Abstract
FhSAP2 is a novel antigen isolated from the adult fluke of Fasciola hepatica. Based on sequence similarity with amoebapores and other related proteins, it belongs to the saposin-like protein (SAPLIP) family. FhSAP2 has been shown to be highly immunogenic and capable of inducing protective immune responses in mice and rabbits challenged with F. hepatica. Moreover, FhSAP2 is also reactive with sera from humans with chronic fascioliasis. In the present study, we investigated the expression of FhSAP2 in various developmental stages of F. hepatica by qPCR and demonstrated that FhSAP2-mRNA species are up-regulated in undeveloped eggs, newly excysted juveniles, and adults, but down-regulated in the miracidium stage. Monoclonal antibodies against FhSAP2 were produced, and two clones that are positive to F. hepatica whole-body extract, but not reactive with extracts from other trematodes, were selected, expanded and used for histolocalization studies. Confocal immunofluorescence revealed the presence of native FhSAP2 in epithelial cells surrounding the gut, toward the outermost part of the tegument, and toward the tegumental cells of both adults and newly excysted juveniles.
Keywords: Fasciola hepatica, monoclonal antibody, qPCR, immunohistochemistry
1. Introduction
FhSAP2 is a novel antigen isolated from the Fasciola hepatica adult fluke, which, by sequence similarity with amoebapores and other related proteins, belongs to the saposin-like protein (SAPLIP) family (Espino and Hillyer, 2003). The saposin signature motif consists of a compact domain of mainly α-helical character that contains six cysteine residues and seven hydrophobic conserved residues and has been implicated in membrane binding, pore formation, and subsequent cell lysis in several family members (Bruhn, 2005; Zhai and Saier, 2000). Although members of the SAPLIP family have different biological functions, they are all able to interact with lipids.
In addition to FhSAP2, four other isoforms of SAPLIPs have been reported in Fasciola species. The F. hepatica isoform is designated as FhSAP1 (Reed et al., 2000), and the isoforms from F. gigantica are termed FgSAP1, FgSAP2 and FgSAP3 (Grams et al., 2006). Because recombinant FhSAP2 has been shown to lyse both erythrocytes and peripheral blood mononuclear cells (Espino and Hillyer, 2003), a possible role in nutrient acquisition has been suggested for Fasciola SAPLIPs. The released cell contents would be available for enzymatic digestion and uptake by the parasite's tegument, as has been demonstrated for small molecules in schistosomes (Skelly et al., 1994). However, a decisive role for FhSAP2 in nutrition remains to be determined by further experiments.
Fascioliasis caused by F. hepatica is an economically important disease that imposes an immense burden on global livestock production, estimated to cost more than US$2 billion per annum, and also a recognized food-borne zoonotic disease affecting approximately 2.4 million people (Mas-Coma, 2005). As an alternative to the chemotherapeutic approach to combat the disease, we have used FhSAP2 as a vaccine and found that it is highly immunogenic and capable of inducing a strong anti-pathologic effect and a reduction in liver fluke burden of 81.2% in rabbits (Espino and Hillyer, 2004) and 60-83.3% in mice (Espino et al., 2010). Furthermore, we also demonstrated that FhSAP2 is a good antigen for antibody detection in chronic human fascioliasis (Figueroa-Santiago et al., 2011) and is highly reactive with sera from animals within 2 weeks of infection (Espino and Hillyer, 2003; Espino and Hillyer, 2004). However, whether FhSAP2 is expressed, as a predominant antigen in juveniles or adults remains unknown. In adult F. gigantica, for example, FgSAP2 is the predominant product, whereas in immature flukes, FgSAP1 predominates (Grams et al., 2006). In the present study, we investigated the differential expression of FhSAP2 within various developmental stages of F. hepatica using qPCR. Moreover, we developed monoclonal antibodies (mAbs) against recombinant FhSAP2 and investigated the specific localizations of the native protein in the adult and juvenile stages of F. hepatica. These mAbs could help to improve the detection of F. hepatica in infected hosts, thus contributing to fascioliasis control.
2. Materials and methods
2.1. Adult parasites
F. hepatica adult flukes were removed from the bile ducts of condemned bovine livers at a local abattoir and transported to the laboratory in warm RPMI 1640-medium. Schistosoma japonicum and S. mansoni were collected by perfusion from the mesenteric region of mice, 8 weeks after infection by skin penetration with 200 cercariae from each species. All parasite specimens were washed several times with PBS (0.1M phosphate-buffered saline pH 7.4) to remove all traces of blood, bile and contaminating residues. Clonorchis sinensis adult flukes were obtained lyophilized from the parasites library of the University of Puerto Rico.
2.2. Miracidia
During transportation, F. hepatica releases a large number of eggs into the medium, which are allowed to settle. After several washes, the eggs were examined at ×100 magnification to confirm the absence of visible contaminants, and then stored at −20°C until use. Some eggs were allowed to mature by incubation in the dark at 22°C for 9-12 days. Miracidia were stimulated to hatch by exposing them to light for 2h at 25°C (Caban-Hernandez et al., 2012). Free-swimming miracidia were collected using a transfer pipette, immediately snap-frozen and stored.
2.3. Newly excysted juveniles (NEJs)
Metacercariae (obtained from Baldwin Aquatics, Inc, Monmouth, Oregon) were washed in distilled water, transferred to watch glasses and pre-incubated in 1.2% sodium bicarbonate, 0.9% sodium chloride, 0.8% sodium tauroglycolate for 30 minutes at 37°C. Metacercariae were then transferred to watch glasses and allowed to excyst for up to 3 h at 37°C in excystment medium freshly prepared by diluting the pre-incubation solution 1:1 with 0.33% HCl and 0.8% L-cysteine. Newly excysted juveniles (NEJs) were removed from the excystment medium and maintained in fresh Fasciola saline (FS; Dulbecco's modified Eagle's medium (DMEM) [w/o NaHPO3 and PO−3] plus 0.5ml/ml distilled water, 2.2mM Ca[C2H3O2]2, 2.7mM MgSO4, 61.1mM glucose, 1μM serotonin, 5μg/ml gentamicin, 15mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), pH 7.4) at 37°C. NEJs were collected, snap-frozen and stored at −80°C until use (Caban-Hernandez et al., 2012).
