Abstract
The liver secretes triglyceride-rich VLDLs, and the triglycerides in these particles are taken up by peripheral tissues, mainly heart, skeletal muscle, and adipose tissue. Blocking hepatic VLDL secretion interferes with the delivery of liver-derived triglycerides to peripheral tissues and results in an accumulation of triglycerides in the liver. However, it is unclear how interfering with hepatic triglyceride secretion affects adiposity, muscle triglyceride stores, and insulin sensitivity. To explore these issues, we examined mice that cannot secrete VLDL [due to the absence of microsomal triglyceride transfer protein (Mttp) in the liver]. These mice exhibit markedly reduced levels of apolipoprotein B-100 in the plasma, along with reduced levels of triglycerides in the plasma. Despite the low plasma triglyceride levels, triglyceride levels in skeletal muscle were unaffected. Adiposity and adipose tissue triglyceride synthesis rates were also normal, and body weight curves were unaffected. Even though the blockade of VLDL secretion caused hepatic steatosis accompanied by increased ceramides and diacylglycerols in the liver, the mice exhibited normal glucose tolerance and were sensitive to insulin at the whole-body level, as judged by hyperinsulinemic euglycemic clamp studies. Normal hepatic glucose production and insulin signaling were also maintained in the fatty liver induced by Mttp deletion. Thus, blocking VLDL secretion causes hepatic steatosis without insulin resistance, and there is little effect on muscle triglyceride stores or adiposity.
Keywords: microsomal triglyceride transfer protein, triglyceride-rich lipoprotein, fatty liver, insulin resistance, obesity, de novo lipogenesis, ceramide, diacylglycerol
Most of the triglycerides in the plasma are carried by chylomicrons, which are secreted from the intestine, and by VLDLs, which are secreted by the liver (1). The triglycerides within chylomicrons and VLDL are hydrolyzed by lipoprotein lipase along the capillary endothelium, mainly in the heart, skeletal muscle, and adipose tissue (2). The lipids released from triglyceride-rich lipoproteins in capillaries are taken up by surrounding parenchymal cells and stored in triglyceride droplets or used for fuel within mitochondria.
We have sought to better define the metabolic impact of the triglyceride secretion from the liver. We reasoned that the delivery of VLDL triglycerides to adipose tissue might have a significant impact on adiposity and on the metabolic activity of adipose tissue. We also suspected that the secretion of triglycerides by the liver might have a significant impact on the stores of triglycerides in skeletal muscle. In the current study, we have begun to address these issues by examining adipose tissue size and activity, as well as skeletal muscle triglyceride stores, in mice that cannot secrete VLDL. For these studies, we took advantage of mice that lack microsomal triglyceride transfer protein (Mttp) in the liver; these mice assemble and secrete chylomicrons normally but cannot assemble triglyceride-rich lipoproteins in the liver.
The inability to secrete VLDL is accompanied by hepatic steatosis (3, 4). In other settings, hepatic steatosis is associated with insulin resistance (5–7). In the current study, we also tested whether the hepatic steatosis accompanying a blockade in VLDL secretion would result in insulin resistance.
