Abstract
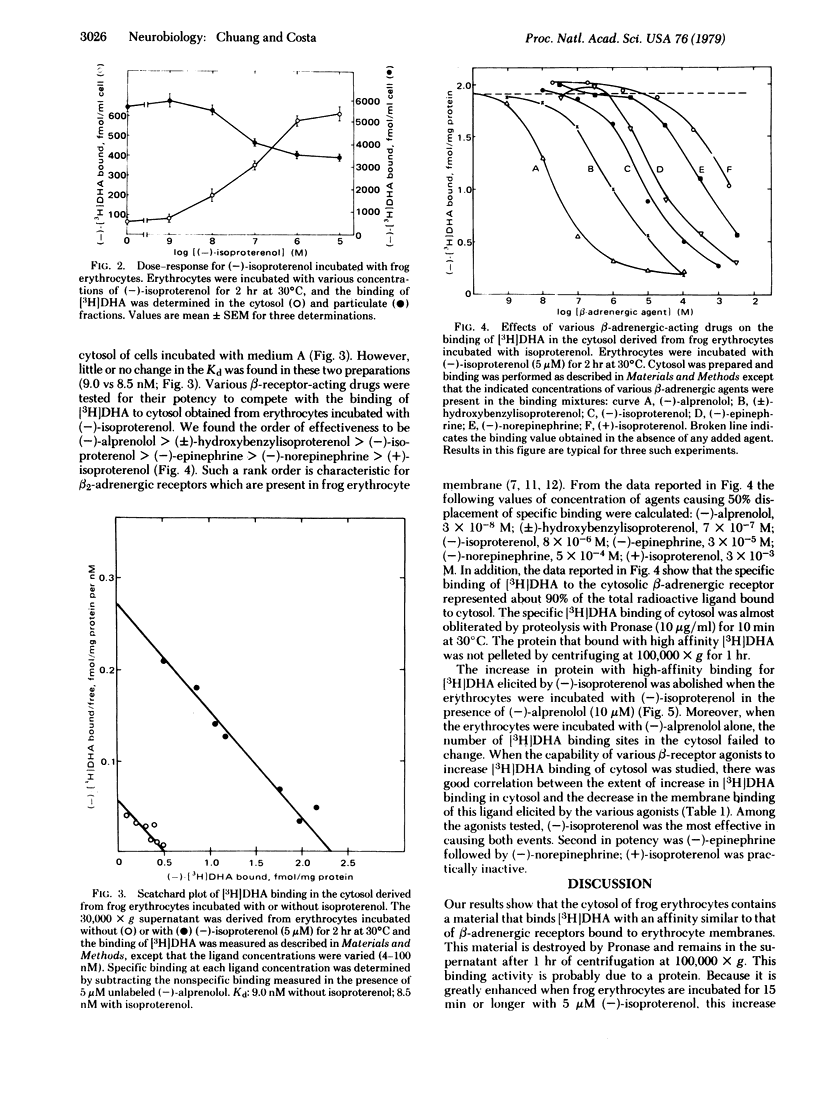
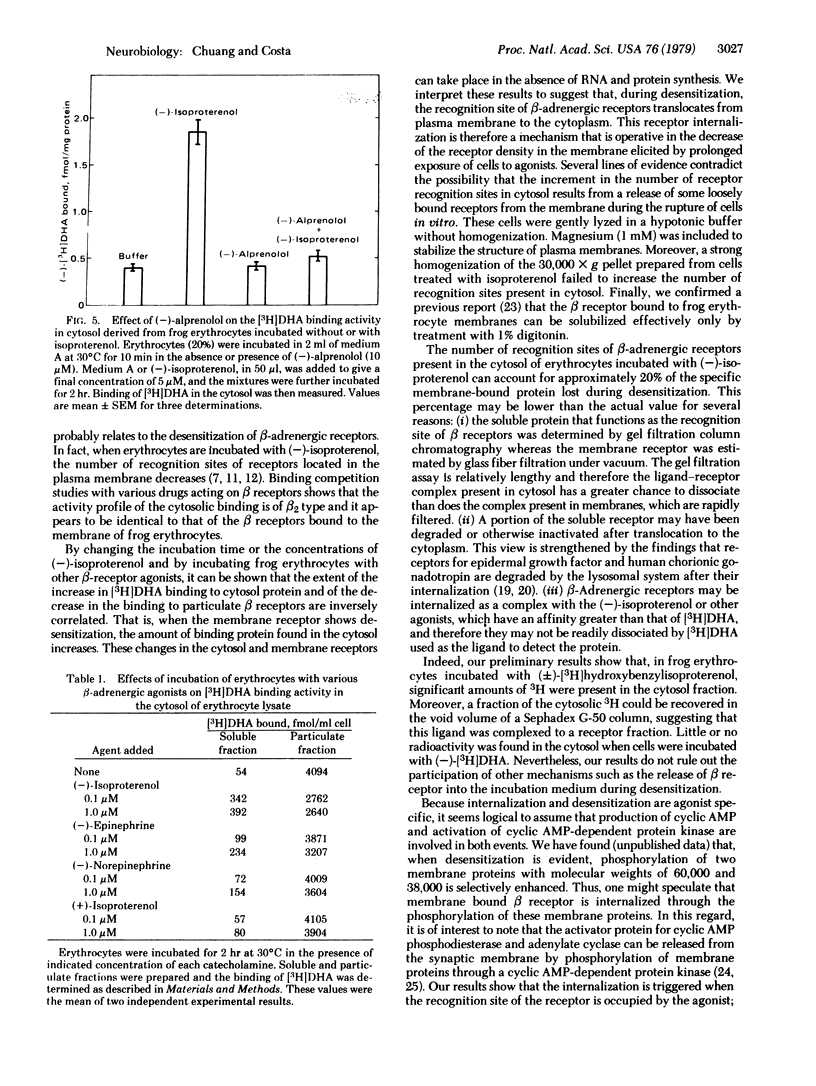
In the supernatant (30,000 × g) of frog erythrocyte homogenates, by using gel filtration we detected a protein that could bind [3H]dihydroalprenolol ([3H]DHA) with high affinity. This binding was greatly enhanced when the erythrocytes were preincubated with (-)-isoproterenol. After various periods of incubation with (-)-isoproterenol, the extent of the increase in the density of [3H]DHA binding sites in the cytosol was paralleled by a proportional decrease in the number of [3H]DHA binding sites in the corresponding pellet; both events peaked after 2-3 hr of incubation with (-)-isoproterenol. The Ka of the (-)-isoproterenol-induced increase in [3H]DHA binding in cytosol and the decrease in this binding in the membrane ranged between 60 and 90 nM. The changes in the cytosol and particulate [3H]DHA binding sites were independent of RNA and protein synthesis. The increase in cytosol binding elicited by (-)-isoproterenol was blocked by exposure of the cells to (-)-alprenolol which per se failed to change the cytosol binding of [3H]DHA. Scatchard analysis revealed that the enhanced [3H]DHA binding to cytosol material was due to a 4-fold increase in the Bmax with little or no change in Kd (≈9 nM). Binding displacement data show that these soluble [3H]DHA binding sites resemble the surface membrane recognition sites. Moreover, the ability of various β-adrenergic agents to increase [3H]DHA binding to cytosol after they were incubated with frog erythrocytes paralleled their affinity for membrane-bound β receptors. These findings support the view that the β-adrenergic receptor desensitization caused by prolonged exposure to (-)-isoproterenol is due, at least in part, to an internalization of the recognition site of β-adrenergic receptors.