2.4. Real-time PCR analysis (qPCR) of FhSAP2 transcript
Total RNA was extracted from undeveloped eggs, miracidia, NEJs, and adult flukes, using a PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA) and then treated with Turbo DNA-free endonuclease (Ambion) to remove contaminating genomic DNA. The RNA was quantified with a Nanodrop spectrophotometer, and equal amounts (100ng) of RNA from each life-cycle stage were used to synthesize cDNA. The reverse transcription reaction was performed with High Capacity RNA to cDNA kit following the manufacturer's instructions (BioAnalytical Instruments, San Juan, PR). qPCR was performed using a SYBR Green PCR Master Mix (BioAnalytical Instruments) in a final volume of 25 μl and 200nM of each primer, and each reaction underwent 40 amplification cycles using an Applied Biosystems StepOne Plus Real-Time PCR system. The cycling conditions were as follows: 95°C/15 min followed by 40 cycles of 94°C/15 s, 55°C/30 s, 72°C/30 s. Dissociation curves were generated for each sample to verify the amplification of a single product. RNA levels were quantified using the 2−ΔΔCt method (Livak and Schmittgen, 2001) with F. hepatica GADPH as the internal control transcript (Rinaldi et al., 2008). The following primers were designed for the qPCR experiments: FhSAP2 (forward: 5’-GCAAATAGCTCGCTCTCAGGAT-3’; reverse: CAGATTTCCGTTGCGTTGTG-3’,); Fh-GAPDH (forward: 5’-GCGCCAATGTTCGTGTTCGG-3’; reverse: 5’-TGGCCGTGTACGAATGCAC-3’).
2.5. Preparation of whole-body antigens (WB) of adult parasites
Whole-body extracts of F. hepatica (FhWB), S. mansoni (SmWB), S. japonicum (SjWB) and C. sinensis (CsWB) adult worms were prepared as previously described (Caban-Hernandez et al., 2012). Briefly, dried worms were homogenized with a Teflon homogenizer in 0.1M PBS with 5 mM EDTA, 8 mM phenylmethylsulfonyl fluoride (PMSF), 2 mM iodoacetamide, and 2 mM leupeptin. The homogenate was centrifuged at 30,000 × g for 30 min at 4°C and the supernatant was collected. Protein concentration was determined by the bicinchoninic acid (BCA) method (Smith et al., 1985). These extracts were stored at −80°C until used in subsequent experiments.
2.6. Preparation of recombinant F. hepatica saposin-like 2 protein (FhSAP2)
cDNA encoding FhSAP2 (GenBank AF286903) was cloned into the restriction sites of pBAD/HisB expression vector (Invitrogen) and this plasmid was used to transform E. coli TOP10 to over-express the polypeptide as a fusion protein containing six histidine residues at the amino terminus. The expressed protein was purified using Ni2+ affinity column chromatography as previously described (Espino and Hillyer, 2003).
2.7. Production and screening of monoclonal antibodies (mAbs) against FhSAP2
BALB/c mice were immunized subcutaneously with 20μg FhSAP2 in complete Freund's adjuvant. The second and third injection of a similar dose of the recombinant protein in incomplete Freund's adjuvant was given at 2-week intervals. Hybridomas were produced by fusion of spleen cells from BALB/c mice immunized with FhSAP2 and mouse myeloma cells (P3x63-Ag8.653) using polyethylene glycol (PEG) (Sigma-Aldrich, St Louis, MO). The limiting dilution method was used to clone the hybridoma cells that successfully grew in culture. Indirect ELISA was used to screen the mAbs produced by the hybridoma clones. Flat-bottomed microtiter plates (Maxisorp, Nunc) were coated with 2.5 μg/ml of FhSAP2, FhWB, SmWB, SjWB or CsWB in 0.05M NaHCO3/Na2CO3 buffer (pH 9.6) overnight (O/N) at 4°C. The plates were blocked with 3% bovine serum albumin (BSA) in PBS containing 0.05% Tween-20 (PBST) for 1 h at 37°C. Hybridoma culture supernatants were incubated for 1 h at 37°C. Bound antibodies were detected after the addition of 100μl/well of a horseradish peroxidase-conjugated rabbit anti-mouse IgG diluted 1:2000 in PBST for 1 h at 37°C and then 20mg O-phenylenediamine, 0.07% H2O2 in citrate buffer (pH 5.5) for 20 min at room temperature (RT). The reaction was stopped with 50μl of 12.5% sulfuric acid and the plates were read at 490nm. All washes between steps were performed with PBST. Each ELISA determination was performed in duplicate and the results expressed as the mean OD for each determination. ELISA was considered positive when the mean OD of a determined mAb was ≥ 0.2, which is the cut-off established based on the mean OD + 3SD of the normal mouse serum (NMS) or myeloma culture fluid (MCF). mAbs with OD values >0.5 in the FhSAP2-ELISA were considered highly reactive and selected for isotype determination. For antibody isotyping, the same protocol was followed but mAbs were diluted 1:10 in PBST and horseradish peroxidase-conjugated rabbit anti-mouse IgG1, IgG2a or IgG2b (Sigma) diluted 1:1000 was used.