MATERIALS AND METHODS
Mice
We bred mice homozygous for a conditional allele of Mttp and the inducible Mx1-Cre transgene (Mttpflox/floxMx1-Cre mice) (4,8,9). Cre expression was induced by injecting 5 week-old Mttpflox/floxMx1-Cre mice with polyinosinic-polycytidylic ribonucleic acid (pI-pC; Sigma, St. Louis, MO; 500 μg every other day for 10 days). The series of pI-pC injections inactivated Mttp in the liver, eliminating VLDL production, but did not cause significant changes in intestinal lipoprotein production (4). In previous studies (4, 10) and in the current study, we refer to the pI-pC-treated Mttpflox/floxMx1-Cre mice as MttpΔ/Δ mice. In the analysis of MttpΔ/Δ mice, we used untreated (i.e., no pI-pC injections) age- and sex-matched Mttpflox/floxMx1-Cre littermates as controls. Mice were housed in a virus-free barrier facility with a 12 h light/dark cycle. In most experiments, mice were fed a chow diet containing 4.5% fat (Ralston Purina, St. Louis, MO). Some mice were fed a high-fat diet (fat, 60% of calories; carbohydrate, 20% of calories; D12492, Research Diets, New Brunswick, NJ) for 4 months. The fat was derived mostly from lard, the carbohydrates were mainly sucrose and maltodextrin (1:2), and the protein was mainly casein. In those studies, the body weight of mice was monitored biweekly. At the conclusion of this study, mice were fasted for 5h, and body fat was measured by dual energy X-ray absorptiometry with a PixiMus2 scanner (GE Healthcare Lunar, Madison, WI). Liver, inguinal and epididymal adipose tissues, and blood were collected. In other experiments, mice were fed a high-carbohydrate diet (960238, INC Biochemicals, Solon, OH) for 1 month. This diet contained vitamin-free casein (21%), sucrose (58.5%), and Alphacel Non-nutritive bulk (16.5%) and salt mixture U.S.P. XIV (4%)
Lipids and insulin measurements, and gene expression studies
Plasma triglycerides, cholesterol, free fatty acids, and insulin were measured by commercial kits [triglycerides, cholesterol, free fatty acids kits were from Roche Diagnostics (Indianapolis, IN); the insulin kit was from ALPCO Diagnostics (Salem, NH)]. The distribution of lipids within the plasma lipoproteins was assessed after overnight fasting by fast protein liquid chromatography (FPLC) with a Superose 6 10/30 column (Pharmacia Fine Chemicals, Piscataway, NJ) (11). Apolipoprotein B-48 (apoB-48) and apoB-100 in fasting plasma were detected by Western blot analysis as described previously (10, 12). Muscle (gastrocnemius) and liver triglyceride levels were measured after overnight fasting by chemical analysis followed by a modified Folch lipid extraction (13, 14). RNA from adipose tissue was extracted with Trizol reagent (Sigma). To assess gene expression in adipose tissue, RNA was pooled from control mice and MttpΔ/Δ mice (n = 9 in each group), and mRNA levels were measured in triplicate as previously described (15).
Studies of glucose metabolism and insulin sensitivity in MttpΔ/Δ mice
Glucose tolerance tests were performed on 8–12 week-old chow-fed mice after a 4 h fast. Glucose (2 g/kg) was injected intraperitoneally, and blood was drawn from a tail vein at 0, 15, 30, 60, and 120 min. Hyperinsulinemic euglycemic clamp studies were performed on 16 week-old mice (n = 5–8). An indwelling catheter for insulin and glucose infusion was placed into a left femoral vein of anesthetized mice. Mice were allowed to recover for 4–7 days. After a 5 h fast, a 180 min hyperinsulinemic euglycemic clamp study was conducted in awake, freely moving mice, as previously described (11, 16–18). In a first set of clamp experiments, mice were infused with high (18 mU/kg/min) doses of insulin, in order to measure systemic insulin sensitivity. Briefly, human insulin was administered as a prime-continuous infusion at the start of the clamp, and 20% glucose was infused at variable rates and adjusted every 10 min to clamp plasma glucose levels at 6 mM. In a second set of clamp experiments, a lower dose of insulin (2.5 mU/kg/min) and [3-3H]glucose (NEN Life Sciences, Boston, MA) were infused [10 μCi bolus, followed by 0.05 μCi/min (basal) and 0.1 μCi/min (clamp)] to estimate insulin-stimulated whole-body glucose flux and to calculate hepatic glucose production. With this dose of insulin, hepatic glucose production is not totally suppressed (19). After a 2 h stabilization period, blood was sampled from the tail every 10 min during the last 40 min of the clamp study. Glucose concentrations in plasma samples were measured using a glucose oxidation method (Thermo Electron, Waltham, MA), and [3H]glucose was quantified as previously described (17). Rates of basal and clamp glucose turnover were determined as the ratio of the [3-3H] glucose infusion rate to the specific activity of plasma glucose at the end of the basal period and during the final 40 min of the clamp period, respectively. Hepatic glucose production was determined by subtracting the rate of glucose infusion from the rate of whole-body glucose uptake. Suppression of hepatic glucose production was calculated compared with basal hepatic glucose production.
