Abstract
Triacylglycerols (triglycerides) (TGs) are the major storage molecules of metabolic energy and FAs in most living organisms. Excessive accumulation of TGs, however, is associated with human diseases, such as obesity, diabetes mellitus, and steatohepatitis. The final and the only committed step in the biosynthesis of TGs is catalyzed by acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes. The genes encoding two DGAT enzymes, DGAT1 and DGAT2, were identified in the past decade, and the use of molecular tools, including mice deficient in either enzyme, has shed light on their functions. Although DGAT enzymes are involved in TG synthesis, they have distinct protein sequences and differ in their biochemical, cellular, and physiological functions. Both enzymes may be useful as therapeutic targets for diseases. Here we review the current knowledge of DGAT enzymes, focusing on new advances since the cloning of their genes, including possible roles in human health and diseases.
Keywords: triacylglycerols, triglycerides, acyl-CoA: diacylglycerol acyltransferase, diacylglycerol, fatty acyl-CoA, lipoprotein, adipose, obesity, intestine, mammary gland
Triacylglycerols (triglycerides) (TGs), a major type of neutral lipid, are a heterogeneous group of molecules with a glycerol backbone and three FAs attached by ester bonds. The physical and chemical properties of TG differ based on chain length and the degree to which their FAs are desaturated. TGs serve multiple important functions in living organisms. Chief among these, they are the major storage molecules of FA for energy utilization and the synthesis of membrane lipids. Because they are highly reduced and anhydrous, TGs store 6-fold more energy than the same amount of hydrated glycogen (1). In plants, TGs are a major component of seed oils, which are valuable resources for dietary consumption and industrial uses. TG from plants and microorganisms can also be used for biofuels. In animals, energy stores of TG are concentrated primarily in adipocytes, although TGs are also found prominently in myocytes, hepatocytes, enterocytes, and mammary epithelial cells. In addition to energy storage, TG synthesis in cells may protect them from the potentially toxic effects of excess FA. In the enterocytes and hepatocytes of most mammals, TGs are synthesized for the assembly and secretion of lipoproteins, which transport dietary and endogenously synthesized FA between tissues. Also, TGs in secreted lipids acts as a component of the skin’s surface water barrier, and collections of TG in adipose tissue provide insulation for organisms.
Although TGs are essential for normal physiology, the excessive accumulation of TG in human adipose tissue results in obesity and, in nonadipose tissues, is associated with organ dysfunction. For example, excessive TG deposition in skeletal muscle and the liver is associated with insulin resistance, in the liver with nonalcoholic steatohepatitis, and in the heart with cardiomyopathy (2, 3). Owing to worldwide increases in the prevalence of obesity and other diseases of excessive TG accumulation, an understanding of the basic processes that govern TG synthesis and storage is of considerable biomedical importance.
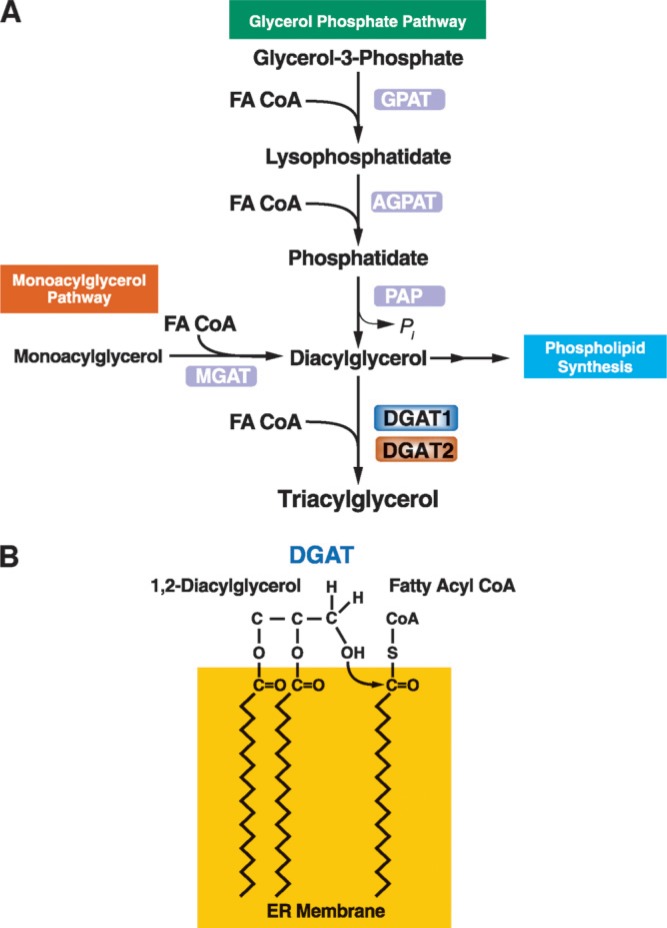
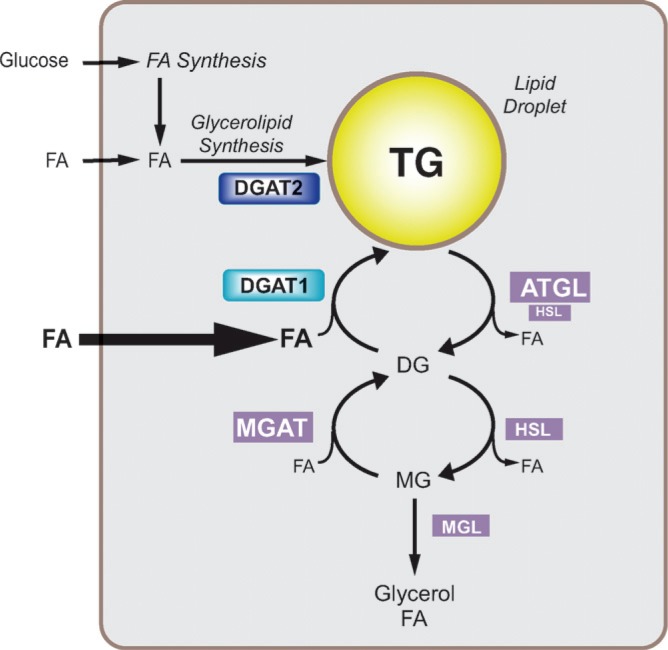
Two major pathways for TG biosynthesis, elucidated in the 1950s and 1960s, are known: the glycerol phosphate or Kennedy pathway (4) and the monoacylglycerol (MG) pathway (Fig. 1A) (for reviews of pathway biochemistry, see Refs. 5–8). Both pathways use fatty acyl-CoAs, the “activated” forms of FA synthesized by intracellular acyl-CoA synthases, as acyl donors (9). The glycerol phosphate pathway is present in most cells. In contrast, the MG pathway is found in specific cell types, such as enterocytes, hepatocytes, and adipocytes, where it may participate in the reesterification of hydrolyzed TG (10). The MG pathway is the dominant mode of TG synthesis in human small intestine, where TGs are synthesized from components of hydrolyzed dietary fats (11, 12). In the final reaction of both pathways, a fatty acyl-CoA and diacylglycerol (DG) molecule are covalently joined to form TG. This reaction (Fig. 1B) is catalyzed by acyl-CoA:diacylglycerol acyltransferase (DGAT, E.C. 2.3.1.20) enzymes. TG biosynthesis is believed to occur mainly at the endoplasmic reticulum (ER) (13). Newly synthesized TGs are thought to be released into the associated lipid bilayer, where they are channeled into cytosolic lipid droplets or, in cells that secrete TG, nascent lipoproteins (Fig. 2). The precise mechanism by which TGs are deposited into lipid droplets is unknown. Several models have been proposed (as reviewed in Ref. 14). Transfer of TG into lipoproteins involves the cotranslational addition of lipids to apolipoprotein B (apoB) in a process catalyzed by the microsomal triglyceride transfer protein (MTP) (as reviewed in Refs. 15–17).
Fig. 1.
Triacylglycerol synthesis and acyl-CoA:diacylglycerol acyltransferase (DGAT) enzymes. A: Triacylglycerols (triglycerides) are the end-product of a multi-step pathway. DGAT1 or DGAT2 catalyzes the final reaction. B: DGAT enzymes catalyze the formation of an ester linkage between a fatty acyl CoA and the free hydroxyl group of diacylglycerol. The model shows this reaction occurring at the surface of the endoplasmic reticulum (ER) bilayer membrane. GPAT, glycerol-phosphate acyltransferase; AGPAT, acylglycerol-phosphate acyltransferase; PAP, phosphatidic acid phosphohydrolase; MGAT, acyl CoA:monoacylglycerol acyltransferase.
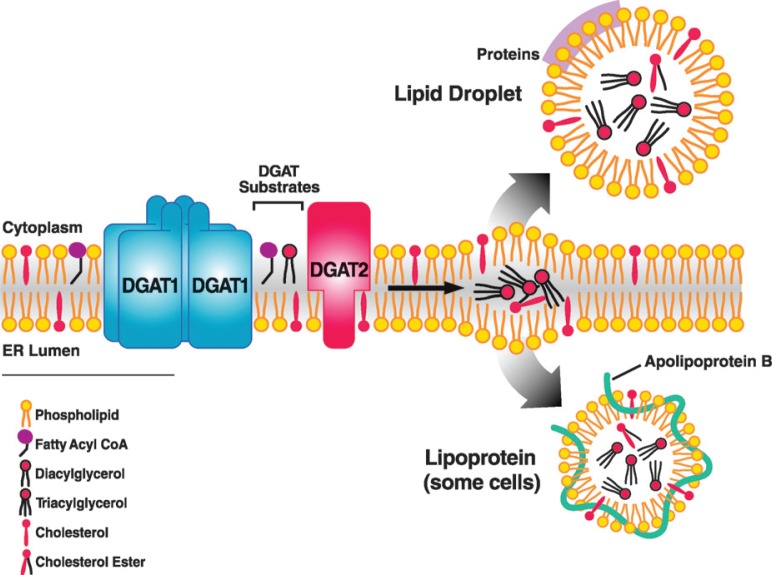
Fig. 2.
Hypothetical model illustrating the role of DGAT enzymes in triacylglycerol synthesis in the ER. Triacylglycerol products of the DGAT reaction may be channeled into the cores of cytosolic lipid droplets or triacylglycerol-rich lipoproteins for secretion in cells such as enterocytes and hepatocytes. Although the model depicts the reaction at the cytosolic surface of the ER, it has been proposed that the reaction may also take place at the luminal surface (see text for discussion). DGAT1 is shown as a homotetramer (59).
DGAT activity was first reported in 1956 (13, 18). Although there was much interest subsequently in the biochemistry of TG synthesis, the purification of a DGAT proved to be difficult. Only in the last decade have DGAT genes been cloned, and the molecular tools for studying TG synthesis become available. At least two DGAT enzymes exist in a wide variety of eukaryotes. Interestingly, these two DGAT enzymes are not similar at the level of DNA or protein sequences. In this review, we summarize progress over the past decade in understanding these two key enzymes of TG synthesis.2
DGAT1 AND DGAT2: DISTINCT GENE FAMILIES
DGAT1
The genes encoding murine and human DGAT1 were identified by their similarity to the sequences of acyl-CoA:cholesterol acyltransferase (ACAT) enzymes (Fig. 3) (19–24) and were shown in 1998 to encode proteins that possess DGAT activity (22). Since then, orthologs have been identified in many species of eukaryotes, including yeast, fungi, plants, and invertebrates. In humans, the DGAT1 gene comprises 17 exons and spans 10.62 kb on chromosome 8 (Table 1). In most species, the gene encodes a protein of about 500 amino acids with a calculated molecular mass of ∼55 kDa (Fig. 4).
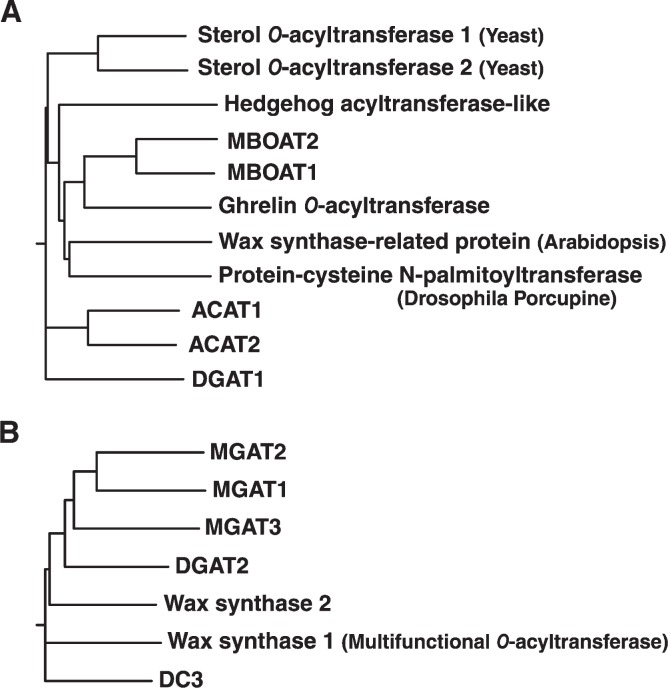
Fig. 3.
Dendrograms of the DGAT gene families. A: The acyl-CoA: cholesterol acyltransferase (ACAT)/DGAT1 gene family is part of a membrane-bound O-acyltransferase (MBOAT) superfamily of enzymes. Selected members shown are human proteins unless indicated. MBOAT1 and MBOAT2 are family members whose activities are unknown. Dendrograms were generated by ClustalW using Biology Workbench (http://workbench.sdsc.edu/). B: The DGAT2 gene family. DC (DGAT candidate) 3 refers to a family member whose activity is unknown.
TABLE 1.
Genes encoding DGAT enzymes
| Enzyme | Gene (UniGene) | Chromosome Localization | Number of Exons | Number of Amino acids | Tissues with High Levels of mRNA Expression |
|---|---|---|---|---|---|
| DGAT1 | |||||
| Human | DGAT1 Hs .512810 | 8q24.3 cM | 17 | 488 | Small intestine, testis, adipose tissues, mammary gland, thymus, skeletal muscle, spleen, heart, skin |
| Mouse | Dgat1 Mm. 22633 | 15 D3 | 17 | 498 | Small intestine, testis, adipose tissues, mammary gland, skin |
| DGAT2 | |||||
| Human | DGAT2 Hs. 334305 | 11q13.3 cM | 8 | 388 | Liver, adipose tissues, mammary gland, testis, peripheral leukocytes, heart |
| Mouse | Dgat2 Mm. 180189 | 7 E1 | 8 | 388 | Liver, testis, heart, kidney, stomach, small intestine, skeletal muscle, skin, uterus |
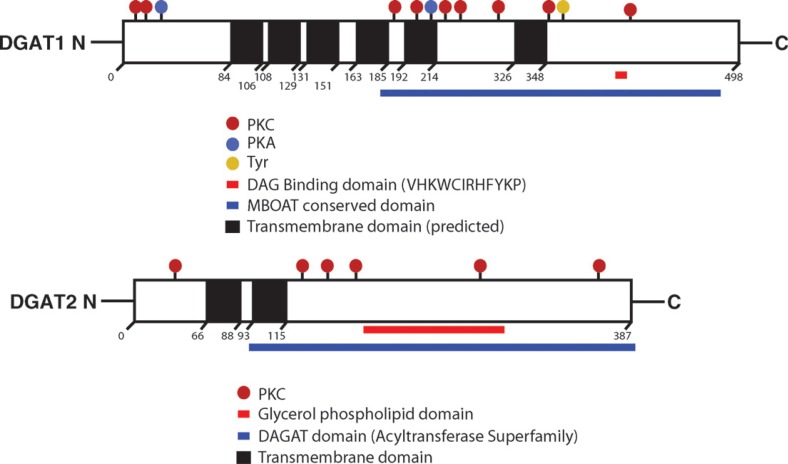
Fig. 4.
