setting (P < 0.01). Only tumor size (HR 1.15, P = 0.009) persisted as a univariately significant variable when considering high-grade tumors alone.
TABLE 4.
Univariate analysis of prognostic factors
| Characteristic | Overall survival |
Local recurrence-free survival |
Distant recurrence-free survival |
|||
|---|---|---|---|---|---|---|
| HR (95 % CI) | P | HR (95 % CI) | P | HR (95 % CI) | P | |
| Age (scaled by 0.1) | 1.80 (1.25–2.58) | 0.002 | 1.28 (0.85–1.92) | 0.231 | 0.88 (0.59–1.30) | 0.520 |
| Age ≥62 y (median)a | 3.68 (1.30–10.40) | 0.014 | 3.27 (0.85–12.60) | 0.084 | 0.88 (0.27–2.88) | 0.829 |
| Grade (3 categories) | 2.03 (0.95–4.33) | 0.069 | 1.27 (0.54–2.99) | 0.582 | 2.92 (0.80–10.70) | 0.106 |
| Negative margin statusb | 1.18 (0.48–2.94) | 0.718 | 4.34 (1.15–16.40) | 0.030 | 0.70 (0.20–2.41) | 0.573 |
| R status | 1.26 (0.49–3.26) | 0.632 | 3.08 (0.94,10.09) | 0.064 | 1.01 (0.26–3.85) | 0.993 |
| Site (LE vs. other) | 2.12 (0.81–5.59) | 0.128 | 0.72 (0.22–2.37) | 0.593 | 4.43 (0.96–20.55) | 0.057 |
| Tumor size | 1.12 (1.04–1.21) | 0.004 | 1.01 (0.87–1.16) | 0.924 | 1.16 (1.05–1.29) | 0.004 |
| Receipt of chemotherapyc | 0.92 (0.27–3.14) | 0.888 | 1.18 (0.25–5.53) | 0.836 | 3.50 (1.01–12.07) | 0.048 |
| Receipt of radiotherapyc | 0.46 (0.18–1.16) | 0.100 | 0.43 (0.12–1.51) | 0.187 | 3.15 (0.68–14.61) | 0.142 |
HR hazard ratio, CI confidence interval
Age modeled as a dichotomous variable of < 62 y or ≥62 y
Modeled as negative vs. close/positive
Modeled as time-dependent variables
DISCUSSION
In this study, we present the treatment and survival for 69 patients with MFS with a median follow-up of 41 months. Liberal use of RT in combination with a high rate of microscopically negative surgical resection margins combined to produce a low rate of amputation (3 %) and a 72 % 5-year LRFS rate.
MFS clinically tend to have lower rates of distant metastasis and higher rates of local recurrence than other types of sarcomas.7 Historically, local recurrence rates for malignant fibrous histiocytoma and MFS has ranged from 22 % to 79 %.2,5,9,14 It also has been associated with higher-grade recurrent disease in 42–50 % of patients.9,14,15 Of the 3 series of MFS utilizing the WHO histologic classification, two series reported 5-year rates of LRFS of 82 % and 30 %, respectively.12,13 The third study reported a 4-year LRFS of 60 % but restricted its reporting to intermediate and highgrade tumors.11 The present study reports a 5-year LRFS rate of 72 % (66 % for high-grade tumors alone), which lies between the rates of the aforementioned series. Multiple local recurrences occurred in 4 (36 %) of the 11 patients in our series. This is less than that reported in other studies, in which patients developed multiple recurrences in approximately 48–54 %.9,11,13,14 This discrepancy cannot be fully explained by a somewhat shorter median follow-up time, as our 41-month median surveillance period is longer or comparable to that of other series.11,13 However, the liberal use of RT may explain the lower rate of multiple recurrences in our study.
Several studies have identified significant predictors of local recurrence and metastases, including tumor size, depth, extent of histologic myxoid areas, mitotic rate, and grade.1,4,5,13 However, these studies are fraught with the inclusion of a diagnostically broad spectrum of myxoid neoplasms and an inconsistent use of multivariable models. In this study, tumor size and age were significantly predictive of OS, while close/positive margin status was predictive of LRFS. Interestingly, tumor grade was not significantly associated with local or distant failure, as reported in other studies.7,12 However, with a median follow-up of 65 months, none of the 9 patients with grade 1 tumors included in this report experienced a recurrence. It is very likely that tumor grade is an important predictor of recurrence; the lack of statistical significance in this series is explained by the small number of patients with grade 1 tumors and the total absence of recurrences in this group. When analyzing the 60 patients with high-grade tumors alone, the 5-year LRFS rate of 66 % is better when compared to the series by Haglund et al., which also looked at high-grade MFS but had a 4-year LRFS of 60 %.11 The patients in both of these series had an approximately 78 % rate of radiation administration and a 20–25 % rate of margin positivity.