To measure hepatic insulin signaling, insulin (5 U/kg) was injected into overnight-fasted mice via the inferior vena cava. Groups of mice were euthanized at baseline and 3 min after the injection, and the liver was harvested. Protein was extracted and blotted with Akt (Ser-473) and total Akt antibody (1:1,000 dilution) (Cell Signaling Technology, Danvers, MA). Western blots of liver proteins (extracted from livers of mice that had not been given insulin) were also performed with an antibody against phosphoenolpyruvate carboxykinase (PEPCK) (1:1,000 dilution; antibody provided by Dr. Françoise Assimacopoulos, University of Geneva). The secondary antibody for the Akt Western blots was an HRP-conjugated donkey anti-rabbit IgG (1:8,000 dilution; Amersham, Piscataway, NJ). The secondary antibody for the PEPCK Western blot was an HRP-conjugated rabbit anti-sheep IgG (1:5,000 dilution). The Western blots were developed with the ECL chemiluminescence system (Amersham) followed by exposure to X-ray film.
Measurements of triglyceride synthesis and de novo lipogenesis
Triglyceride synthesis rate and de novo lipogenesis were measured by mass isotopomer distribution analysis (20, 21). Five days before the end of an experiment, deuterated water (2H2O, 99.8% pure) was injected intraperitoneally (20 μl/g body weight) to achieve ∼3% 2H2O body water. Mice were then given 8% 2H2O in their drinking water for 5 days. Mice were euthanized after 4 h of fasting, and inguinal adipose tissue and the gastrocnemius muscle were removed. Deuterated water enrichment was measured in blood as described previously (20, 21).
Lipids from adipose tissue and skeletal muscle were extracted by a modified Folch method, and triglycerides were separated by TLC with hexane-ethyl ether-acetic acid (80:20:1). Triglycerides were then transmethylated with 3 N methanolic hydrochloric acid (Sigma) at 75°C for 3 h. For the analysis of triglyceride synthesis, glycerol was extracted and derived to glycerol triacetate by 1 h incubation with acetic anhydride-pyridine (2:1) (13, 22). Glycerol triacetate [mass-to-charge ratios 159 (M0), 160 (M1), and 161 (M2)]) was analyzed by GC-MS, and the fractional triglyceride synthesis rate was calculated by isotopomer enrichment (20, 23). The absolute rate of triglyceride synthesis was calculated from the fractional rate and adipose tissue weight. For the analysis of de novo lipogenesis, the fatty acid fraction was extracted from transmethylated triglycerides and measured by GC-MS. The fractional rate of de novo lipogenesis was calculated by mass isotopomer distribution analysis, and the absolute rate was calculated based on the adipose weight (20, 24).
Liver ceramides and diacylglycerols were measured by Acquity Ultra Performance LC coupled to QT of Premier time-of-flight mass spectrometer (Waters, Inc., Milford, MA) as described (25).
Statistical analyses
Results are expressed as means ± SEM. A Student’s t-test was used to compare groups.