2.8. Immunoblotting
SDS-PAGE and immunoblotting were performed as previously described (Gaudier et al., 2012). Briefly, FhSAP2 and WB extracts were separated using 12.5% SDS-PAGE and transferred onto nitrocellulose membranes and probed with the selected mAb clones. Membranes incubated with MCF and NMS were used as negative controls. Nonspecific binding was blocked with 3% BSA in PBST at RT for 1 h. Peroxidase-conjugated rabbit anti-mouse IgG was used to detect the mAb-antigen complex. The reaction was visualized with further incubation in diaminobenzidine as chromogenic substrate until the bands appeared.
2.9. Immunolocalization of FhSAP2 using mAbs
mAbs were used to localize native FhSAP2 in sections of F. hepatica adult flukes and whole mounts of NEJs using immunofluorescence microscopy as previously described, with minor modifications (Gaudier et al., 2012). Briefly, after fixing the specimens, the samples were incubated at RT in 100mM ammonium chloride in PBS for 10 min followed by incubation with PBST for 20 min at RT. Then, non-specific binding was blocked by incubating the sections in 0.1% glycine in PBS (30 min, at RT) followed by a 2-h incubation at RT in 3% BSA in PBS. To block non-specific binding, NEJ whole-mounts were blocked by incubating the preparations in Antibody Diluent Solution (AbD: 0.1M PBS, pH 7.4, containing 0.1% [V/V] Triton X-100, 0.1% [W/V] BSA, and 0.1% [W/V] NaN3). Following the blocking step, the sections and NEJ mounts were incubated overnight with the selected mAb (culture supernatant). After several washes to remove excess antibody, the specimens were incubated in fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG at 1:200 dilution at RT followed by incubation in phalloidintetramethylrhodamine isothiocyanate (TRITC) to counterstain muscle. All specimens were examined using a Zeiss LSM 510 META Laser Scanning Confocal Microscope.
3. Results
3.1. qPCR analysis of FhSAP2 mRNA
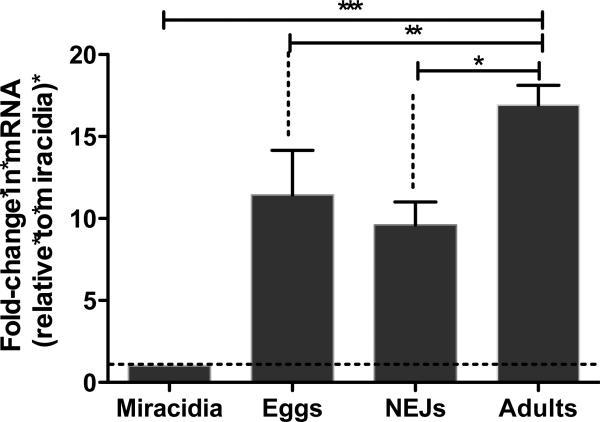
Expression of FhSAP2 at the mRNA level in F. hepatica was evaluated in adults, eggs, miracidia and NEJs by using qPCR. The data were standardized relative to F. hepatica GADPH, and the 2−ΔΔCt method was used to quantify relative FhSAP2 expression. FhSAP2 mRNA was expressed in undeveloped eggs, NEJs and adult parasites, but the expression was significantly lower in miracidia. We therefore used the miracidium stage as a calibrator to determine quantitative differences among levels of expression of FhSAP2. The results demonstrate that FhSAP2 mRNA is elevated 17-fold in adults, 12-fold in-undeveloped eggs, and 10-fold in NEJs compared to miracidia (P<0.001). No statistical differences were found between the expression levels of FhSAP2-mRNA in undeveloped eggs compared to NEJs. However, the difference in expression of FhSAP2-mRNA in adults compared to NEJs (P < 0.0023) or eggs (P < 0.03) was found to be statistically significant (Fig. 1).
Figure 1. Relative FhSAP2–mRNA levels at different stages of the life cycle of F. hepatica as determined by quantitative PCR (qPCR).
Results are shown as the fold change in expression relative to that of the miracidium stage and are the mean ± SEM of a minimum of three experiments, each in triplicate. *Differences in the FhSAP2–mRNA levels were statistically significant between adults and NEJs (P< 0.0023), ** between adults and undeveloped eggs (P<0.03), and *** between adults, NEJs or undeveloped eggs compared to miracidia (P<0.0001).