Schematic representations of murine DGAT1 and DGAT2 proteins. Possible domains and sites of modification are shown. Transmembrane domains were predicted by the program SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui/sosui_submit.html). The two transmembrane domains in DGAT2 have been confirmed experimentally (47). Phosphorylation sites were predicted by PROSEARCH (http://workbench.sdsc.edu/). PKC, PKA, and Tyr represent potential phosphorylation sites for protein kinase C, protein kinase A, and tyrosine kinase, respectively.
DGAT1 expressed in insect or mammalian cells migrates faster than predicted (∼50 kDa) on SDS-PAGE (22, 25). Within species, DGAT1 protein sequences share 15–25% identity, mostly in the C termini, with those of ACAT1 and ACAT2 (22). A motif conserved between DGAT1 and ACATs (FYXDWWN; amino acids 360–366 of human DGAT1) has been implicated in binding fatty acyl-CoA, a common substrate for the enzymes (26). However, experimental evidence suggests that the fatty acyl-CoA binding domain is at the N terminus, because a fragment containing the first 116 amino acids of DGAT1 from the rapeseed plant Brassica napus and a fragment of the first 95 amino acids from mouse DGAT1 directly bind fatty acyl-CoA (27, 28). Animal and plant DGAT1 enzymes share up to 40% amino acid identity, mostly at their C termini. Toward the C-terminal region, DGAT1 also possesses a putative DG binding domain. Both DGATs possess several predicted phosphorylation sites (Fig. 4).
DGAT1, like ACAT enzymes, is part of a large family of membrane-bound O-acyltransferases (MBOAT, National Center for Biotechnology Information (NCBI) Conserved Domains Database accession number: pfam03062; discussed below) (29) (Fig. 3). Other MBOAT family members catalyze reactions that add fatty acyl chains to proteins (30–33). This family of membrane-associated enzymes catalyzes O-acylation reactions, transferring fatty acyl moieties onto the hydroxyl or thiol groups of lipid and protein acceptors, and its members are involved in lipid metabolism, signal transduction, and hormone processing. ACAT enzymes catalyze the joining of cholesterol and fatty acyl-CoA to form cholesterol esters (24). Other members of the MBOAT family include a protein-cysteine N-palmitoyltransferase (skinny hedgehog, or sightless protein) (32); porcupine, a putative acyltransferase implicated in Wnt processing and signaling (30, 31); and an acyltransferase that attaches an essential eight-carbon fatty acyl moiety to ghrelin, a gut-derived hormone that regulates appetite (33). A common feature of the MBOAT family is a long hydrophobic region that contains asparagine and histidine residues of the putative active site (corresponding to amino acids 378 and 415, respectively, of human DGAT1; 389 and 426 of mouse DGAT1) (29, 32).
DGAT2
The existence of DGAT2 was predicted from the finding that mice lacking DGAT1 have abundant TG in tissues (34). In 2001, a second DGAT enzyme was purified from the fungus Mortierella ramanniana (35). This led to the cloning of mammalian DGAT2 and the identification of a seven-member gene family (35, 36) (DAGAT, NCBI Conserved Domain Database accession number: pfam 03982) (Fig. 3). In addition to DGAT2, this family includes acyl-CoA:monoacylglycerol acyltransferase-1 (MGAT1) (37), MGAT2 (38, 39), MGAT3 (40), and wax monoester synthases (41–43).
The human DGAT2 gene comprises eight exons and spans 42.03 kb on chromosome 11. It is located ∼37.5 kb from the MGAT2 gene. In most species, the gene encodes a protein of 350–400 amino acids (Fig. 4), and the calculated molecular mass for DGAT2 enzymes ranges from 40 to 44 kDa. DGAT2 is about 50 residues longer at the N terminus than other non-DGAT2 members of the gene family. This region may include domains that confer substrate specificity or provide regulatory functions. DGAT2 and its family members share 40–45% amino acid identity throughout the entire lengths of the proteins. The most conserved regions, probably containing the catalytic domains of the proteins, are found in the C termini (amino acids 200–360 of human and mouse DGAT2; both are composed of 388 amino acids with 95% identity). All DGAT2 family members, from yeast to human, contain the highly conserved sequence of amino acids HPHG (amino acids 161–164 of mouse DGAT2) (36, 44), and mutations of this sequence in mouse DGAT2 markedly reduce total DGAT activity in vitro (44). Thus, these amino acids may be part of the active site of DGAT2, and these findings also suggest a role for histidine as part of the active site, as in acyltransferase enzymes of the MBOAT family (29).
DGAT2 also contains the consensus sequence (amino acids 80–87; FLXLXXXn, in which n is a nonpolar amino acid), for a neutral lipid binding domain found in a variety of proteins that bind to or metabolize neutral lipids, such as cholesterol ester transfer protein (CETP), hormone-sensitive lipase (HSL), lecithin:cholesterol acyltransferase (LCAT), cholesterol 7α-hydroxylase, cholesterol esterase, and TG hydrolase (45, 46). This domain may be involved in DG binding. Mutations in this region of DGAT2 also markedly reduce in vitro DGAT activity (44).
BIOCHEMISTRY OF DGAT ENZYMES
Several lines of evidence clearly demonstrate that both DGAT1 and DGAT2 function as DGAT enzymes. First, the overexpression of either DGAT1 or DGAT2 in plant, insect, or mammalian cell lines increased in vitro DGAT activity, generating TG from a variety of fatty acyl-CoA and DG substrates (22, 36). Second, in intact cells, the overexpression of either enzyme increased de novo synthesis and accumulation of TG (47) (unpublished observations). In addition, tissues from mice deficient in either enzyme exhibited decreased DGAT activities and TG levels (34, 47).
Because the enzymes have yet to be purified to homogeneity, the enzymology of DGAT1 and DGAT2 has been analyzed using partially purified membrane preparations from cells that overexpress the enzymes. It is unclear whether either enzyme has distinct preferences for fatty acyl-CoA substrates of specific chain length and desaturation. However, in competition assays, DGAT1 preferred a monounsaturated substrate, oleoyl-CoA (18:1), as compared with saturated palmitoyl CoA (16:0) (36). DGAT2 did not exhibit such a preference. The DGAT2 enzyme purified from M. ramanniana showed enhanced DGAT activity toward medium-chain fatty acyl-CoAs (12:0), as compared with long-chain fatty acyl-CoA (18:1) (35) and exhibited higher activities with DGs containing short- and medium-chain fatty acyl moieties (6:0, 8:0, and 10:0) rather than longer chains (12:0, 14:0, 16:0, and 18:0) (35).
The available data suggest that DGAT2 is a more potent DGAT with a higher affinity for its substrates than DGAT1. In studies performed with enzymes overexpressed in insect cells, the two enzymes had similar maximal rates for TG synthesis in the mid-range (∼100 μM) of oleoyl-CoA concentrations. However, DGAT2 tended to be slightly more active at lower oleoyl-CoA concentrations (0–50 μM), and DGAT1 was more active at higher (>100 μM) oleoyl-CoA concentrations (36). These data suggest different Km values for fatty acyl-CoAs between the two enzymes, with DGAT2 more active at lower substrate concentrations. Additionally, in rat hepatoma cells, DGAT2 overexpression resulted in considerably more TG accumulation than did DGAT1 overexpression, despite relatively lower in vitro DGAT activity in the DGAT2-overexpressing cells (47). This observation suggests that DGAT2 may require more optimized assay conditions or an as-yet-unknown cofactor for full activity in vitro. The higher potency of DGAT2 is consistent with results from loss-of-function experiments in model organisms. In yeast, deletion of the DGAT2 ortholog Dga1 showed that it accounts for a major portion of TG synthesis (48, 49), whereas the DGAT1 orthologs, Are1 and Are2, account for only a minor portion (49). In mice, DGAT2 deficiency leads to severe reductions of TG (47), whereas DGAT1 deficiency results in only moderate reductions (34). Little is known about differences in the affinity of DG for the two enzymes, although DGAT2 can utilize endogenous DG associated with membrane preparations to synthesize TG in in vitro assays, whereas DGAT1 appears to be more dependent on added DG (25).
DGAT1 and DGAT2 have different sensitivities to magnesium in in vitro assays. High magnesium concentrations (>50 mM) suppress DGAT2; the effect on DGAT1 activity is much less (36). This characteristic has been exploited to selectively measure DGAT1 activity (36). Conversely, low levels of magnesium (<20 mM) in the assay appear to enhance the activity of DGAT2 (36) (unpublished observations). The significance of the different magnesium sensitivities is unclear.
DGAT1, but not DGAT2, has additional acyltransferase activities beyond that of esterifying DG in vitro. For example, DGAT1 is a potent acyl-CoA:retinol acyltransferase (ARAT) (25), which catalyzes the synthesis of retinyl esters from retinol (vitamin A) and fatty acyl-CoA substrates, and might have been named for this activity had it been discovered first. ARAT is one of two enzymes that catalyze the esterification of retinol in mammalian tissues (50). The other is lecithin:retinol acyltransferase, which accounts for the bulk of retinol ester biosynthesis (51). Several lines of evidence suggest that DGAT1 functions as an ARAT in vivo. The overexpression of DGAT1 in fibroblast COS-7 cells promotes the accumulation of retinyl esters (25). Additionally, Dgat1−/− mice have markedly reduced ARAT activities in liver, testes, and kidneys and perturbed retinol homeostasis in the liver (25). DGAT1 accounts for the majority of ARAT activity in differentiated Caco-2 cells, a model of enterocytes (52). Further, studies of knockout mice lacking LRAT, DGAT1, or both enzymes showed that DGAT1 functions as an intestinal ARAT in vivo and that the ARAT activity of DGAT1 may be important in intestinal absorption of vitamin A, especially when dietary intake is high (53). DGAT1 mRNA occurs at high levels in hepatic stellate cells (54), which store large amounts of retinyl esters. Therefore, DGAT1 seems to have ARAT activity in vivo, especially when intracellular unesterified retinol concentrations are high (25, 53, 54).
DGAT1 also possesses MGAT activity in in vitro assays (25), suggesting that it can catalyze the sequential esterification of MG with two fatty acyl-CoA moieties to convert MG to TG. This sequence of reactions might be particularly important in tissues in which TGs undergo high levels of hydrolysis and reesterification. In in vitro assays, DGAT1 can also catalyze the synthesis of monoester waxes and diester waxes by using fatty acyl alcohols (with one or two hydroxyl groups) as acyl acceptors (25). The wax synthase activity of DGAT1 may explain in part the dry fur observed in Dgat1−/− mice (55). Interestingly, at least two members of the DGAT2 gene family also possess monoester wax synthase activity (41–43), highlighting the overlapping functions of the DGAT1 and DGAT2 gene families.
Judging by maximal activities observed when each of the known acyl acceptors (retinol, MG, DG, and fatty acyl alcohols) is provided over a range of concentrations in in vitro assays, DGAT1 exhibits highest activities for DGAT and MGAT reactions, followed by those for monoester wax synthase, ARAT, and diester wax synthase (25). The apparent Km for each of these acyl acceptors ranges from 15 to 40 μM. Because these substrate concentrations are probably higher than those normally present in tissues, it is likely that DGAT1 activity becomes important when intra-cellular substrate concentrations are high. High substrate concentrations could also occur from channeling of substrates to DGAT1 via other proteins.
That DGAT1, but not DGAT2, possesses multiple acyltransferase activities is consistent with the apparent evolutionary history of the two enzymes. The DGAT2 ortholog in Saccharomyces cerevisiae, Dga1, plays a major role in TG synthesis (49), which has apparently been conserved in many species during evolution. In contrast, the DGAT1 orthologs in S. cerevisiae, Are1 and Are2, act primarily as sterol esterification enzymes (56) and play, at most, minor roles in TG synthesis (49). This suggests that an ancestral gene for the ACAT/DGAT1 gene family evolved to yield enzymes capable of utilizing different substrates as fatty acyl-CoA acceptors and that DGAT1 acquired activity toward substrates with long-chain acyl moieties (i.e., DG) while losing activity for sterols. This evolutionary perspective on the DGAT enzymes predicts that the substrate recognition site of DGAT1 is less discriminating than that of DGAT2, with the former capable of utilizing DG, MG, long-chain alcohols, and retinol as fatty acyl acceptors. A comparison of high-resolution structures of the two DGAT enzymes would allow this prediction to be tested.
TOPOLOGY AND CELLULAR BIOLOGY OF DGAT ENZYMES
DGAT1 and DGAT2 are found mainly in the ER (44, 57), where enriched DGAT activity is found and TG synthesis occurs. In addition to differences in substrate specificities and affinities (discussed above), DGAT1 and DGAT2 may have different functions because of their topology, protein partners, and subcellular localization.
Little is known about the topology of DGAT1. Like ACAT1 and ACAT2, DGAT1 contains many hydrophobic regions and is predicted to contain multiple transmembrane domains. DGAT1 forms homotetramers, and this may occur via interactions between the N termini of each subunit (58), similar to ACAT1 (59). Supporting this model, N-terminal fragments of DGAT1 from the rapeseed plant form multimers and probably face the cytosol, inasmuch as they are sensitive to protease treatment without permeabilizing microsomal membranes (27).
DGAT2 topology is better understood. It is less hydrophobic than DGAT1 and was predicted to have only one or two membrane-spanning domains (35, 36). Mouse DGAT2 expressed in cells is an integral membrane protein with the N and C termini oriented toward the cytosol (44). A long hydrophobic region spanning amino acids 66–115 contains either two transmembrane domains or a single hydrophobic domain that is embedded in the membrane bilayer (44). Most of the protein is on the cytosolic side of the ER, suggesting that its active site is at the surface of the cytosolic leaflet of the ER bilayer.