RESULTS
MttpΔ/Δ mice have low plasma lipid levels and develop hepatic steatosis
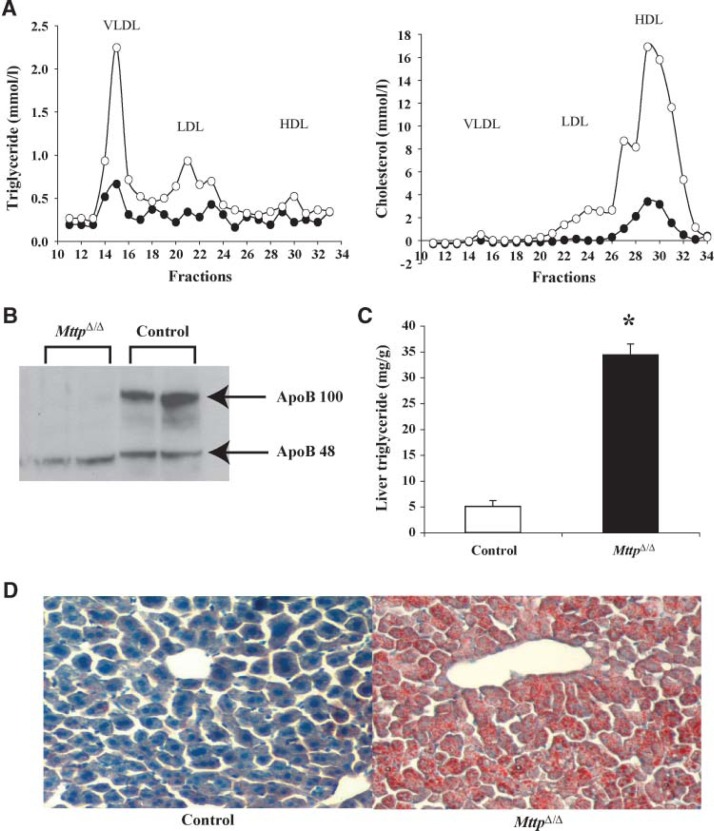
MttpΔ/Δ mice had low plasma levels of triglycerides and cholesterol after a 4 h fast (Table 1). VLDL triglycerides were virtually absent in the plasma of MttpΔ/Δ mice after overnight fasting, as judged by FPLC fractionation of the plasma (Fig. 1A). The amount of apoB-100 in the plasma of MttpΔ/Δ mice was dramatically reduced, whereas the levels of apoB-48 were reduced minimally (Fig. 1B). The amount of triglycerides in the livers of chow-fed MttpΔ/Δ mice was 7-fold greater than in control mice (Fig. 1C). Increased amounts of neutral lipids were observed in the liver sections of MttpΔ/Δ mice (Fig. 1D).
TABLE 1.
Plasma glucose, insulin, and lipid measurements
| Control | MttpΔ/Δ | |
|---|---|---|
| Glucose (mg/dl) | 64.30 ± 2.43 (n = 27) | 56.54 ± 1.79a (n = 24) |
| Insulin (μg/l) | 0.30 ± 0.04 (n = 20) | 0.24 ± 0.04 (n = 20) |
| Free fatty acids (μM) | 272.00 ± 26.66 (n = 8) | 222.56 ± 25.61 (n = 9) |
| Triglycerides (mg/dl) | 58.50 ± 1.69 (n = 8) | 32.56 ± 3.04a (n = 9) |
| Cholesterol (mg/dl) | 94.75 ± 3.75 (n = 8) | 52.44 ± 4.01a (n = 9) |
Results are given as mean ± SEM. Glucose and insulin were measured after an overnight fast. Plasma lipids were measured after a 4 h fast.
P < 0.05.
Fig. 1.
Analysis of MttpΔ/Δ mice. A: Distribution of cholesterol and triglycerides in the plasma lipoproteins of MttpΔ/Δ and control mice that had been fasted overnight. Open circles, control mice; closed circles, MttpΔ/Δ. B: Western blot analysis of apolipoprotein B-100 (apoB-100) and apoB-48 in the plasma of MttpΔ/Δ and control mice that had been fasted overnight. C: Liver triglycerides in MttpΔ/Δ and control mice after overnight fasting (n = 5, * P < 0.05) (mean ± SEM). D: Oil Red O staining of liver sections from MttpΔ/Δ and control mice following overnight fasting (original magnification × 10).
Normal adipose tissue stores in MttpΔ/Δ mice
The body fat contents of MttpΔ/Δ and control mice were not statistically different on a chow diet (Fig. 2A). Even on a high-fat diet, weight gain and fat mass in MttpΔ/Δ mice were identical to controls (Fig. 2A, B) (Table 2). Food intake during the high-fat diet was monitored, and there were no differences between MttpΔ/Δ mice and controls (MttpΔ/Δ, 2.73 ± 0.17 g/day; controls, 2.56 ± 0.10 g/day). The triglyceride synthesis rates in adipose tissue, measured during both the chow and high-fat diet, were similar in MttpΔ/Δ and control mice (Fig. 2C).