The importance of the surgical resection margins has been reported in several studies to affect rates of local recurrence in many types of sarcomas.7,16 If one defines a positive surgical margin as either tumor at ink or within 1 mm of the inked margin, as defined by Lin et al. and Sanfilippo et al., patients in this series with a positive margin had a statistically worse RFS than those patients with negative margins (HR 4.34, P = 0.030).12,13 Both of the aforementioned series, with positive margin rates of 35 % and 18 %, respectively, found positive margins to be predictive not only of local recurrence, but also of distant recurrence and overall and metastasis-free survival. An association between positive margin status and OS has been previously attributed to the infiltrative growth pattern commonly observed in MFS, and subsequent high rate of local recurrence.10,17 These recurrences, in turn, are often of higher grade than the original tumor and may be unresectable and/or metastatic.7 Thus, it is critical that MFS be treated surgically with aggressive attempts to achieve widely negative margins to optimize both local control and survival. Although there are no guidelines defining the optimal extent of the surgical margin in MFS, it seems reasonable to aim for at least a 1–2 cm margin when anatomically feasible. The appropriate extent of surgical resection can be guided by the extent of soft tissue edema seen on preoperative magnetic resonance imaging, the diameter of which is often much greater than that of the actual tumor and may delineate the infiltrative tumor edges.10 Staged procedures (e.g., “slow Mohs” surgery), in which radical resection is followed by temporary wound coverage with a dressing before definitive reconstruction, are often appropriate in the surgical treatment of MFS to allow for careful, detailed pathologic assessment of the circumferential margins on permanent sections.18
The use of RT has been associated with decreased local recurrence rates for several types of sarcomas, but this finding has not been demonstrable in recent series on MFS.10-12,19-21 In the present study, 77 % of patients received radiation as a component of primary therapy, including 6 (55 %) of 11 patients who experienced local recurrence. When examining the descriptive tumor and treatment characteristics of patients who received radiation (53 patients), 91 % had intermediate or high-grade tumors, 20 % had a R1 resection, and the median size was 6.9 cm. There were no significant differences when these patients were compared to those who did not receive radiation (16 patients), whose correlative characteristics were 86 %, 20 %, and 5.0 cm. Although optimal regimens remain to be delineated, the liberal use of radiation in this series, combined with a 80 % R0 resection rate, show that aggressive multimodality treatment for MFS can achieve reasonable rates of local recurrence.
There are limitations to the applicability of this retrospective study. Although this is the largest documented North American cohort of MFS, the numbers of patients and recurrence events are small, and treatment strategies presented represent those of a single institution with inherent selection biases. Therefore, the ability to elucidate prognostic factors with narrow confidence intervals is limited. Furthermore, the follow-up time of 41 months is relatively short, given the promising overall 5-year survival.
In conclusion, MFS is a soft tissue sarcoma that has a propensity for local recurrence as a result of its infiltrative growth pattern. In this study, OS for MFS patients was influenced by tumor size and patient age, and local recurrence was predicted by margin status. The liberal use of RT in 77 % of treated patients, in tandem with aggressive surgical resection aimed at negative microscopic margins, demonstrated promising 5-year rates of local and DRFS and a low rate of multiple local recurrences. In addition, no patient with a grade 1 tumor in this series developed recurrent disease at a median follow-up of 65 months. Future research is needed to elucidate the role of radiation and chemotherapy in the treatment of MFS, but on the basis of the findings of this study, we believe that RT should be considered for all patients diagnosed with grade 2 or 3 MFS and that consideration should be given to staged surgery/reconstruction followed by postoperative RT. Finally, given the complexity and rarity of MFS, these patients should be assessed in a multidisciplinary environment where a team-oriented approach can optimize management and surveillance.
ACKNOWLEDGMENT
Supported in part by Biostatistics Core of Dana-Farber/Harvard Cancer Center, supported by NCI Cancer Center Support Grant NIH 5 P30 CA06516.