An unresolved issue is whether one or both DGAT enzymes catalyze TG synthesison the luminal face of the ER. Several laboratories have reported that two separate DGAT activities are present in hepatic microsomes, an “overt” (cytosolic) activity and a “latent” (luminal) activity that appears after permeabilization (60–62). One model suggests that the overt fraction may reflect DGAT activity on the cytoplasmic side of the ER, which catalyzes the synthesis of TG destined for deposition into cytosolic lipid droplets. The latent fraction is DGAT activity within the ER lumen, where TG bound for secretion is synthesized. However, data confirming this model are lacking, and the overt and latent activities appear not to reflect simply those of the two DGAT enzymes. In livers of DGAT1 knockout mice, the absence of DGAT1 causes a marked reduction in both the overt and latent activities (unpublished observations), indicating that DGAT1 may contribute to both activities. On the other hand, DGAT2 topology suggests that it contributes only to the overt activity (44). Supporting this, studies in hepatoma cells show that niacin selectively inhibits DGAT2 and is more active against the overt activity (63).
DGAT1 and DGAT2 probably interact with different cellular proteins and participate in different pathways of TG synthesis. Loss-of-function studies in yeast and mice indicate that DGAT2 is involved in the bulk of TG synthesis (47) and that it may be closely linked with the pathways of de novo FA biosynthesis. Supporting this conjecture, studies in HeLa cells show that DGAT2 colocalizes with stearoyl-CoA desaturase 1 (SCD1), an ER enzyme catalyzing the monounsaturation of FA (64). These studies also employed immunoprecipitation and fluorescence resonance energy transfer methods to show that DGAT2 and SCD1 physically interact. These findings suggest that DGAT2 is responsible for incorporating endogenously synthesized monounsaturated FA into TG. In contrast, the cumulative evidence suggests that DGAT1 may be involved in esterifying exogenous FA taken up by cells or in a recycling pathway that involves the reesterification of hydrolyzed TG (Fig. 5). This model requires further testing. An interaction between DGAT1 and SCD1 has not been examined.
Fig. 5.
Hypothetical model for intracellular roles of DGAT1 and DGAT2. On the basis of the available evidence, we speculate that DGAT2 function is closely linked to endogenous FA synthesis and esterification, whereas DGAT1 may be involved in the recycling of hydrolyzed triacylglycerols by reesterifying the FAs. DGAT1 may also play an important role in protecting cells from high concentrations of FAs. The model suggests that DGAT2 is most active with low substrate concentrations (e.g., with de novo FA synthesis) and that DGAT1 is most active with high substrate concentrations, such as with exogenous FA or high levels of lipolysis. FAs need to be activated by forming FA CoA (not shown). HSL, hormone-sensitive lipase; ATGL, adipose-tissue triglyceride lipase; MGL, monoacylglycerol lipase. Permission to use Fig. 5 granted by Elsevier Ltd.
The subcellular localization of DGAT1 and DGAT2 appears to be different. Expression of DGAT1 and DGAT2 in the tung tree (Vernicia fordii) in a tobacco cell line showed that each enzyme localized to distinct punctate areas of the ER, suggesting that the enzymes were in different ER subdomains (57). This is consistent with the hypothesis that DGAT1 and DGAT2 may be part of distinct multiprotein complexes of TG synthesis.
Cytosolic lipid droplets, the primary site of intracellular TG storage, may represent another site for TG synthesis (65). Human DGAT2, expressed in cultured mammalian cells, localizes in close proximity to the surfaces of lipid droplets (65). Additionally, Dga1 activity in yeast is enriched in lipid droplet fractions (48). The long hydrophobic region (amino acids 66–115) of DGAT2 could allow the enzyme to bind directly to the surface of the lipid droplet monolayer and to catalyze TG synthesis at this location. However, this region probably contains two trans-membrane domains (44), and neither DGAT1 nor DGAT2 has been identified in proteomic analyses of isolated lipid droplets (66–76). Therefore, instead of being part of the lipid droplets, DGAT2 may reside in the ER bilayer close to the droplets.
TISSUE EXPRESSION OF DGAT ENZYMES
DGAT1 and DGAT2 are expressed in many of the same tissues in mammals. DGAT1 is expressed ubiquitously, with the highest mRNA levels in organs that make large amounts of TG, such as small intestine, liver, adipose tissue, and mammary gland. In humans, the highest mRNA levels were in the small intestine, followed by testis, adipose tissue, thymus, mammary gland, skeletal muscle, heart, spleen, pancreas, and liver (22). The expression pattern is similar in mice, except that mRNA levels are relatively low in liver (22).
DGAT2 is expressed in most tissues, especially those that make large amounts of TG (36). In humans, high levels of DGAT2 mRNA (∼2.4 kb and 1.4 kb) were found in liver, adipose tissues, and mammary gland, and lower levels were found in testis, peripheral leukocytes, and heart (36). It is unknown how the two major transcripts in humans differ functionally. In mice, DGAT2 is expressed in a broader range of tissues with less variation in mRNA levels among tissues (36). The highest levels of mRNA for DGAT2 (∼2.0 kb) were found in adipose tissues, followed by liver and many other tissues, including heart, small intestine, skeletal muscle, stomach, kidney, and skin.
REGULATION OF DGAT ENZYMES
Although glycerol-phosphate acyltranserases (GPATs) are believed to catalyze the rate-limiting step for TG biosynthesis (77, 78), DGAT enzymes may be important in determining the flux of lipids into TG. The DG substrate for the DGAT reaction is also a substrate for phospholipid synthesis, and DGAT activity may divert the flux of DG from phospholipid synthesis toward TG biosynthesis (79). Although DGAT activity may be primarily determined by substrate availability, the overexpression of DGAT enzymes in cultured cells (47) or in liver (80, 81), skeletal muscle (82–84), or adipose tissue (85, 86) of mice leads to intra-cellular TG accumulation. The net accumulation of TG may reflect uncoupling of normally well-regulated TG synthesis and degradation.
Expression of DGAT enzymes is regulated at the mRNA level under specific conditions, although transcription factors regulating DGAT genes have not been studied in detail. Levels of DGAT1 and DGAT2 mRNA increase markedly during the differentiation of NIH 3T3-L1 fibroblasts into adipocytes, corresponding to a large increase in DGAT activity (22, 36, 87, 88). These findings indicate a robust upregulation of DGAT1 and DGAT2 during adipogenesis and suggest the involvement of C/EBPα or PPARγ, transcription factors that control the expression of many genes in adipogenesis. A PPAR binding site occurs in the DGAT1 promoter (89), and thiazolidinediones, which activate PPARγ, increase DGAT1 mRNA levels in adipocyte cell lines and adipose tissues of mice and humans (90). C/ EBPβ and C/EBPα increase the expression of DGAT2 mRNA in adipogenesis (91). Sterol-regulatory element binding proteins (SREBPs) activate the transcription of lipid synthesis enzymes in adipogenesis, but these transcription factors have not been shown to regulate DGAT1 or DGAT2 gene expression (92).
The transcription factor X-box binding protein 1 (XBP1), a key regulator of the unfolded protein response, induces expression of lipogenic genes, including DGAT2, in the liver (93). Overexpression of XBP1 in primary hepatocytes (from either wild-type or XBP1-deficient mice) increased DGAT2 expression, whereas deletion of XBP1 in the liver decreased production of lipids and resulted in hypolipidemia (93).
In cultured adipocytes, mRNA levels of DGAT enzymes are regulated by insulin and glucose. Supplementing the culture medium for differentiated NIH 3T3-L1 adipocytes with either glucose or insulin stimulated DGAT activity, and the effects of glucose and insulin were additive (87). DGAT1 mRNA levels were markedly suppressed by glucose starvation and stimulated by glucose administration, indicating that DGAT1 expression is positively regulated by glucose (87). Also in 3T3-L1 adipocytes, DGAT1 and DGAT2 mRNA levels increased in response to glucose (94). In contrast, insulin treatment increased DGAT2 mRNA levels but did not affect DGAT1 expression (87).
In cultured hepatocytes, levels of DGAT1 and DGAT2 mRNA are regulated by the MEK-ERK signaling pathway (95). Inhibition of this signaling pathway in HepG2 hepatoma cells increased DGAT1 and DGAT2 mRNA levels 2-and 4-fold, respectively, and stimulated VLDL secretion. DGAT activity seems to be inhibited when cells are actively dividing, which may facilitate the flow of FA to phospholipid for membrane biosynthesis instead of TG. Conversely, when cell proliferation is suppressed, DGAT expression might be induced to facilitate storage of excess FA.
In vivo there appears to be a remarkably reciprocal relationship in the physiological regulation of DGAT enzymes. For example, genetic- and chronic diet-induced obesity is associated with increased mRNA levels of DGAT2 but decreased mRNA levels of DGAT1 (87, 96). Similarly, DGAT2 mRNA levels in both the white adipose tissue (WAT) and liver are decreased by fasting and increased by refeeding, whereas the opposite appears to be the case for DGAT1 (87) (unpublished observations). DGAT2 expression may be regulated coordinately with other enzymes involved in the synthesis and storage of FA in the fed state. Furthermore, DGAT2 mRNA levels may be regulated by leptin. DGAT2 mRNA levels were increased 3-fold in the WAT of DGAT1-deficient ob/ob mice (97) and in the skin of ob/ob mice (55), suggesting that leptin suppresses the expression of DGAT2. In support of this, DGAT2 mRNA levels were increased in the WAT, skeletal muscle, and small intestine of two strains of diabetic mice: leptin receptor-deficient db/db and leptin-resistant Agouti strain KK-Ay mice (98). Because leptin deficiency stimulates de novo FA synthesis (99), DGAT2 expression may be coordinately regulated to promote storage of FA as TG.
DGAT mRNA levels have been examined in several other physiological and pathophysiological conditions. For example, DGAT1 mRNA levels are increased in murine skeletal muscle after 2 weeks of swimming (100), and DGAT2 mRNA levels are decreased in skin from patients with psoriasis (98). In a model of nephrotic syndrome, characterized by overproduction of TG-rich lipoproteins by liver, mRNA levels of DGAT1, but not DGAT2, were increased in rat livers (101). In rats with chronic renal failure, hepatic levels of DGAT2 mRNA and DGAT activity were diminished (102).
DGAT enzymes may also be regulated posttranscriptionally. Studies performed decades ago in rat hepatocytes and adipose tissue suggested that DGAT activity can be regulated posttranscriptionally by modulating the phosphorylation state of the enzymes (103–105). In these studies, DGAT activity could be inactivated by a cytosolic factor in the presence of ATP and magnesium. Partial purification of an inhibitory factor from adipose tissue suggested that it could be a tyrosine kinase, because it was inhibited by tyrosine kinase inhibitors but not by serine/threonine kinase inhibitors (106). DGAT1, but not DGAT2, possesses a putative tyrosine phosphorylation site. However, when this site was mutated in human DGAT1 (a T316F mutation) and expressed in cells, no effects on in vitro DGAT activity or TG synthesis were seen (88). Additional evidence that DGAT activity may be posttranscriptionally regulated comes from observations in 3T3-L1 adipocytes, in which levels of DGAT1 or DGAT2 mRNA are disproportionate to changes in DGAT activity during adipogensis (22, 36, 88). Allosteric regulation is found in the DGAT1-related ACAT enzymes (107) but has not been demonstrated for either DGAT.
DGAT activity may also be regulated by hormones or nutritional status. Administration of glucagon or epinephrine to rat tissues decreased DGAT activity (103, 108, 109), and treatment with long-chain FAs increased DGAT activity (110, 111). It is not known whether these treatments regulate DGAT1 or DGAT2 nor whether they exert their effects at the transcriptional or posttranscriptional level. More recently, treatment of rat hepatocytes with 5-amino-4-imidazolecarboxamide riboside, an activator of AMP-activated kinase (AMPK), had no effect on DGAT activity in rat hepatocytes, although it downregulated GPAT activity (112). However, DGAT activity in rat liver increased almost 2-fold when fasted animals had been refed for 24 h. This increase was correlated with AMPK activation, prompting speculation that AMPK is involved in coordinating enzymes of FA and glycerolipid metabolism, including DGAT (111).
PHYSIOLOGICAL FUNCTIONS OF DGAT ENZYMES
Our understanding of the physiologic roles of DGAT enzymes stems largely from studies of genetically modified mice. These studies tested the hypothesis that altering the expression of genes that limit the rate of TG synthesis would have important effects on TG homeostasis and systemic and tissue-specific metabolic processes. Results from several laboratories highlight two emerging themes. First, the distinct biochemistry and regulation of DGAT1 and DGAT2 are reflected in their different physiologic roles in vivo. For DGAT1, in particular, the pleiotropic phenotype of knockout mice (discussed below) may in part reflect its role in the esterification of substrates other than DG. Second, DGAT enzymes modulate complex physiologic processes far removed from their basic biochemical functions, and perturbing the expression of DGAT enzymes in specific tissues has direct implications for diseases such as obesity, insulin resistance, and liver steatosis. Complementary studies with anti-sense oligonucleotides (ASOs) and small-molecule pharmacologic inhibitors have shed further light on the potential therapeutic benefits of modulating DGAT activity.
Functions of DGAT1 and DGAT2 in energy metabolism
DGAT1 and DGAT2 are important modulators of energy metabolism. Compared with DGAT1 and consistent with its biochemical function in cells, DGAT2 appears to be the dominant DGAT enzyme controlling TG homeostasis in vivo (47). DGAT2 deficiency in mice is not compatible with life: DGAT2-deficient (Dgat2−/−) mice survive for only a few hours after birth (Fig. 6). At least two mechanisms contribute to the early mortality. First, DGAT2 deficiency reduces those substrates necessary for energy metabolism. The carcass TG content of Dgat2−/− mice was reduced by ∼90%, and TGs were nearly absent from Dgat2−/− livers. The plasma levels of TG were reduced by 64%, FFA by 80%, and glucose by 60% (47). These findings highlight the importance of DGAT2 in mammalian TG metabolism and indicate a requirement for DGAT2-mediated TG synthesis in the perinatal period. Second, the barrier function of the skin is defective in the Dgat2−/− mice (discussed below), leading to rapid dehydration (47).
Fig. 6.