Fig. 2.
Body weight and adipose tissue studies in MttpΔ/Δ mice. A: Body fat measured by dual energy X-ray absorptiometry on a chow diet (n = 9–10) and after 4 months on a high-fat diet (n = 15–18). Open bar, control mice; closed bar, MttpD/D mice. (mean ± SEM). B: Weight gain on a high-fat diet (n = 16–18). Open circles, control mice; closed circles, MttpΔ/Δ mice. C: Triglyceride synthesis in inguinal fat as judged by mass isotopomer distribution analysis on chow (n = 8) and high-fat diets (n = 7–9) (mean ± SEM). Similar results were observed in epididymal fat. D: Fractional de novo lipogenesis in adipose tissue in MttpΔ/Δ and control mice on chow and high-fat diets, as measured by mass isotopomer distribution analysis (n = 6–10). E: Similar absolute levels of de novo lipogenesis in adipose tissue of MttpΔ/Δ and control mice on chow and high-fat diets. F: Expression of three genes in adipose tissue that are upregulated during lipogenesis [ATP citrate lyase (ATPCL), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD-1)]. Studies were performed on a chow diet and after 1 month on a high-carbohydrate diet (HCHO). Samples from MttpΔ/Δ and control mice were pooled and analyzed in triplicate. Results were normalized to cyclophilin mRNA expression. Fold change was calculated compared with chow-fed control mice.
TABLE 2.
Body and tissue weights after 4 months on a high-fat diet
| Control (n = 18) | MttpΔ/Δ (n = 15) | |
|---|---|---|
| g | ||
| Body weight | 37.66 ± 1.06 | 40.96 ± 2.08 |
| Liver | 2.14 ± 0.14 | 3.43 ± 0.42a |
| Epididymal fat | 1.71 ± 0.16 | 1.37 ± 0.14 |
| Inguinal fat | 0.94 ± 0.10 | 0.95 ± 0.15 |
Results are given as mean ± SEM.
P < 0.05.
We suspected that MttpΔ/Δ mice might maintain normal adipose mass by activating de novo lipogenesis in adipose tissue. However, the fractional rates of de novo lipogenesis on the chow diet were similar in inguinal fat in both groups of mice (Fig. 2D). On the high-fat diet, the fractional de novo lipogenesis rates were reduced to a similar degree in both groups of mice. The absolute rates of de novo lipogenesis on the chow and high-fat diets were similar in both groups of mice (Fig. 2E). These studies suggest that both groups of mice were equally capable of gaining weight, and that the absence of VLDL secretion had little impact on either adiposity or de novo lipogenesis. We also examined gene expression in mice on both the chow and a high-carbohydrate diet. Genes important in de novo lipogenesis were increased to a similar degree in both groups of mice on the high-carbohydrate diet (Fig. 2F).
Normal skeletal muscle triglyceride stores in MttpΔ/Δ mice
We hypothesized that the reduced delivery of VLDL triglycerides to peripheral tissues might be associated with reduced stores of triglycerides in skeletal muscle, but this was not the case. Both MttpΔ/Δ and control mice had similar triglyceride levels in the gastrocnemius (Fig. 3A). Also, the fractional triglyceride synthesis rate in muscle was similar in the two groups of mice (Fig. 3B).
Fig. 3.

Skeletal muscle triglyceride levels in MttpΔ/Δ mice. A: Gastrocnemius triglyceride levels in MttpΔ/Δ and control mice that had been fasted overnight (n = 5) (mean ± SEM). B: Fractional triglyceride synthesis (%) in MttpΔ/Δ and control mice measured by mass isotopomer distribution analysis (n = 4) (mean ± SEM).