3.2. Screening and selection of mAbs to FhSAP2
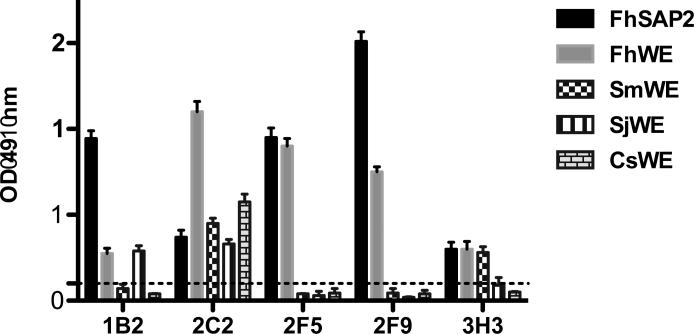
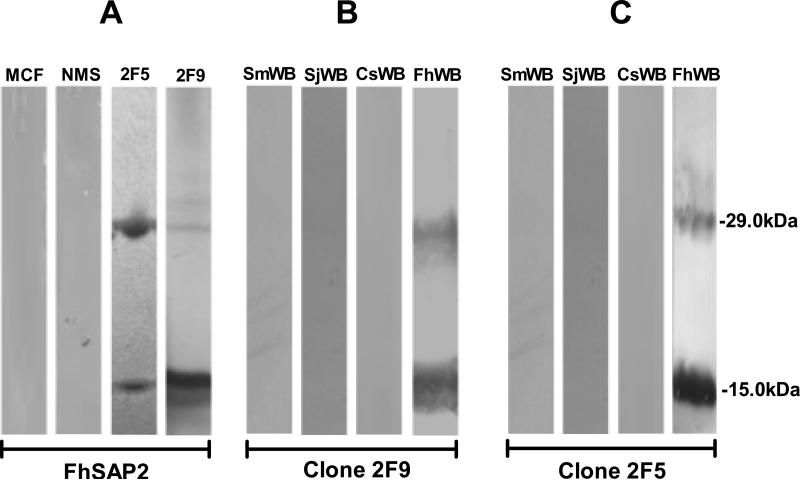
Twenty-two stables clones of mAbs were obtained, of which five (1B2, 2C2, 2F5, 2F9 and 2H3) were found to be highly reactive with FhSAP2 (Fig. 2). Immunoglobulin isotypes of these mAbs were determined to be IgG1 for 2H3 and 1B2, IgG2a for 3D3 and 2F5, and IgG2b for 2F9. All mAbs were κ-light chain. These five mAbs were re-tested against total protein extract of adult F. hepatica and other trematodes to determine possible cross-reactions. Clones 2F5 and 2F9 reacted exclusively with F. hepatica extract whereas clones 1B2, 2C2 and 2H3 showed cross-reactions with extracts from S. mansoni, S. japonicum or C. sinensis (Fig. 2). The specificity of clones 2F5 and 2F9 was further corroborated by Western blot. Both clones reacted strongly with a polypeptide of 14kDa typical of the recombinant FhSAP2, which includes 11.5kDa of the amino acid sequence plus 2.5kDa corresponding to the His-tag located at the amino terminus of the protein moiety (6 × His + the Enterokinase site). In addition, these mAbs reacted with a polypeptide of ~29kDa that corresponds to the oligomeric form of FhSAP2 (Espino and Hillyer, 2003). mAbs 2F5 and 2F9 are also reactive with FhWB and do not cross-react with the extracts of others trematodes (Fig. 3). Therefore, clones 2F5 and 2F9, highly specific for F. hepatica, were selected for large-scale production and used in immunolocalization studies.
Figure 2. ELISA of mAbs against different F. hepatica antigens.
mAbs were tested against recombinant F. hepatica saposin like-protein 2 (FhSAP2), whole body extract (WE) from F. hepatica (FhWE), Schistosoma mansoni (SmWE), S. japonicum (SjWE) and Clonorchis sinensis (CsWE) using indirect ELISA. All mAbs were highly reactive with FhSAP2 (black bar) and FhWE (gray bar) with OD readings > 0.5. However, mAbs 1B2, 2C2 and 2H3 cross-reacted with extracts from S. mansoni, S. japonicum or C. sinensis. Dashed line represents the cut-off point (=0.2), which was calculated as the mean OD + 3SD of the normal mouse serum (NMS) or myeloma culture fluid (MCF).
Figure 3. Specificity of monoclonal antibodies against FhSAP2 analyzed by Western blot.
Proteins were separated by 12.5% SDS-PAGE and transferred onto nitrocellulose membranes. (A) Membranes containing FhSAP2 were incubated with myeloma culture fluid (MCF) and normal mouse serum (NMS) as negative controls or probed with monoclonal antibodies 2F5 and 2F9, which recognize both the monomeric (12-15kDa) and oligomeric forms (29kDa) of FhSAP2. (B) mAb-2F9 and 2F5 (C) are reactive only with F. hepatica whole-body extract (FhWB) and do not react with proteins from whole-body extracts of Schistosoma mansoni (SmWB), S. japonicum (SjWB), Clonorchis sinensis (CsWB). mAb-2F9 and 2F5 recognize native FhSAP2 in both monomeric and oligomeric forms.
3.3. Immunolocalization
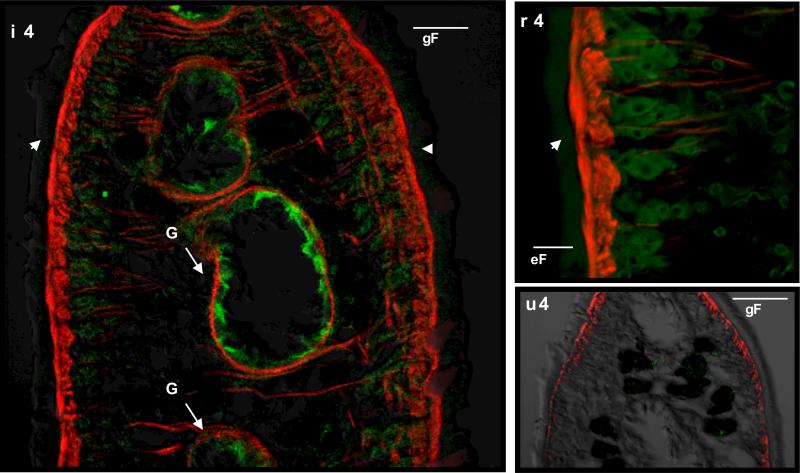
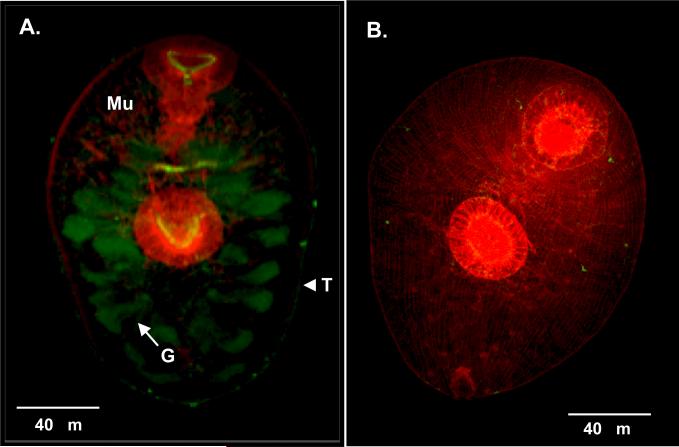
mAbs 2F5 and 2F9 incubated separately with adult sections detected native FhSAP2 in gut epithelial cells. Moreover, in the tegument, weak staining that appeared as a thin line of fluorescence or a green layer surrounding the parasite could be seen, and the tegumental cells underneath the tegument also exhibited immunoreactivity (Fig. 4). In NEJs, FhSAP2 was also localized in the gut and in the outermost part of the tegument, the glycocalyx (Fig. 5). No staining was observed when adult sections or NEJs were incubated with normal mouse serum or myeloma culture fluid. Although both mAbs showed identical recognition patterns, the fluorescence was much more intense with mAb-2F5. Figures 3 and 4 show the staining pattern obtained with clone 2F5 as representative of both mAbs.