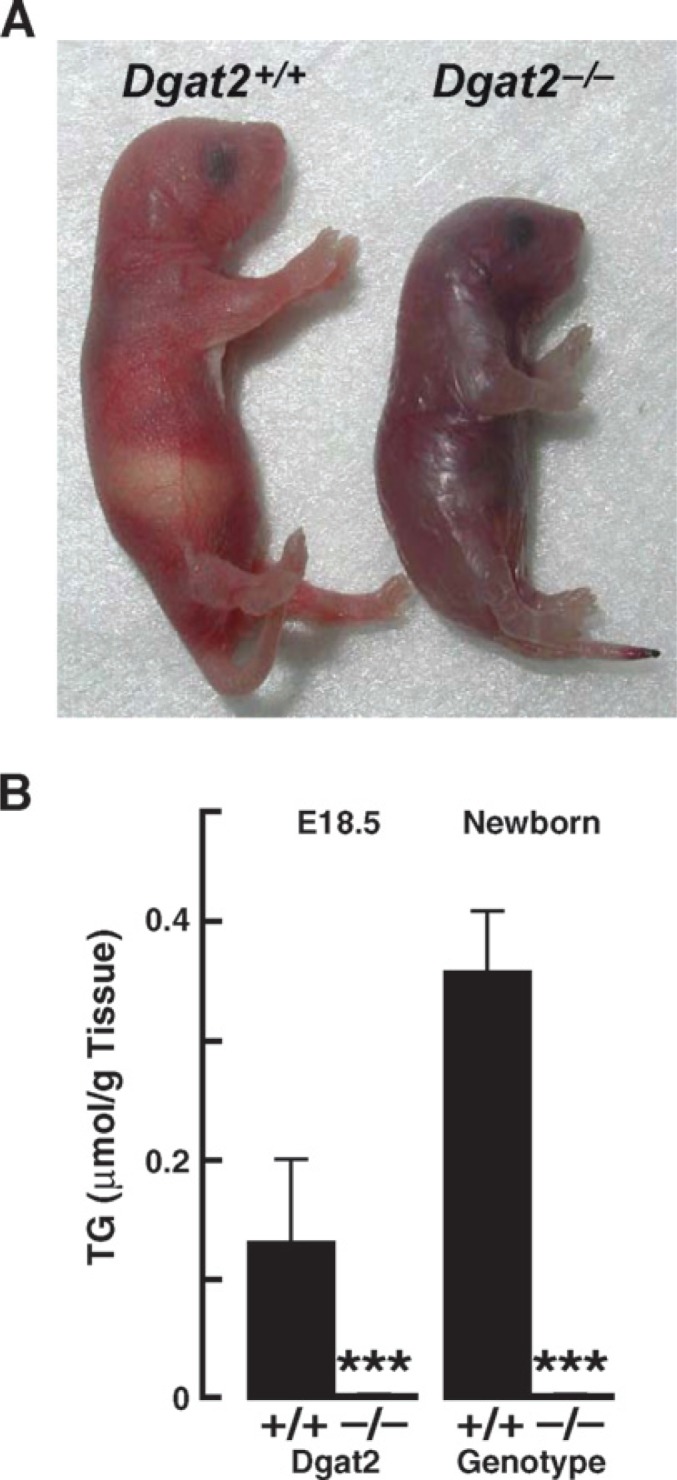
Phenotype of DGAT2 deficiency in mice. A: Mice lacking DGAT2 are smaller, do not feed well, have abnormal skin, and die early in the postnatal period. B: Reduction in carcass triacylglycerols in Dgat2−/− mice. A and B, reproduced from (46).
Heterozygous DGAT2 deficiency, at least in a mixed genetic background (C57BL/6 and 129/SvJae), has few detectable effects in mice. Adult Dgat2+/− mice are viable, healthy, and physically indistinguishable from their litter-mates. Their fat pad weights, TG content, plasma TG levels, and susceptibility to diet-induced obesity are all similar to those of wild-type mice (unpublished observations). DGAT2 heterozygosity also do not protect leptin-deficient (ob/ob) mice from obesity (unpublished observations). These findings suggest that reduction of Dgat2 gene expression by 50% is sufficient for normal life, probably owing to its high apparent catalytic efficiency and ability to function at relatively low substrate concentrations. Supporting this idea, DGAT activity measured in vitro is similar in Dgat2+/− and wild-type brown adipose tissue (BAT) (47). Notably, DGAT1 cannot compensate for DGAT2 deficiency in vivo, and primary hepatocytes from Dgat2−/− mice have normal DGAT1 expression and can synthesize TG only when exogenous FAs are provided (47). This finding suggests that substrate concentrations in vivo in Dgat2−/− are insufficient to drive TG synthesis through DGAT1.
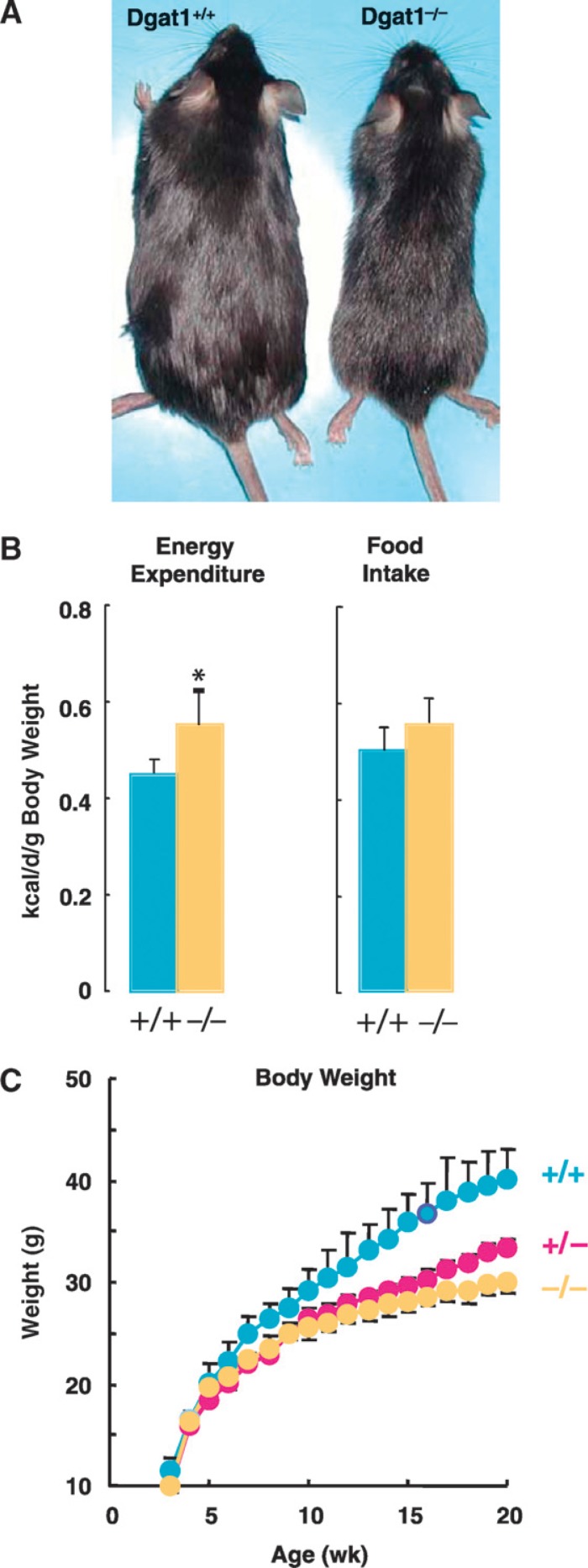
In contrast, Dgat1−/− mice are viable and have a ∼50% reduction in adiposity (34, 95). Interestingly, they maintain a lean phenotype even on a high-fat diet and are resistant to diet-induced obesity (34) (Fig. 7). DGAT1-heterozygous (Dgat1+/−) mice on an inbred (C57BL/6) background exhibit partial resistance to diet-induced weight gain, adipose mass accumulation, and adipocyte hypertrophy (unpublished observations), implying that the level of DGAT1 mRNA expression directly correlates with the degree of fat stores in mice. Further, TG levels in Dgat1−/− mice are also significantly reduced in nonadipose tissues such as the liver and skeletal muscle following a high-fat diet (97). Interestingly, the levels of DG and fatty acyl-CoA, two substrates of the DGAT reaction, are not significantly increased in the skeletal muscle and liver of Dgat1−/− mice on a chow diet (97). This may relate to increased FA oxidation in these tissues or to channeling of these substrates into other metabolic pathways.
Fig. 7.
Effects of DGAT1 deficiency on energy metabolism in mice. A: Male mice (10 weeks old) fed a high-fat diet for 2 weeks. Note the leaner appearance of the Dgat1−/− mouse. Image courtesy of C. Villanueva. B: Energy expenditure and food intake in wild-type and Dgat1−/− mice. C: Weight curves for inbred wild-type, Dgat1+/−, and Dgat1−/− mice fed a high-fat diet (unpublished observations).
The protection against obesity in Dgat1−/− mice does not result from decreased food intake (Dgat1−/− mice eat as much as or more than wild-type mice) or from detectable fat malabsorption (113). Rather, it correlates with at least two factors: a reduced kinetic rate of TG absorption from the gut and an increased rate of energy expenditure. Dgat1−/− mice have slower gastric emptying (unpublished observations), lower chylomicronemia after food intake, and more TG in enterocytes when fed a chronic high-fat diet, suggesting a role for DGAT1 in accelerating assimilation of TG from the diet (114). This conjecture is supported by recent studies of a potent and specific small-molecule inhibitor of DGAT1 that, when orally administered to mice, decreased the rate at which an oral fat bolus appeared as TG in the serum and also reduced weight gain in response to a high-fat diet (115). Increased energy expenditure in Dgat1−/− mice is multi-factorial, with male mice exhibiting increases in physical activity (34) and thermogenesis (97, 113) The increased thermogenesis is probably owing in part to increased expression of uncoupling protein 1, a major mediator of nonshivering thermogenesis, in brown adipose tissue (97, 113). Although the enhanced use of fuel substrates to drive thermogenesis in Dgat1−/− mice protects them from developing obesity, lower levels of substrates may become limiting under conditions of high demand. Indeed, when fasted in a cold environment, Dgat1−/− mice develop significant hypothermia, which is associated with the depletion of tissue glycogen stores and hypoglycemia (113).
Increased sensitivity to leptin may also contribute to the increased energy expenditure seen in DGAT1 deficiency. Dgat1−/− mice lose more weight than wild-type mice in response to subcutaneous leptin infusion, consistent with increased leptin sensitivity (97). DGAT1 deficiency protects against obesity and insulin resistance in Agouti yellow mice, but this protection is lost in ob/ob mice, which lack leptin (97). An intact leptin signaling pathway may be important. How DGAT1 deficiency enhances sensitivity to leptin is unclear. However, the expression of leptin-responsive genes was increased in adipose tissues, suggesting that the effects are mediated at least in part by peripheral tissues (97). In contrast, transgenic mice overexpressing Dgat1 in WAT under control of the aP2 promoter on a C57BL6/J background (aP2-Dgat1 mice) have increased body weight, adiposity, and food intake in the face of elevated circulating leptin levels (85). These mice may be resistant to leptin. The data from mice with altered Dgat1 expression suggest a functional correlation between DGAT1 expression and leptin sensitivity.
Functions of DGAT1 and DGAT2 in glucose metabolism
In addition to enhancing leptin sensitivity, DGAT1 deficiency also enhances insulin sensitivity in mice, as shown by an improved glucose tolerance and an increase in glucose infusion rate during hyperinsulinemic-euglycemic clamp studies (97). DGAT1 expression appears to have quantitative effects on insulin sensitivity in mice, as demonstrated by an intermediate improvement in insulin and glucose tolerance in Dgat1+/− mice (unpublished observations). Insulin-stimulated glucose uptake and the activities of phosphatidylinositol-3 kinase, protein kinase B, and protein kinase Cλ, three key molecules in the insulin signaling pathway, were increased in skeletal muscle and WAT of Dgat1−/− mice (116). Interestingly, in ex vivo studies of insulin-stimulated glucose uptake in skeletal muscle, DGAT1 deficiency exacerbates the detrimental effects of FA inhibition (83). Although systemic DGAT1 deficiency enhances energy expenditure and improves insulin sensitivity in tissues, DGAT1 deficiency in a specific tissue may promote the toxic effects of lipids on tissue physiology (lipotoxicity).
The mechanism by which DGAT1 deficiency enhances glucose metabolism in mice may also involve an alteration in the endocrine function of the WAT. Increased insulin-stimulated signaling and glucose disposal in Dgat1−/− mice was observed in wild-type mice that received transplanted WAT from Dgat1−/− mice (116, 117). Moreover, Dgat1−/− mice have altered circulating levels of several adipocyte-derived factors, such as decreases in leptin and increases in adiponectin (117). However, adiponectin is not required for this phenotype, because the enhanced insulin sensitivity of Dgat1−/− mice is maintained in an adiponectin-deficient background (118). How DGAT1 deficiency in mice enhances glucose metabolism through the endocrine effects of the WAT is unknown but of obvious interest from a therapeutic standpoint.
Interestingly, just as whole-body DGAT1 deficiency enhances glucose metabolism, specifically increasing Dgat1 expression in the WAT of mice also appears to improve insulin sensitivity. Transgenic mice overexpressing Dgat1 under control of the aP2 promoter (aP2-Dgat1) and generated on a C57BL6/J background are prone to diet-induced obesity but protected against glucose intolerance and nonadipose tissue steatosis in response to a high-fat diet (117). Of note, this result was not observed in a similar model of aP2-Dgat1 mice generated in an inbred FVB genetic background (86). However, these mice had no increase in adiposity or body weight despite the over-expression of Dgat1, and the high-fat diet studies were performed in older mice.
The phenotype of Dgat1−/− mice is strikingly similar to that of mice that lack SCD1 (119), an enzyme that catalyzes the synthesis of Δ9-monounsaturated FA. Shared features include increased energy expenditure, resistance to diet-induced obesity, increased sensitivity to insulin, sebaceous gland atrophy, and hair loss (120). SCD1 deficiency protects against obesity induced by leptin deficiency (121), whereas DGAT1 deficiency does not (97). Dgat1−/− mice have reduced SCD1 mRNA levels in several tissues (unpublished observations). We speculate that a decrease in SCD1 expression or activity may underlie some of the downstream effects of DGAT1 deficiency on lipid and energy metabolism. This suggests a model in which SCD1 functionally interacts with DGAT1. As noted above, however, a physical interaction with SCD1 has been shown only for DGAT2 (64).
DGAT enzymes and lipoprotein metabolism
In enterocytes and hepatocytes, DGAT enzymes may also mediate lipoprotein metabolism and regulate the flux of TG throughout the body. How DGAT1 and DGAT2 modulate the transit of dietary fats through the enterocytes into chylomicrons is still not clear. Data are only available for Dgat1−/− mice. DGAT1 deficiency in the small intestine does not cause fat malabsorption but does alter processing of dietary fat. Fecal fat and caloric contents are normal in Dgat1−/− mice(34), even when the mice are fed a high-fat diet, indicating a quantitatively normal absorption of fat. Furthermore, Dgat1−/− enterocytes can assemble chylomicron-sized particles (11). However, when acutely or chronically challenged with high amounts of dietary fat, lipids accumulate in Dgat1−/− enterocytes, and the transit of fat through the enterocytes into the circulation appears to be delayed (114). Thus, although the precise kinetics of chylomicron secretion in Dgat1−/− mice has not been studied, a dietary fat bolus probably empties into the circulation of these mice at a decreased but more sustained rate. This finding was reproduced in mice treated orally with a selective DGAT1 inhibitor (115). Whether these qualitative alterations in lipid absorption have effects on gut-derived hormones or other aspects of systemic metabolism is unknown but may be relevant to the actions of DGAT1 inhibitors.