MttpΔ/Δ mice have normal insulin sensitivity despite liver steatosis
Hepatic steatosis is often associated with insulin resistance (5). Despite the presence of hepatic steatosis, fasting glucose levels in MttpΔ/Δ mice were lower than in control mice (Table 1). The insulin levels tended to be lower in MttpΔ/Δ, mice but this difference did not achieve statistical significance (Table 1). The MttpΔ/Δ mice and control mice had similar glucose levels after an intraperitoneal glucose load (Fig. 4A). A hyperinsulinemic euglycemic clamp study with a high dose of insulin revealed that a similar amount of glucose was required to maintain plasma glucose levels in both MttpΔ/Δ mice and control mice (Fig. 4B), indicating that MttpΔ/Δ mice did not develop systemic insulin resistance despite a fatty liver. To determine whether the liver exhibited normal insulin sensitivity, we measured hepatic glucose production during hyperinsulinemic euglycemic clamp with a lower dose of insulin and also analyzed hepatic Akt phosphorylation after insulin injection. The suppression of hepatic glucose production during the clamp was similar in both groups (Fig. 4C). Also, there was no difference in phosphorylation of Akt (Ser-473) in the different groups of mice, relative to total Akt (Fig. 4D). PEPCK levels were similar in MttpΔ/Δ and control mice (Fig. 4D). Despite an absence of insulin resistance, MttpΔ/Δ mice had higher content of ceramides and diacylglycerols in the liver (Fig. 4E).
Fig. 4.
Normal sensitivity to insulin in MttpΔ/Δ mice. A: Glucose levels in MttpΔ/Δ and control mice during a glucose tolerance test (n = 10) (mean ± SEM). Open circles, control mice; closed circles, MttpΔ/Δ mice. B: Glucose infusion rate during hyperinsulinemic euglycemic clamp study with a high insulin dose (n = 5–8). C: Suppression of hepatic glucose production (HGP) during a clamp with a low dose of insulin infusion (n = 9) (mean ± SEM). D: Akt phosphorylation before and after insulin infusion and hepatic phosphoenolpyruvate carboxykinase (PEPCK) protein levels in the liver of MttpΔ/Δ and control mice. E: Ceramides and diacylglycerols in the liver of MttpΔ/Δ and control mice after an overnight fast (n = 6) (mean ± SEM). * P < 0.05, significantly different from a control group.
DISCUSSION
Triglycerides are delivered to adipose tissue by intestinal chylomicrons and hepatic VLDLs. In an adult mouse, significant amounts of triglycerides, perhaps 25 to 50 mg/day, are secreted by the liver (26), and these triglycerides are mainly delivered to the muscle, heart, and adipose tissue (27). In the current study, we sought to better define the in vivo importance of VLDL triglycerides for peripheral tissues. To approach this issue, we studied mice that secreted chylomicrons from the intestine normally but were unable to assemble or secrete triglyceride-rich lipoproteins in the liver. We suspected that the inability to deliver hepatic triglycerides to peripheral tissues would significantly affect adiposity and/or result in secondary changes in de novo lipogenesis in adipose tissue or adipose tissue gene expression. However, the absence of VLDL secretion had almost no effect on body weight or adiposity, either on a chow or a high-fat diet. Also, there was no significant effect on de novo lipogenesis in adipose tissue or on the expression of genes associated with de novo lipogenesis. Similarly, eliminating VLDL production had no significant effect on skeletal muscle triglyceride stores. These findings suggest that the inability to assemble and secrete triglyceride-rich lipoproteins from the liver has little effect on adipose tissue or skeletal muscle triglyceride metabolism.
Because the rodent liver constantly produces VLDL triglycerides for distribution to peripheral tissues, the absence of peripheral effects in mice lacking VLDL secretion was somewhat unexpected. However, MttpΔ/Δ mice have no defect in their ability to absorb fat or produce chylomicrons. On a chow diet, mice consume approximately 200 mg of fats per day, and most of this lipid is presumably delivered into the circulation by chylomicrons. It seems possible that the ability to absorb dietary fats and produce chylomicrons in the intestine, along with the ability to carry fatty acids on albumin, outweighs the contribution of liver triglycerides and is sufficient to prevent major metabolic perturbations in adipose tissue and skeletal muscle. Another factor that could limit the peripheral consequences of absent VLDL could be the liver’s ability to release some lipids into the circulation on apoA-I-containing particles.