REFERENCES
- 1.World Health Organization classification of tumours. Mentzel TMD, Calonje EMD, Wadden CFIBMS, et al. Myxofibrosarcoma: clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;17:595–600. doi: 10.1097/00000478-199604000-00001. 1. [DOI] [PubMed] [Google Scholar]
- 2.Merck C, Angervall L, Kindblom L, Oden A. Myxofibrosarcoma. A malignant soft tissue tumor of fibroblastic–histiocytic origin. A clinicopathologic and prognostic study of 110 cases using multivariate analysis. Acta Pathol Microbiol Immunol Scand. 1983;282(Suppl.):1–40. [PubMed] [Google Scholar]
- 3.Fletcher CD, Gustafson P, Rydholm A, Willen H, Akerman M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol. 2001;19:3045–50. doi: 10.1200/JCO.2001.19.12.3045. [DOI] [PubMed] [Google Scholar]
- 4.Angervall L, Kindblom LG, Merck C. Myxofibrosarcoma: a study of 30 cases. Acta Pathol Microbiol Scand. 1977;85A:127–40. [PubMed] [Google Scholar]
- 5.Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977:1685. doi: 10.1002/1097-0142(197704)39:4<1672::aid-cncr2820390442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumours. IARC Press; Lyon: 2002. Pathology and genetics of tumors of soft tissue and bone; pp. 102–3. [Google Scholar]
- 7.Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251:506–11. doi: 10.1097/SLA.0b013e3181cf87fa. [DOI] [PubMed] [Google Scholar]
- 8.Waters B, Panicek DM, Lefkowitz RA, et al. Low-grade myxo-fibrosarcoma: CT and MRI patterns in recurrent disease. Am J Roentgenol. 2007;188:W193–8. doi: 10.2214/AJR.05.1130. [DOI] [PubMed] [Google Scholar]
- 9.Huang HY, Lal P, Qin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Hum Pathol. 2004;35:612–21. doi: 10.1016/j.humpath.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Manoso MW, Pratt J, Healey JH, Boland PJ, Athanasian EA. Infiltrative MRI pattern and incomplete initial surgery compromise local control of myxofibrosarcoma. Clin Orthop. 2006;450:89–94. doi: 10.1097/01.blo.0000229292.98850.14. [DOI] [PubMed] [Google Scholar]
- 11.Haglund KE, Raut CP, Nascimento AF, Wang Q, George S, Baldini EH. Recurrence patterns and survival for patients with intermediate and high grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2010;82:361–7. doi: 10.1016/j.ijrobp.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Sanfilippo R, Miceli R, Grosso F, et al. Myxofibrosarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2011;18:720–5. doi: 10.1245/s10434-010-1341-4. [DOI] [PubMed] [Google Scholar]
- 13.Lin CN, Chou SC, Li CF, et al. Prognostic factors of myxofibrosarcomas: implications of margin status, tumor necrosis, and mitotic rate on survival. J Surg Oncol. 2006;93:294–303. doi: 10.1002/jso.20425. [DOI] [PubMed] [Google Scholar]
- 14.Mentzel TMD, Calonje EMD, Wadden CFIBMS, et al. Myxofibrosarcoma: clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;17:595–600. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Fukunaga N, Fukunaga M. Low grade myxofibrosarcoma: progression in recurrence. Pathol Int. 1997;47:161–5. doi: 10.1111/j.1440-1827.1997.tb03734.x. [DOI] [PubMed] [Google Scholar]
- 16.Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft tissue sarcoma treated with conservative surgery and radiation therapy: an analysis of 1,225 patients. Cancer. 2003;97:2530–43. doi: 10.1002/cncr.11365. [DOI] [PubMed] [Google Scholar]
- 17.Kaya M, Wada T, Nagoya S, et al. MRI and histological evaluation of the infiltrative growth pattern of myxofibrosarcoma. Skeletal Radiol. 2008;37:1085–90. doi: 10.1007/s00256-008-0542-4. [DOI] [PubMed] [Google Scholar]
- 18.Hafner J, Schütz K, Morgenthaler W, Steiger E, Meyer V, Burg G. Micrographic surgery (“slow Mohs”) in cutaneous sarcomas. Dermatology. 1999;198:37–43. doi: 10.1159/000018062. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft tissue sarcomas of the extremities: prospective randomized evaluations of limb-sparing surgery plus radiation therapy compared with amputation and the role of adjuvant chemotherapy. Cancer. 1982;196:305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–68. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]