In the liver, the complex effects of DGAT1 and DGAT2 on TG homeostasis and on the assembly and secretion of VLDL have been studied in cell cultures and animal models. As discussed above, DGAT activities are compartmentalized into an overt fraction and a latent fraction (60–62), which may catalyze the synthesis of TG designated, respectively, for storage in lipid droplets or for secretion in nascent lipoproteins. Based on their topology, DGAT1, but not DGAT2, could contribute to the luminal (latent) activity. Overexpression of human DGAT1 in rat hepatoma McA-RH7777 cells decreased apoB degradation, increased cellular TG accumulation, and increased secretion of TG and apoB-containing lipoproteins (122; unpublished observations). When tested in vivo with adenovirus, short-term overexpression of DGAT1, but not DGAT2, in the livers of mice resulted in an increase in ER luminal DGAT activity and hepatic TG secretion (123). In a different study, in mice studied 4 days after adenoviral infection, overexpression of both DGAT1 and DGAT2 increased cytosolic TG levels in hepatocytes, but did not alter microsomal or VLDL TG levels, suggesting that neither enzyme is involved in determining the rate of TG secretion in VLDL and that factors other than DGAT1 or DGAT2 activity are limiting (81). Thus, the existing data are mixed concerning whether the overexpression of DGAT1 increases the capacity for TG secretion from the liver.
Studies in transgenic mice confirm that overexpression of either DGAT enzyme can cause TG accumulation in cytosolic lipid droplets in the liver (80). Interestingly, small increases in DGAT2 mRNA in murine liver were sufficient to promote marked TG accumulation and hepatic steatosis in vivo, whereas relatively larger increases in DGAT1 mRNA levels produced a smaller increase in hepatic TG storage. In agreement with one of the adenovirus studies discussed above, these transgenic mice also did not have increased plasma TG levels (80).
Although increasing DGAT gene expression transiently or stably in the liver may not alter hepatic TG secretion, the knockdown of DGAT2 mRNA levels in the liver with specific ASO lowered fasting plasma TG levels in mice with diet-induced obesity and reduced the hepatic secretion of VLDL TG and apoB (124, 125). Whether DGAT1 activity is rate limiting for TG secretion is unknown but could be tested in DGAT1 knockout mice.
DGAT enzymes and lipotoxicity
Obesity is characterized by the storage of excess TG in WAT and is often accompanied by aberrant storage of TG in nonadipose tissues, such as skeletal muscle and liver. In addition to TG, FAs and their metabolites, such as DG and ceramides, can also be elevated in these tissues. The deleterious effects of these FAs on cell survival and signaling pathways, including insulin signaling, have been termed lipotoxicity (as reviewed in Ref. 2). Altering the expression of DGAT enzymes in cells may modulate the lipotoxic effects of FAs and FA-derived mediators by controlling the degree to which they are esterified and stored in the inert TG pools of cells.
Monounsaturated oleic acid is efficiently taken up and stored as TG by cultured cells (126). In comparison, saturated palmitic acid is poorly incorporated into TG and is toxic to multiple cell types in culture (126–128). Interestingly, murine embryonic fibroblasts lacking DGAT1 can incorporate oleate into TG-containing lipid droplets and, when treated with oleate, are prone to cell death (126). These data suggest that the storage of FA within TG is key to cell survival and that DGAT1 protects cells against the toxic effects of FA.
The overexpression of DGAT1 in WAT appears to protect against lipotoxicity in nonadipose tissues. aP2-Dgat1 transgenic mice with a C57BL/6 genetic background have increased deposition of TG in the WAT, but reduced TG in nonadipose tissues in association with protection against developing glucose intolerance when fed a high-fat diet (85). Another line of aP2-Dgat1 transgenic mice (FVB genetic background) did not develop obesity or increased TG storage in the WAT and was consequently not protected against hepatic steatosis when fed a high-fat diet (86). The protective effects correlate with the sequestering of TG in WAT. The levels of Dgat2 (96, 129) and Dgat1 (129) mRNA and total DGAT activity (129) in the WAT were increased in two different rodent models of diet-induced obesity, suggesting that DGAT enzymes may be involved in the adaptation of WAT to chronically elevated dietary fat content.
Does lowering DGAT activity in the liver protect against or promote lipotoxicity? The role of DGAT enzymes in the development of diet-induced hepatic steatosis, fibrosis, tissue damage, and impaired glucose tolerance has been studied by using an ASO directed against either DGAT1 or DGAT2 (124, 130, 131). Short-term reduction of Dgat2 mRNA levels by ASO treatment reduced hepatic steatosis and TG content in ob/ob mice (124) and in both mice (124) and rats (130) fed a high-fat diet. These effects were not found with ASO against Dgat1 (130). Decreased hepatic TG content in DGAT2-ASO treated rats was paradoxically associated with reduced levels of DG and lysophosphatidic acid and of lipogenic gene expression (SREBP1c, ACC1, SCD1, mtGPAT) (130). Interestingly, these rats had increased hepatic insulin signaling and expression of oxidative genes. These findings were corroborated in vitro. DGAT2-ASO treatment increases FA oxidation rates in primary murine hepatocytes (124). Thus, short-term reduction of Dgat2 expression in the liver protects against hepatic steatosis and lipotoxic alterations in insulin sensitivity, suggesting that this strategy may be beneficial in human obesity.
Long-term DGAT2-ASO treatment also protected against hepatic steatosis in mice chronically fed a methionine/ choline-deficient diet. However, this protection was associated with increased hepatic levels of free FA, markers of oxidant stress, fibrosis, and hepatocyte necrosis (131). This finding dissociates chronic steatohepatitis and consequent fibrosis from hepatic TG accumulation per se and suggests that DGAT2 activity in the liver is necessary to prevent the lipotoxic consequences of a chronically fatty liver.
Accumulation of lipids in tissues such as liver and skeletal muscle clearly can be associated with insulin resistance. But does DGAT overexpression in these tissues promote such lipotoxicity? Transgenic mice with increased expression of DGAT2 in the liver had hepatic steatosis with increased content of TG, DG, ceramides, and long-chain fatty acyl-CoA (80), but not insulin resistance. Similar findings were reported in mice overexpressing DGAT1 in the liver (81). These data indicate that DGAT-mediated lipid accumulation in the liver does not cause insulin resistance and that hepatic steatosis per se is not always sufficient to cause insulin resistance, which has been validated in other animal models. Rather, an augmented capacity to synthesize and store TG in hepatocytes may protect against lipotoxic metabolic abnormalities associated with hepatic steatosis.
In skeletal muscle, the effects of DGAT overexpression appear to differ, depending on which enzyme is involved. For example, DGAT1 overexpression in skeletal muscle by electroporation promoted a striking increase in diet-induced intramyocellular lipid storage (84), a finding that was corroborated in transgenic mice overexpressing DGAT1 in skeletal muscle [MCK-Dgat1 (83)]. However, increased myocellular TG in MCK-Dgat1 mice was associated with reduced levels of DG and ceramides and with improved total body glucose tolerance and insulin signaling in isolated skeletal muscle specimens (83). Interestingly, exercise training increases intramyocellular lipid storage, particularly in oxidative (type I) skeletal muscle, and increases DGAT1 activity (84). Thus, DGAT1 may be partly responsible for the “exercise paradox” (i.e., enhanced insulin sensitivity with increased TG in the type I skeletal muscles of people who exercise). In this case, DGAT1 may protect against lipotoxicity in skeletal muscle.
This appears not to be the case for DGAT2, at least when it is overexpressed in glycolytic (type II) skeletal muscle. In transgenic mice, increased expression of human DGAT2 primarily in type II skeletal muscle increases the TG content of this muscle type in association with reduced DG and increased ceramide and long-chain fatty acyl-CoA levels (82). When young, these mice had decreased insulin signaling and glucose uptake in isolated muscle and had impaired whole-body glucose and insulin tolerance (82). These studies suggest that more DGAT1 in type I muscle does not lead to lipotoxicity and may even be protective, but more DGAT2 in type II muscle can induce lipotoxicity.
DGAT enzymes and inflammation
Because DGAT enzymes esterify fatty acyl moieties and may help protect cells against toxicity from excess FA, these enzymes may modulate the inflammatory effects of FA. For example, transgenic MCK-Dgat1 mice fed a high-fat diet had reduced phosphorylation of JNK1 (p-JNK), a key component of inflammatory activation, in skeletal muscle, suggesting that increased Dgat1 expression may protect against inflammation in muscle (83). A similar protective effect was observed with liver-specific overexpression of DGAT2, which induced hepatic steatosis without inducing p-JNK and p-NFkB as it does in mice with steatosis induced by a high-fat diet (80). Conversely, chronic suppression of Dgat2 expression in mice by a DGAT2-specific ASO increases circulating levels of ALT, a marker of hepatic inflammation, when hepatic steatosis is induced by a methionine-choline-deficient diet (131). Thus, DGAT expression can modulate the inflammatory response to FAs and their toxic metabolites.
DGAT enzymes and tissue development
Beyond its role in energy and glucose metabolism, DGAT1 may have additional functions. Its deficiency affects developmental processes in tissues, such as hair growth in the skin and lactation in mammary glands. Dgat1−/− mice have dry fur and lose hair after puberty (55). These abnormalities are associated with atrophic sebaceous glands and the absence of wax diesters, a major species of fur lipids in rodents. As a result, water repulsion and thermoregulation after water immersion are impaired in Dgat1−/− mice (55). Instead of producing milk low in fat, female Dgat1−/− mice do not lactate (34). They have atrophic mammary epithelia resulting from defects in both glandular proliferation and differentiation (132). These skin and mammary gland abnormalities are not observed in Dgat1+/− mice, suggesting that the mechanisms involved are different than those for energy and glucose metabolism.
The skin and mammary gland phenotypes of Dgat1−/− mice may relate to DGAT1’s different acyltransferase activities. The wax diester synthase activity of DGAT1 may underlie the missing fur lipids in DGAT1-deficient mice (25, 55), and the ARAT activity of DGAT1 may contribute to the hair phenotype of Dgat1−/− mice. Retinol is the precursor for retinoic acids (all-trans and its isomer 9-cis retinoic acid), which are ligands for the nuclear hormone receptors retinoic acid receptor and retinoid-X receptor (133–135). Retinoids affect sebaceous gland function (136, 137), hair growth (138), and proliferation of mammary gland epithelial cells (139, 140), all of which are part of the phenotypic changes observed in Dgat1−/− mice (34, 55, 132). Whether the ARAT activity of DGAT1 is important in the skin remains to be determined.
DGAT2 also plays an important role in the skin, and Dgat2−/− mice exhibit severe skin abnormalities (Fig. 6A). The skin of newborn Dgat2−/− mice is shiny, lacks elasticity, and exhibits increased transepidermal water loss, which leads to rapid dehydration (47). These phenotypes strikingly resemble those of oed mutant mice (141). The oed mutation arose spontaneously and has an autosomal recessive mode of inheritance. Interestingly, like Dgat2−/− mice, homozygous oed mice had markedly reduced total-body TG levels and died soon after birth, while heterozygous mice did not have distinct phenotypes (141). It is unknown whether the underlying mutation in oed mice was in DGAT2 or a related protein, and the mice are no longer available. In Dgat2−/− mice, the increased water loss was associated with reduced levels of the lipid acylceramide in the skin. Acylceramide is believed to be essential for maintaining the permeability barrier in the skin, and its synthesis requires linoleic acid, an essential FA. Dgat2−/− mice are deficient in this essential FA, and this probably contributes to a 60% reduction in their skin acylceramide levels. Skin grafting experiments suggest that the skin defects in Dgat2−/− mice result, at least in part, from a systemic lack of DGAT2 rather than a loss of DGAT2 specifically in the skin (47). Interestingly, mice deficient in SCD2 exhibit a skin phenotype similar to that of Dgat2−/− mice during the neonatal period (∼30% of Scd2−/− mice survive to adulthood) (142). These findings suggest that SCD2 may provide DGAT2 with monounsaturated FA required for maintaining normal epidermal permeability and for lipid biosynthesis during early skin development. In addition to DGAT2 and SCD2, mice deficient in other genes involved in lipid metabolism, such as ACAT1 and FA transport protein 4 (143–145), also exhibit skin defects, highlighting the importance of lipid metabolism for skin functions and the potential toxicity of unmetabolized lipid substrates.
DGAT ENZYMES IN HUMANS
In humans, DGAT1 and DGAT2 are generally expressed in tissues similar to those in mice (22, 36). Humans with DGAT deficiency have not been identified, nor have coding sequence mutants for DGAT1 or DGAT2 been reported to cause genetic diseases. Homozygosity for deficiency alleles for either enzyme may be selected against: in mice, female mice homozygous for DGAT1 deficiency cannot lactate (34), and mice homozygous for DGAT2 deficiency die in the early postnatal period (47).
Because the level of DGAT1 expression modulates adiposity in inbred mice, alterations in DGAT1 expression might affect body mass in humans. Several common sequence polymorphisms are present in the 5′ noncoding sequences of the DGAT1 gene (89). One of these polymorphisms, C79T, affects promoter activity in cultured cells and has been associated with alterations in body mass index, diastolic blood pressure, and HDL cholesterol levels in Turkish women (89). However, this polymorphism was not associated with increased body mass index or with obesity in a French population that included obese adults and children (146). Thus, this relatively common polymorphism may have a rather small phenotypic effect.
Human DGAT2 is located on chromosome 11q13, a region that was linked to obesity in a genome-wide association study (147, 148). In a subsequent study, several DGAT2 variants were identified in a similar population of obese adolescents (149). However, no association between these polymorphisms and obesity phenotypes was found in case-control and family-based association studies involving several hundred subjects (149). Therefore, it remains to be determined whether expression of either enzyme modulates human adiposity.
DGAT INHIBITORS
TG synthesis plays important roles in many biological processes involving storage and transport of energy and FA substrates. It is indeed essential for life in mammals, as demonstrated in mice lacking DGAT2. However, the potentially beneficial effects of DGAT1 deficiency on energy and glucose metabolism have generated considerable interest in DGAT1 inhibition as a potential therapy for obesity and diabetes in humans. Insights into the prospects of DGAT1 inhibition from DGAT1-deficient mice were recently reviewed in detail (150). Natural and synthetic inhibitors of DGAT1 are being developed (as reviewed in Ref. 151). Numerous agents have been reported to inhibit DGAT activity, including a variety of substances isolated from microorganisms (152–156) and fish oils (157), but it is unclear whether these agents inhibit one or both DGAT enzymes. Recently, a selective small-molecule inhibitor of DGAT1 was reported to reduce weight gain and hepatic TG when administered to mice fed a high-fat diet (115). These findings indicate that pharmacologic inhibition of DGAT1 may have metabolic effects similar to those seen with inactivation of its gene in mice, raising the promise of DGAT1 inhibitors for the treatment of obesity, hepatic steatosis, and possibly diabetes mellitus. Another potential use of DGAT1 inhibitors is as a topical treatment for acne vulgaris, because DGAT1 deficiency causes atrophy of sebaceous glands (25, 55).