Figure 4. Immunolocalization of native FhSAP2 in F. hepatica adult worm.
(A) Cross-section of the middle part of the body of an adult fluke (×20) stained with supernatant of 2F5 mAb showing a thin line of fluorescence at the outermost part of the tegument (glycocalyx) (T) and intense staining toward the gut (G). Phalloidin-TRITC was used to counter-stain muscle (Mu). (B) A ×100 view of a tegument section showing strong fluorescence staining toward the tegumental cells (TC) underneath the tegument (T). (C) No staining was observed when mAb 2F5 was substituted by myeloma culture fluid.
Figure 5. Immunolocalization of native FhSAP2 in F. hepatica newly excysted juveniles.
(A) Whole-worm mount of an NEJ (×40) stained with supernatant of 2F5 showing intense staining in the gut and a thin line of fluorescence surrounding the tegument (T) of the parasite. Phalloidin-TRITC was used to counter-stain muscle (Mu). (B) No staining was observed in controls in which supernatant of 2F5 was substituted by myeloma culture fluid.
4. Discussion
In the present study, we demonstrate that Fasciola hepatica saposin like-protein 2 (FhSAP2) mRNA is present in varying amounts during parasite development. The observation that FhSAP2 is highly expressed in undeveloped eggs suggests that this protein could have a role in the development of the miracidium or in miracidial hatching, which would be consistent with the pore-forming and lytic functions of an amoebapore-like protein (Hecht et al., 2004) and various members of the saposin-like protein family, including FhSAP2 (Bruhn, 2005; Espino and Hillyer, 2003). After hatching, the miracidium must penetrate the appropriate snail intermediate host to continue its life cycle. Down-regulation of the FhSAP2-gene in the miracidium indicates a lack of requirement for FhSAP2 during the short life span of this larva in the aquatic environment as well as during its penetration into the snail host. Once inside the snail, the miracidium transforms sequentially into a sporocyst, a redia and a cercaria, which emerges from the snail and encysts on aquatic plants as a metacercaria. After excystment, both the NEJs and adult flukes exhibit high levels of FhSAP2 mRNA expression. These data indicate that FhSAP2 has an important role in adaptation and survival of the parasite to its new environment, when F. hepatica enters the mammalian host. Here, we did not assess FhSAP2 mRNA expression in juvenile stages; however, we had previously demonstrated by northern blot that mRNA species obtained from 4-week-old juveniles hybridize with FhSAP2 cDNA (Espino and Hillyer, 2003). Collectively, these results indicate that FhSAP2 is continuously expressed by parasites from the metacercaria excystment stage until they become a mature egg-laying fluke, which is in agreement with the finding that high antibody levels against FhSAP2 are produced from early stages (2 weeks) to chronic stages in mice and rabbits infected with F. hepatica (Espino et al., 2010; Espino and Rivera, 2010). Interestingly, the homolog of FhSAP2 in the species F. gigantica (FgSAP2) that shares 89.1% identity and 93.1% similarity with FhSAP2 was found to be poorly expressed in NEJs (Grams et al., 2006). In contrast, the isoforms FgSAP1 and FhSAP1, which share almost 60% identity and 78% similarity with FhSAP2, are the dominant products of immature flukes (Grams et al., 2006; Reed et al., 2000). Such different expression patterns observed among molecules belonging to the same protein family in two closely related species suggest different functions for the FhSAP2 and FgSAP2 isoforms and could explain why lytic activity has so far been observed only in the case of FhSAP2 (Espino and Hillyer, 2003). Presumably, FhSAP2 could be involved in the lysis of hepatocytes during juvenile migration in the hepatic phase and of cells contained in blood during the adult stage.
In agreement with the qPCR results, the immunostaining studies localized native FhSAP2 in both NEJs and adult stages. The protein localization not only confirmed the tissue-specificity, but it also revealed that FhSAP2 is concentrated in the gut. This is consistent with the localization of various SAPLIPs of F. gigantica (Grams et al., 2006) and S. mansoni (Don et al., 2008). Given that many components of the excretory-secretory antigens are derived mainly from the cecum and tegument of the parasites, this localization also confirms the existence of a functional secretory signal-sequence in FhSAP2, first predicted by computational analysis to be at the N-terminal end of the protein. Indeed, we previously reported the presence of this protein in the excretion-secretion material of F. hepatica (Espino and Hillyer, 2003). Interestingly, native FhSAP2 was also localized toward the tegument surface of both NEJs and adult F. hepatica, as demonstrated by weak fluorescence staining, which is a localization that has not been reported for any other members of the parasite SAPLIP family (Don et al., 2008; Don et al., 2007; Grams et al., 2006; Kueakhai et al., 2011). The tegument of flukes is a surface syncytial layer covering the parasite and bounded externally by an outer membrane coated with a carbohydrate-rich glycocalix (Anuracpreeda et al., 2009; Dalton, 2004; Dangprasert et al., 2001). This interfacing layer is believed to play essential roles such as renewal of the surface plasma membrane, active uptake of nutrients, and evasion of the host immune system (Wilson et al., 2011). Localization of FhSAP2 in the tegument suggests that FhSAP2 could contribute to the maintenance and turnover of the surface membrane to prevent the attachment of host immune effectors cells, as suggested for some tegumental proteins of schistosomes (Tran et al., 2010); however this needs to be explored with further studies. Also, the localization of FhSAP2 in the gut led us to speculate that FhSAP2 could be primarily produced in the cecum of the parasite, and a portion of the protein might be further transported to the surface to be excreted through the tegument where it could act externally in the host environment.