MttpΔ/Δ mice develop liver steatosis but no evidence of hepatic inflammation (3). Hepatic steatosis is often associated with insulin resistance in humans (28, 29) and in mousemodels (30–32). We suspected that the MttpΔ/Δ mice might develop insulin resistance, but this was not the case. Glucose tolerance tests and hyperinsulinemic euglycemic clamp tests indicated normal systemic sensitivity to insulin in MttpΔ/Δ mice. Their hepatic insulin sensitivity was also normal, as judged by a hyperinsulinemic euglycemic clamp with a lower dose of insulin. Increased levels of lipid intermediates, such as ceramide and diacylglycerols, have been shown to interfere with insulin signaling (33, 34). However, the MttpΔ/Δ mice had no insulin resistance despite increased levels of ceramide and diacylglycerols in the liver. Of note, these results are consistent with recent results on mice with overexpressing acyl-CoA:diacylglycerol acyltransferase 2 (DGAT2) in the liver (35). Both models exhibit elevated levels of lipid intermediates, but they do not have hepatic inflammation or develop insulin resistance. Thus, increased levels of these lipid intermediates are not sufficient to induce insulin resistance. Increased lipid intermediates in the setting of inflammation or other unknown factors may be required to induce hepatic insulin resistance.
In summary, blocking VLDL secretion in mice did not affect skeletal muscle triglyceride stores or adiposity and did not affect de novo lipogenesis in adipose tissue. Despite hepatic steatosis, blocking VLDL secretion in MttpΔ/Δ mice was not associated with insulin resistance. We conclude that VLDL does not play a major role in the delivery of triglyceride fuel to peripheral tissues, at least under the conditions that we studied. On the other hand, VLDL secretion is clearly important for protecting the liver from hepatic steatosis.
Acknowledgments
The authors thank Dr. Larry Rudel for help in estimating the amount of triglyceride carried in VLDL and chylomicrons in the mouse. The authors also thank Drs. Scott Turner and Ryan Streeper for scientific discussions; David Tarussio, Jerry Chen, Ben Hunrichs, Martin Decaris, and Tiffany Thomas, for technical assistance; Tuulikki Seppänen-Laakso for lipid analytical measurements; and the Mouse Metabolic Evaluation Facility at University of Lausanne for hyperinsulinemic euglycemic clamp studies.
Abbreviations:
- apo
apolipoprotein
- DGAT2
acyl-CoA:diacylglycerol acyltransferase 2
- FPLC
fast protein liquid chromatography
- Mttp
the gene for microsomal triglyceride transfer protein
- PEPCK
phosphoenolpyruvate carboxykinase
- pI-pC
polyinosinic-polycytidylic ribonucleic acid
Footnotes
This work was supported by the Swiss National Science Foundation, by a fellowship award from the American Heart Association, Western States Affiliate, and by l’association de Langue Francaise pour l’Etude du Diabete et des Maladies Metaboliques (ALFEDIAM).