DGAT1 has a broad tissue expression pattern and possible physiological functions beyond those involving TG synthesis. Thus, inhibition of DGAT1 could lead to detrimental effects, as suggested by the altered retinol metabolism, skin abnormalities, and defects in mammary gland development in DGAT1-deficient mice. Interestingly, in these mice, the beneficial effects on energy balance and glucose metabolism were seen with heterozygous deficiency, whereas the abnormalities in skin and mammary glands were only observed in the absence of DGAT1. Thus, it may be possible to pharmacologically inhibit DGAT1 sufficiently to yield beneficial metabolic effects without causing the adverse effects of complete deficiency.
Inhibiting DGAT2 as a therapy for metabolic diseases may prove to be more challenging, because DGAT2 deficiency is not compatible with life. However, recent studies suggest that this strategy may be promising. Niacin, which can pharmacologically raise levels of HDL and decrease levels of plasma TG and LDL, apparently inhibits DGAT2, but not DGAT1 in in vitro assays (63). Also, inhibiting hepatic DGAT2 with ASO had the desirable effects of decreasing plasma TG levels and insulin resistance induced by a high-fat diet (125, 130). Thus, inhibiting DGAT2, particularly in tissues such as the liver, may be beneficial.
DGAT ENZYMES IN OTHER ORGANISMS
Genes encoding DGAT enzymes have been identified in many other species, including Arabidopsis thaliana, Drosophila melanogaster, and most mammalian species. In plants, DGAT1 and DGAT2 orthologs occur in several tissues, including leaf, flower, and seeds (57), and are important for seed oil production (158, 159). For example, an ethyl methan sulfonate-induced mutation resulting in an 81 bp insertion of exon 2 in the A. thaliana DGAT1 reduces seed oil and delays seed maturation (160). Recently, a high-oil quantitative trait locus, determining the content and composition of maize seed oil, was attributed to a mutation in DGAT1 (161). A phenylalanine insertion (F469) increases seed oil and oleic acid content. This phenylalanine is conserved in most plant DGAT1 sequences and was probably deleted in some strains of maize during domestication or breeding (161).
Likewise, DGAT2 may mediate the content and composition of seed oils in some species. In tung trees, which are important for industry because they produce large quantities of TG and a high percentage (80%) of the unusual eleostearic acid, both enzymes are expressed in various organs (57), but only DGAT2 is highly induced during seed development at the onset of TG synthesis and preferentially catalyzes synthesis of trieleostearin, the main component of tung oil (57). Increased DGAT2 expression increases oil content in transgenic oilseed crops, including soybean (162, 163).
Both enzymes occur in flies, and a strain of D. melanogaster with a mutant DGAT1 gene has been described (164). This mutant, called midway, exhibits premature nurse cell death in eggs, indicating an essential role for the DGAT1 gene in fly egg development.
In cows, variations at the DGAT1 locus are emerging as important regulators of milk fat content. Polymorphisms in both the coding and noncoding sequences of DGAT1 have been linked to milk fat content (52, 165–171) and possibly to muscle fat content (172). The best-characterized polymorphism, K232A, occurs at a lysine of DGAT1 that is conserved across other mammalian DGAT1 proteins and has been consistently linked to milk production traits. In in vitro assays, the K allele was associated with a 1.5-fold higher Vmax than the A allele for bovine DGAT1 expressed in insect cells, supporting its effect on increasing milk fat content (167).
FUTURE RESEARCH
With the cloning of genes for DGAT1 and DGAT2, considerable progress has been made toward an understanding of the molecular processes involved in TG biosynthesis and how these processes relate to physiology, metabolism, and disease. Nonetheless, important areas of investigation remain. How does the biochemistry of DGAT enzymes differ, including the molecular mechanisms of their catalytic reactions? How do the two enzymes relate to different aspects of TG metabolism in cells? How do the two enzymes differ with respect to their regulation and binding partners? Do other mammalian DGAT enzymes exist? Insights into the molecular structure of the enzymes would greatly enhance our understanding of how the enzymes work and would provide a fascinating comparison of apparently unrelated enzymes that catalyze the same reaction. We can expect the next decade to considerably advance our understanding of how these enzymes function in the fundamental processes of energy storage and utilization in living organisms. Currently, research in mammals is focused on exploring ways to block DGAT enzymes to limit TG and energy storage. Other studies are attempting to utilize DGAT enzymes to engineer organisms to increase the production of oils for food consumption and for biofuels. The molecular age of DGAT investigation has begun and promises many more basic biological insights as well as breakthroughs in therapeutic applications.
Acknowledgments
The authors thank Hubert Chen for early contributions to this review; members of the Farese laboratory for their many contributions to the field and to perspectives provided in this review; Daryl Jones for manuscript preparation; Gary Howard and Stephen Ordway for editorial assistance; and David W. Nelson for comments on the manuscript. We also acknowledge the many colleagues in the field whose work we were not able to include.
Abbreviations:
- ACAT
acyl-CoA:cholesterol acyltransferase
- AMPK
AMP-activated kinase
- apoB
apolipoprotein B
- ARAT
acyl-CoA:retinol acyltransferase
- ASO
anti-sense oligonucleotide
- DG
diacylglycerol
- DGAT
acyl-CoA:diacylglycerol acyltransferase
- ER
endoplasmic reticulum
- GPAT
glycerol-phosphate acyltranserase
- MG
monoacylglycerol
- MGAT
acyl-CoA: monoacylglycerol acyltransferase
- SCD
stearoyl-CoA desaturase
- SREBP
sterol-regulatory element binding protein
- TG
triacylglycerol (triglyceride)
- WAT
white adipose tissue
- XBP1
X-box binding protein 1
Footnotes
TG biosynthesis can also occur through acyl-CoA-independent enzymes. For example, DG transacylase, an enzyme present in rodent small intestine, catalyses the direct transfer of a fatty acyl group from one DG to a second DG, yielding TG and MG products (173), and phospholipid:diacylglycerol transacylase catalyses the production of TG in a similar reaction, in which a fatty acyl group from the sn-2 position of phosphatidylcholine is transferred to DG. This latter pathway accounts for a substantial fraction of TG biosynthesis in yeast and plants (174). These acyl-CoA-independent mechanisms of TG synthesis will not be reviewed further here. We also will not review bacterial DGAT enzymes, which utilize acyl-CoA but are unrelated by sequence homology to mammalian DGAT1 and DGAT2 (175).
This work was supported by American Heart Association Scientist Development Grants (C-L.E.Y., S.J.S.), an A.P. Giannini Foundation Award (S.K.), National Institutes of Health Grant DK-56084 (R.V.F.), the Sandler Family Supporting Foundation, and the J. David Gladstone Institutes.
REFERENCES
- 1.Stryer L. Biochemistry. W.H. Freeman & Co.; New York, NY: 1988. p. 471. [Google Scholar]
- 2.Unger RH. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 3.Friedman J. Fat in all the wrong places. Nature. 2002;415:268–269. doi: 10.1038/415268a. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy E. Metabolism of lipides. Annu. Rev. Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- 5.Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu. Rev. Biochem. 1980;49:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]
- 6.Brindley DN. Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier; Amsterdam, The Netherlands: 1991. pp. 171–203. [Google Scholar]
- 7.Lehner R, Kuksis A. Biosynthesis of triacylglycerols. Prog. Lipid Res. 1996;35:169–201. doi: 10.1016/0163-7827(96)00005-7. [DOI] [PubMed] [Google Scholar]
- 8.Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 9.Coleman R, Lewin T, Van Horn C, Gonzalez-Baró M. Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J. Nutr. 2002;132:2123–2126. doi: 10.1093/jn/132.8.2123. [DOI] [PubMed] [Google Scholar]
- 10.Xia T, Mostafa N, Bhat BG, Florant GL, Coleman RA. Selective retention of essential fatty acids: The role of hepatic monoacylglycerol acyltransferase. Am. J. Physiol. 1993;265:R414–R419. doi: 10.1152/ajpregu.1993.265.2.R414. [DOI] [PubMed] [Google Scholar]
- 11.Kayden HJ, Senior JR, Mattson FH. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 1967;46:1695–1703. doi: 10.1172/JCI105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansbach CM, II, Gorelick F. Development and physiological regulation of intestinal lipid absorption. II. Dietary lipid absorption, complex lipid synthesis, and the intracellular packaging and secretion of chylomicrons. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G645–G650. doi: 10.1152/ajpgi.00299.2007. [DOI] [PubMed] [Google Scholar]
- 13.Weiss SB, Kennedy EP, Kiyasu JY. The enzymatic synthesis of triglycerides. J. Biol. Chem. 1960;235:40–44. [PubMed] [Google Scholar]
- 14.Walther T, Farese RV., Jr The life of lipid droplets. Biochim. Biophys. Acta. 2008. In press. [DOI] [PMC free article] [PubMed]
- 15.Shelness G, Ledford A. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr. Opin. Lipidol. 2005;16:325–332. doi: 10.1097/01.mol.0000169353.12772.eb. [DOI] [PubMed] [Google Scholar]
- 16.Shelness GS, Sellers JA. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 2001;12:151–157. doi: 10.1097/00041433-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Hussain M, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 18.Weiss S, Kennedy E. The enzymatic synthesis of triglycerides. J. Biol. Chem. 1960;235:40–44. [PubMed] [Google Scholar]
- 19.Chang CCY, Huh HY, Cadigan KM, Chang TY. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 1993;268:20747–20755. [PubMed] [Google Scholar]
- 20.Cases S, Novak S, Zheng Y-W, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 21.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of Acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 1998;273:26747–26754. doi: 10.1074/jbc.273.41.26747. [DOI] [PubMed] [Google Scholar]
- 22.Cases S, Smith SJ, Zheng Y-W, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oelkers P, Behari A, Cromley D, Billheimer JT, Sturley SL. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 1998;273:26765–26771. doi: 10.1074/jbc.273.41.26765. [DOI] [PubMed] [Google Scholar]
- 24.Buhman KK, Chen HC, Farese RV., Jr The enzymes of neutral lipid synthesis. J. Biol. Chem. 2001;276:40369–40372. doi: 10.1074/jbc.R100050200. [DOI] [PubMed] [Google Scholar]
- 25.Yen C-LE, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Cromley D, Billheimer J, Sturley S. Identification of potential substrate-binding sites in yeast and human acyl-CoA sterol acyltransferases by mutagenesis of conserved sequences. J. Lipid Res. 2001;42:1282–1291. [PubMed] [Google Scholar]
- 27.Weselake R, Madhavji M, Szarka S, Patterson N, Wiehler W, Nykiforuk C, Burton T, Boora P, Mosimann S, Foroud N, et al. Acyl-CoA-binding and self-associating properties of a recombinant 13.3 kDa N-terminal fragment of diacylglycerol acyltransferase-1 from oilseed rape. BMC Biochem. 2006;7:24. doi: 10.1186/1471-2091-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siloto R, Madhavji M, Wiehler W, Burton T, Boora P, Laroche A, Weselake R. An N-terminal fragment of mouse DGAT1 binds different acyl-CoAs with varying affinity. Biochem. Biophys. Res. Commun. 2008;373:350–354. doi: 10.1016/j.bbrc.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhai L, Chaturvedi D, Cumberledge S. Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J. Biol. Chem. 2004;279:33220–33227. doi: 10.1074/jbc.M403407200. [DOI] [PubMed] [Google Scholar]
- 31.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 32.Chamon Z, Mann R, Nellen D, von Kessler D, Bellotto M, Beachy P, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Brown M, Liang G, Grishin N, Goldstein J. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat. Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 35.Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ. DGAT2 is a new diacylglycerol acyltransferase gene family. Purification, cloning, and expression in insect cells of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J. Biol. Chem. 2001;276:38862–38869. doi: 10.1074/jbc.M106168200. [DOI] [PubMed] [Google Scholar]
- 36.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J. Biol. Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- 37.Yen C-LE, Stone SJ, Cases S, Zhou P, Farese RV., Jr Identification of a gene encoding MGAT1, a monoacylglylcerol acyltransferase. Proc. Natl. Acad. Sci. USA. 2002;99:8512–8517. doi: 10.1073/pnas.132274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao J, Lockwood J, Burn P, Shi Y. Cloning and functional characterization of a mouse intestinal acyl-CoA: monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 2003;278:13860–13866. doi: 10.1074/jbc.M300139200. [DOI] [PubMed] [Google Scholar]
- 39.Yen C-LE, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 40.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, Meegalla R, Taub R, Billheimer JT, Ramaker M, Feder JN. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 2003;278:13611–13614. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 41.Cheng JB, Russell DW. Mammalian waxbio-synthesis. II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J. Biol. Chem. 2004;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turkish AR, Henneberry AL, Cromley D, Padamsee M, Oelkers P, Bazzi H, Christiano AM, Billheimer JT, Sturley SL. Identification of two novel human acyl-CoA wax alcohol acyltransferases: members of the diacylglycerol acyltransferase 2 (DGAT2) gene superfamily. J. Biol. Chem. 2005;280:14755–14764. doi: 10.1074/jbc.M500025200. [DOI] [PubMed] [Google Scholar]
- 43.Yen C-LE, Brown CH, IV, Monetti M, Farese RV., Jr A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes, and retinyl esters. J. Lipid Res. 2005;46:2388–2397. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stone S, Levin M, Farese RV., Jr Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J. Biol. Chem. 2006;281:40273–40282. doi: 10.1074/jbc.M607986200. [DOI] [PubMed] [Google Scholar]