The evolutionarily conserved arrangement of disulfide-linked amphipathic helices of SAPLIPs supports a wide array of biological functions. Protein–lipid interactions lead to cell-lysis activity by amoebapores, and, in other related groups, to stimulation of lipid catabolism (Munford et al., 1995). However, it is difficult at this stage to identify a specific role for FhSAP2 in F. hepatica. If FhSAP2 has an amoebapore-like capacity and forms ion channels within lipid bilayer membranes, it may contribute to the lymphotoxic activities attributed to F. hepatica excretory-secretory material (Cervi, 2009; Goose, 1978). Reed and colleagues also suggested a role for FhSAP1 in lipid metabolism, another SAPLIP in F. hepatica, since insufficiency in de novo fatty acid and cholesterol synthesis is a trait of trematodes (Reed et al., 2000). Also, we cannot discount a possible role in parasite tegument formation, as seen for other trematode tegumental proteins (Tran et al., 2010). Thus, it is reasonable to assume that F. hepatica expresses a cluster of SAPLIPs, as has been seen in Entamoeba histolytica (Leippe et al., 1994a; Leippe et al., 1994b), where some members of the SAPLIP family could have a role in parasite feeding or migration in juveniles and others could have a role in adult establishment in bile ducts and survival within the host.
Aside from the studies of Hanna and colleagues, who developed mAbs against F. hepatica somatic and tegumental antigens (Hanna and Trudgett, 1983; Hanna et al., 1988), the majority of mAbs developed for Fasciola have been produced against antigens of F. gigantica. These include mAbs against partially purified native proteins derived from tegument (Anuracpreeda et al., 2006; Anuracpreeda et al., 2009; Chaithirayanon et al., 2002; Krailas et al., 2002) to mAbs against recombinant proteins of well-known biological function such as fatty acid binding proteins (Sirisriro et al., 2002) and a member of the SAPLIP protein family from F. gigantica (FgSAP2) (Kueakhai et al., 2013). Two F. hepatica mAbs (ES78 and MM3), produced against excretory-secretory antigens, have been well characterized (Espino et al., 1998; Mezo et al., 2007) and both have been shown to be excellent diagnostic reagents for detecting coproantigens in humans and sheep with acute or chronic infection (Dumenigo et al., 2000; Espino et al., 1998; Ubeira et al., 2009; Valero et al., 2009). The mAbs developed in the present study are highly specific for FhSAP2 and do not cross-react with antigens from S. mansoni, S. japonicum or C. sinensis, trematodes closely related to F. hepatica. Another interesting observation is the demonstration by Western blot of both monomeric and oligomeric forms of FhSAP2 in the crude extract of F. hepatica adult flukes. This indicates that oligomer formation is a natural event during the synthesis of FhSAP2 in F. hepatica, which seems to be a common characteristic of members of the SAPLIP family. Oligomerization is one of the proposed mechanisms by which these molecules are thought to interact with membranes and lipids to exert their functions (Willis et al., 2011).
Recently, we reported that FhSAP2 is a useful antigen for antibody detection by ELISA in sera of chronic human fascioliasis (Figueroa-Santiago et al., 2011). However, this method could only detect circulating antibodies, which precludes detection of active infection. Conversely, the mAbs 2F5 and 2F9 developed here against FhSAP2 could be used to detect both circulating or coproantigens of the parasite. mAbs are more specific than a polyclonal antibody for antigen capturing (Cordova et al., 1999), which would help to improve the immunodiagnosis of human fascioliasis. Because of the abundant expression of FhSAP2 at different stages of parasite development and its release into excretory-secretory parasite products (Espino and Hillyer, 2003), FhSAP2 should be an antigen easily detected in body fluids by these monoclonal antibodies.
In conclusion, in the present study we provide direct evidence that FhSAP2 is differentially expressed in different developmental stages of F. hepatica. Quantitative-PCR analyses showed up-regulation of FhSAP2 transcript in undeveloped eggs, NEJs and adult worms, which suggests a role for this protein during mammalian host infection. Second, anti-FhSAP2 mAbs revealed the protein to be localized in the gut and tegument of NEJs and adult parasites. These results confirmed the qPCR data and reinforced the potential role of this antigen in parasite biology. Finally, because of the proven specificity of the mAbs developed in this study, these antibodies could be used to develop a mAb-based antigen-capturing assay to detect active infection. This assay will complement the FhSAP2-based antibody detection ELISA previously developed, thus improving substantially the immunodiagnosis of human fascioliasis. Studies are in progress to develop a mAb-based capture sandwich ELISA to detect FhSAP2 in body fluids using these mAbs.
Research Highlights.