REFERENCES
- 1.White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br. J. Nutr. 1998;80:219–229. [PubMed] [Google Scholar]
- 2.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J. Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 3.Bjorkegren J, Beigneux A, Bergo MO, Maher JJ, Young SG. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J. Biol. Chem. 2002;277:5476–5483. doi: 10.1074/jbc.M108514200. [DOI] [PubMed] [Google Scholar]
- 4.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann. Med. 2005;37:347–356. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]
- 6.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 7.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 2006;6:177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 8.Rajewsky K, Gu H, Kuhn R, Betz UA, Muller W, Roes J, Schwenk F. Conditional gene targeting. J. Clin. Invest. 1996;98:600–603. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raabe M, Kim E, Veniant M, Nielsen LB, Young SG. Using genetically engineered mice to understand apolipoprotein-B deficiency syndromes in humans. Proc. Assoc. Am. Physicians. 1998;110:521–530. [PubMed] [Google Scholar]
- 10.Minehira-Castelli K, Leonard SW, Walker QM, Traber MG, Young SG. Absence of VLDL secretion does not affect alpha-tocopherol content in peripheral tissues. J. Lipid Res. 2006;47:1733–1738. doi: 10.1194/jlr.M600125-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Tsao TS, Burcelin R, Katz EB, Huang L, Charron MJ. Enhanced insulin action due to targeted GLUT4 overexpression exclusively in muscle. Diabetes. 1996;45:28–36. doi: 10.2337/diab.45.1.28. [DOI] [PubMed] [Google Scholar]
- 12.McCormick SP, Ng JK, Veniant M, Boren J, Pierotti V, Flynn LM, Grass DS, Connolly A, Young SG. Transgenic mice that overexpress mouse apolipoprotein B. Evidence that the DNA sequences controlling intestinal expression of the apolipoprotein B gene are distant from the structural gene. J. Biol. Chem. 1996;271:11963–11970. doi: 10.1074/jbc.271.20.11963. [DOI] [PubMed] [Google Scholar]
- 13.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 14.Snyder F, Stephens N. A simplified spectrophotometric determination of ester groups in lipids. Biochim. Biophys. Acta. 1959;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- 15.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 16.Burcelin R, Eddouks M, Maury J, Kande J, Assan R, Girard J. Excessive glucose production, rather than insulin resistance, accounts for hyperglycaemia in recent-onset streptozotocin-diabetic rats. Diabetologia. 1995;38:283–290. doi: 10.1007/BF00400632. [DOI] [PubMed] [Google Scholar]
- 17.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 18.Burcelin R, Kamohara S, Li J, Tannenbaum GS, Charron MJ, Friedman JM. Acute intravenous leptin infusion increases glucose turnover but not skeletal muscle glucose uptake in ob/ob mice. Diabetes. 1999;48:1264–1269. doi: 10.2337/diabetes.48.6.1264. [DOI] [PubMed] [Google Scholar]
- 19.Shen HQ, Zhu JS, Baron AD. Dose-response relationship of insulin to glucose fluxes in the awake and unrestrained mouse. Metabolism. 1999;48:965–970. doi: 10.1016/s0026-0495(99)90191-9. [DOI] [PubMed] [Google Scholar]
- 20.Turner SM, Murphy EJ, Neese RA, Antelo F, Thomas T, Agarwal A, Go C, Hellerstein MK. Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am. J. Physiol. Endocrinol. Metab. 2003;285:E790–E803. doi: 10.1152/ajpendo.00402.2002. [DOI] [PubMed] [Google Scholar]
- 21.Chen JL, Peacock E, Samady W, Turner SM, Neese RA, Hellerstein MK, Murphy EJ. Physiologic and pharmacologic factors influencing glyceroneogenic contribution to triacylglyceride glycerol measured by mass isotopomer distribution analysis. J. Biol. Chem. 2005;280:25396–25402. doi: 10.1074/jbc.M413948200. [DOI] [PubMed] [Google Scholar]
- 22.Siler SQ, Neese RA, Parks EJ, Hellerstein MK. VLDL-triglyceride production after alcohol ingestion, studied using [2-13C1] glycerol. J. Lipid Res. 1998;39:2319–2328. [PubMed] [Google Scholar]
- 23.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am. J. Physiol. 1999;276:E1146–E1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- 24.Turner SM, Neese RA, Roohk DJ, Murphy EJ, Roy S, Samandi W, Hellerstein MK. Dissociation between adipose tissue fluxes and lipogenic gene expression in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2006;292:E1101–E1109. doi: 10.1152/ajpendo.00309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee RG, Shah R, Sawyer JK, Hamilton RL, Parks JS, Rudel LL. ACAT2 contributes cholesteryl esters to newly secreted VLDL, whereas LCAT adds cholesteryl ester to LDL in mice. J. Lipid Res. 2005;46:1205–1212. doi: 10.1194/jlr.M500018-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Havel RJ. Lipoproteins and lipid transport. Adv. Exp. Med. Biol. 1975;63:37–59. doi: 10.1007/978-1-4684-3258-9_3. [DOI] [PubMed] [Google Scholar]
- 28.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 29.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 30.Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc. Natl. Acad. Sci. USA. 2004;101:10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol. Behav. 2007;94:242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]