- 45.Au-Young J, Fielding CJ. Synthesis and secretion of wild-type and mutant human plasmacholesteryl ester transfer protein in baculovirus-transfected insect cells: the carboxyl-terminal region is required for both lipoprotein binding and catalysis of transfer. Proc. Natl. Acad. Sci. USA. 1992;89:4094–4098. doi: 10.1073/pnas.89.9.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alam M, Gilham D, Vance DE, Lehner R. Mutation of F417 but not of L418 or L420 in the lipid binding domain decreases the activity of triacylglycerol hydrolase. J. Lipid Res. 2006;47:375–383. doi: 10.1194/jlr.M500344-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Stone SJ, Myers H, Brown BE, Watkins SM, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 48.Sorger D, Daum G. Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J. Bacteriol. 2002;184:519–524. doi: 10.1128/JB.184.2.519-524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oelkers P, Cromley D, Padamsee M, Billheimer JT, Sturley SL. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- 50.Ross AC. Retinol esterification by rat liver microsomes. Evidence for a fatty acyl coenzyme A: retinol acyltransferase. J. Biol. Chem. 1982;257:2453–2459. [PubMed] [Google Scholar]
- 51.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orland MD, Anwar K, Cromley D, Chu CH, Chen L, Billheimer JT, Hussain MM, Cheng D. Acyl coenzyme A dependent retinol esterification by acyl coenzyme A: diacylglycerol acyltransferase 1. Biochim. Biophys. Acta. 2005;1737:76–82. doi: 10.1016/j.bbalip.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg I, Johnston T, Li E, Blaner W. The molecular basis of retinoid absorption: a genetic dissection. J. Biol. Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi K, Yang L, McCall S, Huang J, Yu X, Pandey S, Bhanot S, Monia B, Li Y, Diehl A. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
- 55.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J. Clin. Invest. 2002;109:175–181. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang H, Bard M, Bruner DA, Gleeson A, Deckelbaum RJ, Aljinovic G, Pohl TM, Rothstein R, Sturley SL. Sterol esterification in yeast: a two-gene process. Science. 1996;272:1353–1356. doi: 10.1126/science.272.5266.1353. [DOI] [PubMed] [Google Scholar]
- 57.Shockey J, Gidda S, Chapital D, Kuan J, Dhanoa P, Bland J, Rothstein S, Mullen R, Dyer J. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell. 2006;18:2294–2313. doi: 10.1105/tpc.106.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng D, Meegalla RL, He B, Cromley DA, Billheimer JT, Young PR. Human acyl-CoA:diacylglycerol acyltransferase is a tetrameric protein. Biochem. J. 2001;359:707–714. doi: 10.1042/0264-6021:3590707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu C, Chen J, Lin S, Liu J, Chang CCY, Chang TY. Human acyl-CoA:cholesterol acyltransferase-1 is a homotetrameric enzyme in intact cells and in vitro. J. Biol. Chem. 1999;274:36139–36145. doi: 10.1074/jbc.274.51.36139. [DOI] [PubMed] [Google Scholar]
- 60.Waterman IJ, Price NT, Zammit VA. Distinct ontogenic patterns of overt and latent DGAT activities of rat liver microsomes. J. Lipid Res. 2002;43:1555–1562. doi: 10.1194/jlr.m200051-jlr200. [DOI] [PubMed] [Google Scholar]
- 61.Owen MR, Corstorphine CC, Zammit VA. Overt and latent activities of diacylglycerol acytransferase in rat liver microsomes: possible roles in very-low-density lipoprotein triacylglycerol secretion. Biochem. J. 1997;323:17–21. doi: 10.1042/bj3230017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abo-Hashema KAH, Cake MH, Power GW, Clarke D. Evidence for triacylglycerol synthesis in the lumen of microsomes via a lipolysis-esterification pathway involving carnitine acyltransferases. J. Biol. Chem. 1999;274:35577–35582. doi: 10.1074/jbc.274.50.35577. [DOI] [PubMed] [Google Scholar]
- 63.Ganji S, Tavintharan S, Zhu D, Xing Y, Kamanna V, Kashyap M. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J. Lipid Res. 2004;45:1835–1845. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Man WC, Miyazaki M, Chu K, Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. J. Lipid Res. 2006;47:1928–1939. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 65.Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 66.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 67.Beller M, Riedel D, Jansch L, Dieterich G, Wehland J, Jackle H, Kuhnlein RP. Characterization of the Drosophila lipid droplet subproteome. Mol. Cell. Proteomics. 2006;5:1082–1094. doi: 10.1074/mcp.M600011-MCP200. [DOI] [PubMed] [Google Scholar]
- 68.Wu CC, Howell KE, Neville MC, Yates JR, III, McManaman JL. Proteomics reveal a link between the endoplasmic reticulum and lipid secretory mechanisms in mammary epithelial cells. Electrophoresis. 2000;21:3470–3482. doi: 10.1002/1522-2683(20001001)21:16<3470::AID-ELPS3470>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 69.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim. Biophys. Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 70.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J. Biol. Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 71.Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R. Association of stomatin with lipid bodies. J. Biol. Chem. 2004;279:23699–23709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 72.Cermelli S, Guo Y, Gross S, Welte M. The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr. Biol. 2006;16:1783–1795. doi: 10.1016/j.cub.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 73.Sato S, Fukasawa M, Yamakawa Y, Natsume T, Suzuki T, Shoji I, Aizaki H, Miyamura T, Nishijima M. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J. Biochem. 2006;139:921–930. doi: 10.1093/jb/mvj104. [DOI] [PubMed] [Google Scholar]
- 74.Turro S, Ingelmo-Torres M, Estanyol JM, Tebar F, Fernandez MA, Albor CV, Gaus K, Grewal T, Enrich C, Pol A. Identification and characterization of associated with lipid droplet protein 1: a novel membrane-associated protein that resides on hepatic lipid droplets. Traffic. 2006;7:1254–1269. doi: 10.1111/j.1600-0854.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 75.Wan HC, Melo RC, Jin Z, Dvorak AM, Weller PF. Roles and origins of leukocyte lipid bodies: proteomic and ultra-structural studies. FASEB J. 2007;21:167–178. doi: 10.1096/fj.06-6711com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: protein phosphorylation and GTP-mediated protein translocation. J. Proteome Res. 2007;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 77.Lewin T, de Jong H, Schwerbrock N, Hammond L, Watkins S, Combs T, Coleman R. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim. Biophys. Acta. 2008;1781:352–358. doi: 10.1016/j.bbalip.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dricks L, Sul H. Mammalian mitochondrial glycerol-3-phosphate acyltransferase. Biochim. Biophys. Acta. 1997;1348:17–26. doi: 10.1016/s0005-2760(97)00106-9. [DOI] [PubMed] [Google Scholar]
- 79.Bagnato C, Igal RA. Overexpression of diacylglycerol acyltransferase-1 reduces phospholipid synthesis, proliferation, and invasiveness in simian virus 40-transformed human lung fibroblasts. J. Biol. Chem. 2003;278:52203–52211. doi: 10.1074/jbc.M305760200. [DOI] [PubMed] [Google Scholar]
- 80.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV., Sr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 81.Millar J, Stone S, Tietge U, Tow B, Billheimer J, Wong J, Hamilton R, Farese RV, Jr, Rader D. Short-term over-expression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. J. Lipid Res. 2006;47:2297–2305. doi: 10.1194/jlr.M600213-JLR200. [DOI] [PubMed] [Google Scholar]
- 82.Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Farese RV., Jr Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1772–E1781. doi: 10.1152/ajpendo.00158.2007. [DOI] [PubMed] [Google Scholar]
- 83.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roorda B, Hesselink M, Schaart G, Moonen-Kornips E, Martinez-Martinez P, Losen M, DeBaets M, Menslink R, Schrauwen P. DGAT1 overexpression in muscle by in vivo DNA electroporation increases intramyocellular lipid content. J. Lipid Res. 2005;46:230–236. doi: 10.1194/jlr.M400416-JLR200. [DOI] [PubMed] [Google Scholar]
- 85.Chen HC, Stone SJ, Zhou P, Buhman KK, Farese RV., Jr Dissociation of obesity and impaired glucose disposal in mice overexpressing acyl coenzyme a:diacylglycerol acyltransferase 1 in white adipose tissue. Diabetes. 2002;51:3189–3195. doi: 10.2337/diabetes.51.11.3189. [DOI] [PubMed] [Google Scholar]
- 86.Chen N, Liu L, Zhang Y, Ginsberg HN, Yu YH. Whole-body insulin resistance in the absence of obesity in FVB mice with overexpression of Dgat1 in adipose tissue. Diabetes. 2005;54:3379–3386. doi: 10.2337/diabetes.54.12.3379. [DOI] [PubMed] [Google Scholar]
- 87.Meegalla RL, Billheimer JT, Cheng D. Concerted elevation of acyl-coenzyme A:diacylglycerol acyltransferase (DGAT) activity through independent stimulation of mRNA expression of DGAT1 and DGAT2 by carbohydrate and insulin. Biochem. Biophys. Res. Commun. 2002;298:317–323. doi: 10.1016/s0006-291x(02)02466-x. [DOI] [PubMed] [Google Scholar]
- 88.Yu YH, Zhang Y, Oelkers P, Sturley SL, Rader DJ, Ginsberg HN. Posttranscriptional control of the expression and function of diacylglycerol acyltransferase-1 in mouse adipocytes. J. Biol. Chem. 2002;277:50876–50884. doi: 10.1074/jbc.M207353200. [DOI] [PubMed] [Google Scholar]
- 89.Ludwig EH, Mahley RW, Palaoglu E, Balestra ME, Borecki IB, Innerarity TL, Farese RV., Jr DGAT1 promoter polymorphism associated with alterations in body mass index, high density lipoprotein levels and blood pressure in Turkish women. Clin. Genet. 2002;62:68–73. doi: 10.1034/j.1399-0004.2002.620109.x. [DOI] [PubMed] [Google Scholar]
- 90.Ranganathan G, Unal R, Pokrovskaya I, Yao-Borengasser A, Phanavanh B, Lecka-Czernik B, Rasouli N, Kern P. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. J. Lipid Res. 2006;47:2444–2450. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Payne V, Au W, Gray S, Nora E, Rahman S, Sanders R, Hadaschik D, Friedman J, O'Rahilly S, Rochford JJ. Sequential regulation of diacylglycerol acyltransferase 2 expression by CAAT/enhancer-binding protein beta (C/EBPbeta) and C/ EBPalpha during adipogenesis. J. Biol. Chem. 2007;282:21005–21014. doi: 10.1074/jbc.M702871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horton J, Shah N, Warrington J, Anderson N, Park S, Brown M, Goldstein J. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee A, Scapa E, Cohen D, Glimcher L. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirata T, Unoki H, Bujo H, Ueno K, Saito Y. Activation of diacylglycerol O-acyltransferase 1 gene results in increased tumor necrosis factor-alpha gene expression in 3T3-L1 adipocytes. FEBS Lett. 2006;580:5117–5121. doi: 10.1016/j.febslet.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 95.Tsai J, Qiu W, Kohen-Avramoglu R, Adeli K. MEKERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler. Thromb. Vasc. Biol. 2007;27:211–218. doi: 10.1161/01.ATV.0000249861.80471.96. [DOI] [PubMed] [Google Scholar]
- 96.Suzuki R, Tobe K, Aoyama M, Sakamoto K, Ohsugi M, Kamei N, Nemoto S, Inoue A, Ito Y, Uchida S, et al. Expression of DGAT2 in white adipose tissue is regulated by central leptin action. J. Biol. Chem. 2005;280:3331–3337. doi: 10.1074/jbc.M410955200. [DOI] [PubMed] [Google Scholar]
- 97.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakimoto K, Chiba H, Michibata H, Seishima M, Kawasaki S, Okubo K, Mitsui H, Torii H, Imai Y. A novel diacylglycerol acyltransferase (DGAT2) is decreased in human psoriatic skin and increased in diabetic mice. Biochem. Biophys. Res. Commun. 2003;310:296–302. doi: 10.1016/j.bbrc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 99.Hems D, Rath E, Verrinder T. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem. J. 1975;150:167–173. doi: 10.1042/bj1500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem. Biophys. Res. Commun. 2002;296:395–400. doi: 10.1016/s0006-291x(02)00883-5. [DOI] [PubMed] [Google Scholar]
- 101.Vaziri ND, Kim CH, Phan D, Kim S, Liang K. Up-regulation of hepatic Acyl CoA: Diacylglycerol acyltransferase-1 (DGAT-1) expression in nephrotic syndrome. Kidney Int. 2004;66:262–267. doi: 10.1111/j.1523-1755.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- 102.Vaziri ND, Kim CH, Dang B, Zhan CD, Liang K. Downregulation of hepatic acyl-CoA:diglycerol acyltransferase in chronic renal failure. Am. J. Physiol. Renal Physiol. 2004;287:F90–F94. doi: 10.1152/ajprenal.00358.2003. [DOI] [PubMed] [Google Scholar]
- 103.Haagsman HP, de Haas CG, Geelen MJ, van Golde LM. Regulation of triacylglycerol synthesis in the liver: a decrease in diacylglycerol acyltransferase activity after treatment of isolated rat hepatocytes with glucagon. Biochim. Biophys. Acta. 1981;664:74–81. doi: 10.1016/0005-2760(81)90029-1. [DOI] [PubMed] [Google Scholar]
- 104.Haagsman HP, de Haas CGM, Geelen MJH, van Golde LMG. Regulation of triacylglycerol synthesis in the liver. Modulation of diacylglycerol acyltransferase activity in vitro. J. Biol. Chem. 1982;257:10593–10598. [PubMed] [Google Scholar]
- 105.Rodriguez MA, Dias C, Lau TE. Reversible ATP-dependent inactivation of adipose diacylglycerol acyltransferase. Lipids. 1992;27:577–581. doi: 10.1007/BF02536113. [DOI] [PubMed] [Google Scholar]
- 106.Lau TE, Rodriguez MA. A protein tyrosine kinase associated with the ATP-dependent inactivation of adipose diacylglycerol acyltransferase. Lipids. 1996;31:277–283. doi: 10.1007/BF02529874. [DOI] [PubMed] [Google Scholar]
- 107.Liu J, Chang C, Westover E, Covey D, Chang T. Investigating the allosterism of acyl-CoA:cholesterol acyltransferase (ACAT) by using various sterols: in vitro and intact cell studies. Biochem. J. 2005;391:389–397. doi: 10.1042/BJ20050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sooranna SR, Saggerson ED. A decrease in diacylglycerol acyltransferase after treatment of rat adipocytes with adrenaline. FEBS Lett. 1978;95:85–87. doi: 10.1016/0014-5793(78)80057-x. [DOI] [PubMed] [Google Scholar]
- 109.Schoonderwoerd K, Broekhoven-Schokker S, Hulsmann WC, Stam H. Properties of phosphatidate phosphohydrolase and diacylglycerol acyltransferase activities in the isolated rat heart. Effect of glucagon, ischaemia and diabetes. Biochem. J. 1990;268:487–492. doi: 10.1042/bj2680487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hillmar I, Henze K, Zollner N. Influence of fatty acids on microsomal diacylglycerol acyltransferase activity in primary cultures of hepatocytes. Hoppe Seylers Z. Physiol. Chem. 1983;364:173–178. doi: 10.1515/bchm2.1983.364.1.173. [DOI] [PubMed] [Google Scholar]