✓ F. hepatica saposin-like 2 protein is differentially expressed
✓ FhSAP2 is strongly expressed in the gut and tegument
✓ Specific monoclonal antibodies (mAbs) to FhSAP2 are κ-light IgG2a and IgG2b
✓ Both mAbs recognize monomeric and/or dimeric forms of FhSAP2
✓ Both mAbs show identical recognition patterns of FhSAP2 by immunofluorescence
Acknowledgments
This research was supported by grants from the NIH-1SC1AI096108-01A2 and MBRS-RISE of the University of Puerto Rico R25GM061838-13. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors thank Dr. Daryl Henderson for proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anuracpreeda P, Wanichanon C, Chaithirayanon K, Preyavichyapugdee N, Sobhon P. Distribution of 28.5 kDa antigen in the tegument of adult Fasciola gigantica. Acta Trop. 2006;100:31–40. doi: 10.1016/j.actatropica.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Anuracpreeda P, Wanichanon C, Sobhon P. Fasciola gigantica: immunolocalization of 28.5 kDa antigen in the tegument of metacercaria and juvenile fluke. Exp. Parasitol. 2009;122:75–83. doi: 10.1016/j.exppara.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Bruhn H. A short guided tour through functional and structural features of saposin-like proteins. Biochem. J. 2005;389:249–257. doi: 10.1042/BJ20050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caban-Hernandez K, Gaudier JF, Espino AM. Characterization and differential expression of a ferritin protein from Fasciola hepatica. Mol. Biochem. Parasitol. 2012;182:54–61. doi: 10.1016/j.molbiopara.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervi L, Serradel MC, Guasconi L, Masih DT. New insights into the modulation of immune response by Fasciola hepatica excretory-secretory products. Curr. Immunol. Rev. 2009;5:277–284. [Google Scholar]
- Chaithirayanon K, Wanichanon C, Vichasri-Grams S, Ardseungneon P, Grams R, Viyanant V, Upatham ES, Sobhon P. Production and characterization of a monoclonal antibody against 28.5 kDa tegument antigen of Fasciola gigantica. Acta Trop. 2002;84:1–8. doi: 10.1016/s0001-706x(02)00138-9. [DOI] [PubMed] [Google Scholar]
- Cordova M, Reategui L, Espinoza JR. Immunodiagnosis of human fascioliasis with Fasciola hepatica cysteine proteinases. Trans R Soc Trop Med Hyg. 1999;93:54–57. doi: 10.1016/s0035-9203(99)90178-5. [DOI] [PubMed] [Google Scholar]
- Dalton J, Skelly P, Halton DW. Role of the tegument and gut in nutrient uptake by parasitic platyhelminths. Can. J. Zool. 2004;82:211–232. [Google Scholar]
- Dangprasert T, Khawsuk W, Meepool A, Wanichanon C, Viyanant V, Upatham ES, Wongratanacheevin S, Sobhon P. Fasciola gigantica: surface topography of the adult tegument. J. Helminthol. 2001;75:43–50. doi: 10.1079/joh200041. [DOI] [PubMed] [Google Scholar]
- Don TA, Bethony JM, Loukas A. Saposin-like proteins are expressed in the gastrodermis of Schistosoma mansoni and are immunogenic in natural infections. Int. J. Infect. Dis. 2008;12:e39–47. doi: 10.1016/j.ijid.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Don TA, Oksov Y, Lustigman S, Loukas A. Saposin-like proteins from the intestine of the blood-feeding hookworm, Ancylostoma caninum. Parasitol. 2007;134:427–436. doi: 10.1017/S003118200600148X. [DOI] [PubMed] [Google Scholar]
- Dumenigo BE, Espino AM, Finlay CM, Mezo M. Kinetics of antibody-based antigen detection in serum and faeces of sheep experimentally infected with Fasciola hepatica. Vet. Parasitol. 2000;89:153–161. doi: 10.1016/s0304-4017(00)00206-5. [DOI] [PubMed] [Google Scholar]
- Espino AM, Diaz A, Perez A, Finlay CM. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J. Clin. Microbiol. 1998;36:2723–2726. doi: 10.1128/jcm.36.9.2723-2726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J. Parasitol. 2003;89:545–552. doi: 10.1645/GE-3113. [DOI] [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. A novel Fasciola hepatica saposin-like recombinant protein with immunoprophylactic potential. J. Parasitol. 2004;90:876–879. doi: 10.1645/GE-215R. [DOI] [PubMed] [Google Scholar]
- Espino AM, Morales A, Delgado B, Rivera FM, Figueroa O, Suarez E. Partial immunity to Fasciola hepatica in mice after vaccination with FhSAP2 delivered as recombinant protein or DNA construct. Ethn. Dis. 2010;20(1 Suppl 1):S1–17-23. [PMC free article] [PubMed] [Google Scholar]
- Espino AM, Rivera F. Quantitation of cytokine mRNA by real-time RT-PCR during a vaccination trial in a rabbit model of fascioliasis. Vet. Parasitol. 2010;169:82–92. doi: 10.1016/j.vetpar.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Santiago O, Delgado B, Espino AM. Fasciola hepatica saposin-like protein-2-based ELISA for the serodiagnosis of chronic human fascioliasis. Diagn. Microbiol. Infect. Dis. 2011;70:355–361. doi: 10.1016/j.diagmicrobio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudier JF, Caban-Hernandez K, Osuna A, Espino AM. Biochemical characterization and differential expression of a 16.5-kilodalton tegument-associated antigen from the liver fluke Fasciola hepatica. Clin. Vacc. Immunol. 2012;19:325–333. doi: 10.1128/CVI.05501-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goose J. Possible role of excretory/secretory products in evasion of host defences by Fasciola hepatica. Nature. 1978;275:216–217. doi: 10.1038/275216a0. [DOI] [PubMed] [Google Scholar]