Keywords: receptor desensitization, receptor internalization, frog erythrocytes, adenylate cyclase
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Puett D. Intracellular uptake and catabolism of lutropin by testicular tissue in vivo. FEBS Lett. 1977 Mar 15;75(1):77–82. doi: 10.1016/0014-5793(77)80057-4. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Conti M., Harwood J. P., Dufau M. L., Catt K. J. Internalisation of gonadotrophin--receptor complex in ovarian luteal cells. Nature. 1978 Aug 10;274(5671):598–600. doi: 10.1038/274598a0. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVellis J., Brooker G. Reversal of catecholamine refractoriness by inhibitors of RNA and protein synthesis. Science. 1974 Dec 27;186(4170):1221–1223. doi: 10.1126/science.186.4170.1221. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Hormone-induced desensitisation of hormonal control of cyclic AMP levels in human diploid fibroblasts. Nat New Biol. 1973 Dec 5;246(153):146–148. doi: 10.1038/newbio246146a0. [DOI] [PubMed] [Google Scholar]
- Gnegy M. E., Costa E., Uzunov P. Regulation of transsynaptically elicited increase of 3':5'-cyclic AMP by endogenous phosphodiesterase activator. Proc Natl Acad Sci U S A. 1976 Feb;73(2):352–355. doi: 10.1073/pnas.73.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy M. E., Uzunov P., Costa Regulation of dopamine stimulation of striatal adenylate cyclase by an endogenous Ca++ -binding protein. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3887–3890. doi: 10.1073/pnas.73.11.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. The fate of insulin bound to adipocytes. Evidence for compartmentalization and processing. J Biol Chem. 1978 Jul 25;253(14):4900–4906. [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L. A., Butcher R. W. The effects of epinephrine and prostaglandin E-1 on cyclic adenosine 3':5'-monophosphate levels in WI-38 fibroblasts. J Biol Chem. 1974 May 25;249(10):3098–3102. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauzon G. J., Kulshrestha S., Starr L., Bär H. P. On the accumulation of adenosine 3':5'-monophosphate in Ehrlich cells and adenylate cyclase desensitization in response to epinephrine. J Cyclic Nucleotide Res. 1976;2(2):99–114. [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Williams L. T. A desensitized state of the beta adrenergic receptor not associated with high-affinity agonist occupancy. Mol Pharmacol. 1978 Mar;14(2):376–380. [PubMed] [Google Scholar]
- Limbird L. E., Lefkowitz R. J. Agonist-induced increase in apparent beta-adrenergic receptor size. Proc Natl Acad Sci U S A. 1978 Jan;75(1):228–232. doi: 10.1073/pnas.75.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makman M. H. Properties of adenylate cyclase of lymphoid cells. Proc Natl Acad Sci U S A. 1971 May;68(5):885–889. doi: 10.1073/pnas.68.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J. V., Tate R., Mullikin D., Lefkowitz R. J. Regulation of adenylate cyclase-coupled beta adrenergic receptor binding sites by beta adrenergic catecholamines in vitro. Mol Pharmacol. 1976 May;12(3):409–419. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee C., Lefkowitz R. J. Desensitization of beta-adrenergic receptors by beta-adrenergic agonists in a cell-free system: resensitization by guanosine 5'-(beta, gamma-imino)triphosphate and other purine nucleotides. Proc Natl Acad Sci U S A. 1976 May;73(5):1494–1498. doi: 10.1073/pnas.73.5.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe D. S., Ciosek C. P., Jr, Ishikawa Y., Fahey J. V. Human synoviocytes: activation and desensitization by prostaglandins and 1-epinephrine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3124–3128. doi: 10.1073/pnas.72.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike L. J., Lefkowitz R. J. Agonist-specific alterations in receptor binding affinity associated with solubilization of turkey erythrocyte membrane beta adrenergic receptors. Mol Pharmacol. 1978 Mar;14(2):370–375. [PubMed] [Google Scholar]
- Su Y. F., Cubeddu L., Perkins J. P. Regulation of adenosine 3':5'-monophosphate content of human astrocytoma cells: desensitization to catecholamines and prostaglandins. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):257–270. [PubMed] [Google Scholar]
- Williams L. T., Lefkowitz R. J. Slowly reversible binding of catecholamine to a nucleotide-sensitive state of the beta-adrenergic receptor. J Biol Chem. 1977 Oct 25;252(20):7207–7213. [PubMed] [Google Scholar]