- 111.Assifi M, Suchankova G, Constant S, Prentki M, Saha A, Ruderman N. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am. J. Physiol. Endocrinol. Metab. 2005;289:E794–E800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- 112.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem. J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 113.Chen HC, Ladha Z, Smith SJ, Farese RV., Jr Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am. J. Physiol. Endocrinol. Metab. 2003;284:E213–E218. doi: 10.1152/ajpendo.00248.2002. [DOI] [PubMed] [Google Scholar]
- 114.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J. Biol. Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 115.Zhao G, Souers A, Voorbach M, Falls H, Droz B, Brodjian S, Lau Y, Iyengar R, Gao J, Judd A, et al. Validation of diacyl glycerolacyltransferase I as a novel target for the treatment of obesity and dyslipidemia using a potent and selective small molecule inhibitor. J. Med. Chem. 2008;51:380–383. doi: 10.1021/jm7013887. [DOI] [PubMed] [Google Scholar]
- 116.Chen HC, Rao M, Sajan MP, Standaert M, Kanoh Y, Miura A, Farese RV, Jr, Farese RV., Sr The role of adipocyte-derived factors in enhancing insulin signaling in skeletal muscle and white adipose tissue of mice lacking acyl coenzyme A:diacylglycerol acyltransferase 1. Diabetes. 2004;53:1445–1451. doi: 10.2337/diabetes.53.6.1445. [DOI] [PubMed] [Google Scholar]
- 117.Chen HC, Jensen DR, Myers HM, Eckel RH, Farese RV., Jr Obesity resistance and enhanced glucose metabolism in mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J. Clin. Invest. 2003;111:1715–1722. doi: 10.1172/JCI15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Streeper RS, Koliwad SK, Villanueva CJ, Farese RV., Jr Effects of DGAT1 deficiency on energy and glucose metabolism are independent of adiponectin. Am. J. Physiol. Endocrinol. Metab. 2006;291:E388–E394. doi: 10.1152/ajpendo.00621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr. Opin. Lipidol. 2003;14:255–261. doi: 10.1097/00041433-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- 122.Liang J, Oelkers P, Guo C, Chu P, Dixon J, Ginsberg H, Sturley S. Overexpression of human diacylglycerol acyltransferase 1, acyl-coa:cholesterol acyltransferase 1, or acyl-CoA: cholesterol acyltransferase 2 stimulates secretion of apolipoprotein B-containing lipoproteins in McA-RH7777 cells. J. Biol. Chem. 2004;279:44938–44944. doi: 10.1074/jbc.M408507200. [DOI] [PubMed] [Google Scholar]
- 123.Yamazaki T, Sasaki E, Kakinuma C, Yano T, Miura S, Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. J. Biol. Chem. 2005;280:21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- 124.Yu X, Murray S, Pandey S, Booten S, Bao D, Song X, Kelly S, Chen S, McKay R, Monia BP, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005;42:362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Millar J, Cromley D, Graham M, Crooke R, Billheimer J, Rader D. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim. Biophys. Acta. 2008;1781:97–104. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 126.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuck A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 128.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2007;293:E576–E586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 129.Casaschi A, Maiyoh G, Adeli K, Theriault A. Increased diacylglycerol acyltransferase activity is associated with triglyceride accumulation in tissues of diet-induced insulin-resistant hyperlipidemic hamsters. Metabolism. 2005;54:403–409. doi: 10.1016/j.metabol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 130.Choi C, Savage D, Kulkarni A, Yu X, Liu Z, Morino K, Kim S, Distefano A, Samuel V, Neschen S, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 2007;282:22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 131.Yamaguchi K, Yang L, McCall S, Huang J, Yu X, Pandey S, Bhanot S, Monia B, Li Y, Diehl A. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steato-hepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 132.Cases S, Zhou P, Schillingford J, Wiseman B, Fish J, Angle CS, Hennighausen L, Werb Z, Farese RV., Jr Development of the mammary gland requires DGAT1 expression in stromal and epithelial tissues. Development. 2004;131:3047–3055. doi: 10.1242/dev.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 134.Giguere V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 135.Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 136.Zouboulis CC, Korge B, Akamatsu H, Xia LQ, Schiller S, Gollnick H, Orfanos CE. Effects of 13-cis-retinoic acid, all-trans-retinoic acid, and acitretin on the proliferation, lipid synthesis and keratin expression of cultured human sebocytes in vitro. J. Invest. Dermatol. 1991;96:792–797. doi: 10.1111/1523-1747.ep12471782. [DOI] [PubMed] [Google Scholar]
- 137.Strauss JS, Stewart ME, Downing DT. The effect of 13-cis-retinoic acid on sebaceous glands. Arch. Dermatol. 1987;123:1538a–1541. [PubMed] [Google Scholar]
- 138.Bazzano G, Terezakis N, Attia H, Bazzano A, Dover R, Fenton D, Mandir N, Celleno L, Tamburro M, Jaconi S. Effect of retinoids on follicular cells. J. Invest. Dermatol. 1993;101(Suppl.):138–142. doi: 10.1111/1523-1747.ep12363264. [DOI] [PubMed] [Google Scholar]
- 139.Mehta RG, Cerny WL, Moon RC. Retinoids inhibit prolactin-induced development of the mammary gland in vitro. Carcinogenesis. 1983;4:23–26. doi: 10.1093/carcin/4.1.23. [DOI] [PubMed] [Google Scholar]
- 140.Kistler A. Structure-activity relationship of retinoids on lobuloalveolar differentiation of cultured mouse mammary glands. Carcinogenesis. 1986;7:1175–1182. doi: 10.1093/carcin/7.7.1175. [DOI] [PubMed] [Google Scholar]
- 141.Schiffman M, Santorineou M, Lewis S, Turchin H, Gluecksohn-Waelsch S. Lipid deficiencies, leukocytosis, brittle skin—a lethal syndrome caused by a recessive mutation, edematous (oed), in the mouse. Genetics. 1975;81:525–536. doi: 10.1093/genetics/81.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miyazaki M, Dobrzyn A, Elias P, Ntambi J. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc. Natl. Acad. Sci. USA. 2005;102:12501–12506. doi: 10.1073/pnas.0503132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Accad M, Smith S, Newland D, Sanan D, King LE, Jr, Linton M, Fazio S, Farese RV., Jr Massive xanthomatosis and altered composition of atherosclerotic lesions in hyper-lipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moulson C, Martin D, Lugus J, Schaffer J, Lind A, Miner J. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc. Natl. Acad. Sci. USA. 2003;100:5274–5279. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herrmann T, van der Hoeven F, Grone H, Stewart A, Langbein L, Kaiser I, Liebisch G, Gosch I, Buckkremer F, Drobnik W, et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J. Cell Biol. 2003;161:1105–1115. doi: 10.1083/jcb.200207080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Coudreau SK, Tounian P, Bonhomme G, Froguel P, Girardet JP, Guy-Grand B, Basdevant A, Clement K. Role of the DGAT gene C79T single-nucleotide polymorphism in French obese subjects. Obes. Res. 2003;11:1163–1167. doi: 10.1038/oby.2003.160. [DOI] [PubMed] [Google Scholar]
- 147.Saar K, Geller F, Rüschendorf F, Reis A, Friedel S, Schäuble N, Nürnberg P, Siegfried W, Goldschmidt H, Schäfer H, et al. Genome scan for childhood and adolescent obesity in German families. Pediatrics. 2003;111:321–327. doi: 10.1542/peds.111.2.321. [DOI] [PubMed] [Google Scholar]
- 148.Bouchard C, Pérusse L, Chagnon Y, Warden C, Ricquier D. Linkage between markers in the vicinity of the uncoupling protein 2 gene and resting metabolic rate in humans. Hum. Mol. Genet. 1997;6:1887–1889. doi: 10.1093/hmg/6.11.1887. [DOI] [PubMed] [Google Scholar]
- 149.Friedel S, Reichwald K, Scherag A, Brumm H, Wermter A, Fries H, Koberwitz K, Wabitsch M, Meitinger T, Platzer M, et al. Mutation screen and association studies in the diacylglycerol O-acyltransferase homolog 2 gene (DGAT2), a positional candidate gene for early onset obesity on chromosome 11q13. BMC Genet. 2007;8:17. doi: 10.1186/1471-2156-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen HC, Farese RV., Jr Inhibition of triglyceride synthesis as a treatment strategy for obesity: lessons from DGAT1-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:482–486. doi: 10.1161/01.ATV.0000151874.81059.ad. [DOI] [PubMed] [Google Scholar]
- 151.Matsuda D, Tomoda H. DGAT inhibitors for obesity. Curr. Opin. Investig. Drugs. 2007;8:836–841. [PubMed] [Google Scholar]
- 152.Tabata N, Ito M, Tomoda H, Omura S. Xanthohumols, diacylglycerol acyltransferase inhibitors, from Humulus lupulus. Phytochemistry. 1997;46:683–687. doi: 10.1016/s0031-9422(97)00157-x. [DOI] [PubMed] [Google Scholar]
- 153.Tomoda H, Ohyama Y, Abe T, Tabata N, Namikoshi M, Yamaguchi Y, Masuma R, Omura S. Roselipins, inhibitors of diacylglycerol acyltransferase, produced by Gliocladium roseum KF-1040. J. Antibiot. 1999;52:689–694. doi: 10.7164/antibiotics.52.689. [DOI] [PubMed] [Google Scholar]
- 154.Chung MY, Rho MC, Ko JS, Ryu SY, Jeune KH, Kim K, Lee HS, Kim YK. In vitro inhibition of diacylglycerol acyltransferase by prenylflavonoids from Sophora flavescens. Planta Med. 2004;70:258–260. doi: 10.1055/s-2004-815545. [DOI] [PubMed] [Google Scholar]
- 155.Lee SW, Kim K, Rho MC, Chung MY, Kim YH, Lee S, Lee HS, Kim YK. New polyacetylenes, DGAT inhibitors from the roots of Panax ginseng. Planta Med. 2004;70:197–200. doi: 10.1055/s-2004-815534. [DOI] [PubMed] [Google Scholar]
- 156.Ko JS, Ryu SY, Kim YS, Chung MY, Kang JS, Rho MC, Lee HS, Kim YK. Inhibitory activity of diacylglycerol acyltransferase by tanshinones from the root of Salvia miltiorrhiza. Arch. Pharm. Res. 2002;25:446–448. doi: 10.1007/BF02976599. [DOI] [PubMed] [Google Scholar]
- 157.Rustan AC, Nossen JØ, Christiansen EN, Drevon CA. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J. Lipid Res. 1988;29:1417–1426. [PubMed] [Google Scholar]
- 158.Hobbs DH, Lu C, Hills MJ. Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett. 1999;452:145–149. doi: 10.1016/s0014-5793(99)00646-8. [DOI] [PubMed] [Google Scholar]
- 159.Lu CL, de Noyer SB, Hobbs DH, Kang J, Wen Y, Kratchus D, Hills MJ. Expression pattern of diacylglycerol acyltransferase-1, an enzyme involved in triacylglycerol biosynthesis, in Arabidopsis thaliana. Plant Mol. Biol. 2003;52:31–41. doi: 10.1023/a:1023935605864. [DOI] [PubMed] [Google Scholar]
- 160.Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 161.Zheng P, Allen W, Roesler K, Williams M, Zhang S, Li J, Glassman K, Ranch J, Nubel D, Solawetz W, et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat. Genet. 2008;40:367–372. doi: 10.1038/ng.85. [DOI] [PubMed] [Google Scholar]
- 162.Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol. J. 2008. 2008. Jul 17, Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 163.Lardizabal K, Effertz R, Levering C, Mai J, Pedroso M, Jury T, Aasen E, Gruys K, Bennett K. Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol. 2008;148:89–96. doi: 10.1104/pp.108.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Buszczak M, Lu X, Segraves WA, Chang TY, Cooley L. Mutations in the midway gene disrupt a Drosophila acyl coenzyme A:diacylglycerol acyltransferase. Genetics. 2002;160:1511–1518. doi: 10.1093/genetics/160.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grisart B, Coppieters W, Farnir F, Karim L, Ford C, Berzi P, Cambisano N, Mni M, Reid S, Simon P, et al. Positional candidate cloning of a QTL in dairy cattle: identification of a missense mutation in the bovine DGAT1 gene with major effect on milk yield and composition. Genome Res. 2002;12:222–231. doi: 10.1101/gr.224202. [DOI] [PubMed] [Google Scholar]
- 166.Winter A, Kramer W, Werner FA, Kollers S, Kata S, Durstewitz G, Buitkamp J, Womack JE, Thaller G, Fries R. Association of a lysine-232/alanine polymorphism in a bovine gene encoding acyl-CoA:diacylglycerol acyltransferase (DGAT1) with variation at a quantitative trait locus for milk fat content. Proc. Natl. Acad. Sci. USA. 2002;99:9300–9305. doi: 10.1073/pnas.142293799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grisart B, Farnir F, Karim L, Cambisano N, Kim JJ, Kvasz A, Mni M, Simon P, Frere JM, Coppieters W, et al. Genetic and functional confirmation of the causality of the DGAT1 K232A quantitative trait nucleotide in affecting milk yield and composition. Proc. Natl. Acad. Sci. USA. 2004;101:2398–2403. doi: 10.1073/pnas.0308518100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaupe B, Winter A, Fries R, Erhardt G. DGAT1 polymorphism in Bos indicus and Bos taurus cattle breeds. J. Dairy Res. 2004;71:182–187. doi: 10.1017/s0022029904000032. [DOI] [PubMed] [Google Scholar]
- 169.Kühn C, Thaller G, Winter A, Bininda-Emonds O, Kaupe B, Erhardt G, Bennewitz J, Schwerin M, Fries R. Evidence for multiple alleles at the DGAT1 locus better explains a quantitative trait locus with major effect on milk fat content in cattle. Genetics. 2004;167:1873–1881. doi: 10.1534/genetics.103.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fürbass R, Winter A, Fries R, Kühn C. Alleles of the bovine DGAT1 variable number of tandem repeat associated with a milk fat QTL at chromosome 14 can stimulate gene expression. Physiol. Genomics. 2006;25:116–120. doi: 10.1152/physiolgenomics.00145.2005. [DOI] [PubMed] [Google Scholar]
- 171.Kuehn C, Edel C, Weikard R, Thaller G. Dominance and parent-of-origin effects of coding and non-coding alleles at the acylCoA-diacylglycerol-acyltransferase (DGAT1) gene on milk production traits in German Holstein cows. BMC Genet. 2007;8:62. doi: 10.1186/1471-2156-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thaller G, Kuhn C, Winter A, Ewald G, Bellmann O, Wegner J, Zuhlke H, Fries R. DGAT1, a new positional and functional candidate gene for intramuscular fat deposition in cattle. Anim. Genet. 2003;34:354–357. doi: 10.1046/j.1365-2052.2003.01011.x. [DOI] [PubMed] [Google Scholar]
- 173.Lehner R, Kuksis A. Triacylglycerol synthesis by an sn-1,2(2,3)-diacylglycerol transacylase from rat intestinal microsomes. J. Biol. Chem. 1993;268:8781–8786. [PubMed] [Google Scholar]
- 174.Oelkers P, Tinkelenberg A, Erdeniz N, Cromley D, Billheimer JT, Sturley SL. A lecithin cholesterol acyltransferase-like gene mediates diacylglycerol esterification in yeast. J. Biol. Chem. 2000;275:15609–15612. doi: 10.1074/jbc.C000144200. [DOI] [PubMed] [Google Scholar]
- 175.Kalscheuer R, Steinbüchel A. A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 2003;278:8075–8082. doi: 10.1074/jbc.M210533200. [DOI] [PubMed] [Google Scholar]