- Grams R, Adisakwattana P, Ritthisunthorn N, Eursitthichai V, Vichasri-Grams S, Viyanant V. The saposin-like proteins 1, 2, and 3 of Fasciola gigantica. Mol. Biochem. Parasitol. 2006;148:133–143. doi: 10.1016/j.molbiopara.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hanna RE, Trudgett AG. Fasciola hepatica: development of monoclonal antibodies and their use to characterize a glycocalyx antigen in migrating flukes. Parasite Immunol. 1983;5:409–425. doi: 10.1111/j.1365-3024.1983.tb00756.x. [DOI] [PubMed] [Google Scholar]
- Hanna RE, Trudgett AG, Anderson A. Fasciola hepatica: development of monoclonal antibodies against somatic antigens and their characterization by ultrastructural localization of antibody binding. J. Helminthol. 1988;62:15–28. doi: 10.1017/s0022149x00011147. [DOI] [PubMed] [Google Scholar]
- Hecht O, Van Nuland NA, Schleinkofer K, Dingley AJ, Bruhn H, Leippe M, Grotzinger J. Solution structure of the pore-forming protein of Entamoeba histolytica. J. Biol. Chem. 2004;279:17834–17841. doi: 10.1074/jbc.M312978200. [DOI] [PubMed] [Google Scholar]
- Krailas D, Panomsuk S, Janecharat T, Ukong S. Production of monoclonal antibodies against partially purified surface tegument antigens of Fasciola gigantica. Southeast Asian J. Trop. Med. Public Health 33 Suppl. 2002;3:92–96. [PubMed] [Google Scholar]
- Kueakhai P, Changklungmoa N, Chaithirayanon K, Songkoomkrong S, Riengrojpitak S, Sobhon P. Production and characterization of a monoclonal antibody against recombinant saposin-like protein 2 of Fasciola gigantica. Acta Trop. 2013;125:157–162. doi: 10.1016/j.actatropica.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kueakhai P, Meemon K, Changklungmoa N, Chaithirayanon K, Riengrojpitak S, Sobhon P. Characterization and localization of saposin-like protein-2 (SAP-2) in Fasciola gigantica. Parasitol Res. 2011;108:1493–1500. doi: 10.1007/s00436-010-2201-7. [DOI] [PubMed] [Google Scholar]
- Leippe M, Andra J, Muller-Eberhard HJ. Cytolytic and antibacterial activity of synthetic peptides derived from amoebapore, the pore-forming peptide of Entamoeba histolytica. Proc. Natl. Acad. Sci. U. S. A. 1994a;91:2602–2606. doi: 10.1073/pnas.91.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leippe M, Andra J, Nickel R, Tannich E, Muller-Eberhard HJ. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol. Microbiol. 1994b;14:895–904. doi: 10.1111/j.1365-2958.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J. Helminthol. 2005;79:207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- Mezo M, Gonzalez-Warleta M, Ubeira FM. The use of MM3 monoclonal antibodies for the early immunodiagnosis of ovine fascioliasis. J. Parasitol. 2007;93:65–72. doi: 10.1645/GE-925R.1. [DOI] [PubMed] [Google Scholar]
- Munford RS, Sheppard PO, O'Hara PJ. Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 1995;36:1653–1663. [PubMed] [Google Scholar]
- Reed MB, Strugnell RA, Panaccio M, Spithill TW. A novel member of the NK-lysin protein family is developmentally regulated and secreted by Fasciola hepatica. Mol. Biochem. Parasitol. 2000;105:297–303. doi: 10.1016/s0166-6851(99)00185-1. [DOI] [PubMed] [Google Scholar]
- Rinaldi G, Morales ME, Cancela M, Castillo E, Brindley PJ, Tort JF. Development of functional genomic tools in trematodes: RNA interference and luciferase reporter gene activity in Fasciola hepatica. PLoS Negl. Trop. Dis. 2008;2:e260. doi: 10.1371/journal.pntd.0000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisriro A, Grams R, Vichasri-Grams S, Ardseungneon P, Pankao V, Meepool A, Chaithirayanon K, Viyanant V, Tan-Ariya P, Upatham ES, Sobhon P. Production and characterization of a monoclonal antibody against recombinant fatty acid binding protein of Fasciola gigantica. Vet Parasitol. 2002;105:119–129. doi: 10.1016/s0304-4017(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Skelly PJ, Kim JW, Cunningham J, Shoemaker CB. Cloning, characterization, and functional expression of cDNAs encoding glucose transporter proteins from the human parasite Schistosoma mansoni. J. Biol. Chem. 1994;269:4247–4253. [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tran MH, Freitas TC, Cooper L, Gaze S, Gatton ML, Jones MK, Lovas E, Pearce EJ, Loukas A. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010;6:e1000840. doi: 10.1371/journal.ppat.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeira FM, Muino L, Valero MA, Periago MV, Perez-Crespo I, Mezo M, Gonzalez-Warleta M, Romaris F, Paniagua E, Cortizo S, Llovo J, Mas-Coma S. MM3-ELISA detection of Fasciola hepatica coproantigens in preserved human stool samples. Am J Trop Med Hyg. 2009;81:156–162. [PubMed] [Google Scholar]
- Valero MA, Ubeira FM, Khoubbane M, Artigas P, Muino L, Mezo M, Perez-Crespo I, Periago MV, Mas-Coma S. MM3-ELISA evaluation of coproantigen release and serum antibody production in sheep experimentally infected with Fasciola hepatica and F. gigantica. Vet. Parasitol. 2009;159:77–81. doi: 10.1016/j.vetpar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Willis C, Wang CK, Osman A, Simon A, Pickering D, Mulvenna J, Riboldi-Tunicliffe A, Jones MK, Loukas A, Hofmann A. Insights into the membrane interactions of the saposin-like proteins Na-SLP-1 and Ac-SLP-1 from human and dog hookworm. PLoS One. 2011;6:e25369. doi: 10.1371/journal.pone.0025369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RA, Wright JM, de Castro-Borges W, Parker-Manuel SJ, Dowle AA, Ashton PD, Young ND, Gasser RB, Spithill TW. Exploring the Fasciola hepatica tegument proteome. Int. J. Parasitol. 2011;41:1347–1359. doi: 10.1016/j.ijpara.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Saier MH., Jr. The amoebapore superfamily. Biochim. Biophys. Acta. 2000;1469:87–99. doi: 10.1016/s0304-4157(00)00003-4. [DOI] [PubMed] [Google Scholar]